Methods for evaluating penetration of drug into the skin: A review
Abstract
Background
Skin being the largest organ of the human body plays a very important role in the permeation and penetration of the drug. In addition, the transdermal drug delivery system (TDDS) plays a major role in managing dermal infections and attaining sustained plasma drug concentration. Thus, evaluation of percutaneous penetration of the drug through the skin is important in developing TDDS for human use.
Material and methods
Various techniques are used for getting the desired drug penetration, permeation, and absorption through the skin in managing these dermal disorders. The development of novel pharmaceutical dosage forms for dermal use is much explored in the current era. However, it is very important to evaluate these methods to determine the bioequivalence and risk of these topically applied drugs, which ultimately penetrate and are absorbed through the skin.
Results
Currently, numerous skin permeation models are being developed and persuasively used in studying dermatopharmacokinetic (DPK) profile and various models have been developed, to evaluate the TDD which include ex vivo human skin, ex vivo animal skin, and artificial or reconstructed skin models.
Conclusion
This review discusses the general physiology of the skin, the physiochemical characteristics affecting particle penetration, understand the models used for human skin permeation studies and understanding their advantages, and disadvantages.
1 INTRODUCTION
Skin is the largest organ of human body, which perpetually performs homeostatic barrier function with respect to outward loss of water. Due to its easy accessibility and large surface area, it plays a major role in drug delivery.1 Many compounds, such as sunscreens, insect repellents, and antiseptics, are meant to remain on the skin surface (topical) while others penetrate the skin layers (transdermal) to target sites within or just below the skin. Transdermal delivery is a concept that can be restricted to the situation where a substance diffuses through the various layers of the skin and into the systemic bloodstream with a medicinal result to be performed, for example, withdrawal effects through nicotine therapy. It shows added benefit in managing dermal infections like psoriasis, various fungal infections, cutaneous and visceral leishmaniasis, and ulcers. If we consider the treatment of cutaneous leishmaniasis, the basic criteria are to get localized and sustained plasma drug concentration for which TDD is useful.2 TDDS is generally preferred for delivering drugs which show hepatic first pass effect and are unstable in gastrointestinal (GI) system.3, 4 Whereas, dermal (topical) distribution should be used only to establish a targeting of the pathological sites in the skin, ensuring minimum systemic absorption. This type of drug localization is important in the treatment of dermatological conditions like skin cancer, psoriasis, eczema and microbial infections where the seat of the disease is in the skin.5 In certain cases, drug permeation is aimed at body regions near the site of action where a specific effect is expected, for example, in the muscles, blood vessels, and articulations.
Evaluation of percutaneous penetration of drug at the site of action is important in developing TDDS for human use. Due to ethical considerations and feasibility, fundamental absorption data from human and animal skin are difficult to obtain by in vivo study.6 Thus, various other techniques are used as a substitute for getting the desired information, especially during the development of novel pharmaceutical dosage forms for dermal use.7-10 These methods are important instruments for evaluating the effectiveness and quality of the topical and transdermal formulations. One of the major hurdles in developing transdermal formulation is correlation between ex vivo, animal and human studies for percutaneous absorption and drug penetration through the skin.11, 12 Pharmaceutical formulators, however, need to be aware of all options in the selection of methods for the intended formulations.13, 14 Thus, now a days numerous skin permeation models are being developed and persuasively used in studying dermatopharmacokinetic (DPK) profile of the formulations. Many such models have been developed, to evaluate the TDDS, which include ex vivo human skin, ex vivo animal skin, and artificial or reconstructed skin models. Although some procedures are reported for carrying out skin permeation studies, no formal standardization is available.15, 16
Through this review, we would like to discuss the general physiology of skin, followed by physiochemical characteristics affecting particle penetration through the skin, understand how effectively various models are used as surrogate for human skin, what are their advantages, and disadvantages considering the importance of different skin parts for percutaneous absorption and penetration study.
2 PHYSIOLOGY OF SKIN
Number of topical or dermatological products are applied to the skin or mucous membrane, which enhance the fundamental function or pharmacologically alter the action in the underlined tissues.17 Thus, to utilize the phenomenon of percutaneous absorption successfully, it is important to understand the anatomy, physiology, physicochemical properties of skin.
The skin of an average adult covers a surface area of approximately 2 m2 and receives about one-third of the blood circulating through the body. Microscopically skin constitutes three main histological layers: epidermis, dermis, and hypodermis (subcutaneous layer).18 The epidermis is 0.1-1.5 mm thick which is further divided into five parts: stratum germinativum, stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum. It is responsible for the formation of the melanin pigment. The squamous cell layer is the thickest layer of the epidermis that helps to in movement of certain substances in and out of the body. The stratum corneum (“horny layers”) is made up of 10 to 30 thin layers of the dead cells where the outermost layer is replaced by new layer.19 Just beneath the epidermis, lies the dermis, which is 1.5 to 4 mm thick. It contains collagen, elastin, sweat, oil glands, hair follicles, nerve endings, and blood and lymph vessels. Dermis acts as a water reservoir and contains scavenger cells from the immune system that engulf the foreign organisms. The hypodermis (subcutaneous tissue) is the deepest layer of the skin. Subcutaneous tissue acts as an insulator and shock absorber protecting internal organs from injury. It stores fat and the blood vessels, nerves, lymph vessels, and hair follicles cross through these layers. The stratum corneum, which is the outermost layer of the skin, is an effective barrier for penetration of drugs into deeper layers.20, 21
Skin from different body parts of numerous animals is used for testing any new pharmaceutical dermal formulation or for comparative study of dermatopharmacokinetics of two dermal formulations. The animal models are chosen depending upon the purpose of experiment and data to be obtained with availability of the model. Skin from various parts of body like flank, ear, arms, and legs is used. For the penetration study of formulations intended to use in human, animals are selected with reference to their resemblance to human skin. (Table 1)22 For example, the porcine ear skin is most often used as surrogate to human skin due to average of 20 hairs per cm2 as porcine ear skin is similar to 14-32 hairs per cm2 in humans.23 Physiological and anatomical similarities between skins of these animals make them to be used as surrogate for human skin.
| Sr no. | Species | Anatomic site | SC (μm) | Epidermis (μm) | Whole skin (mm) |
|---|---|---|---|---|---|
| 1. | Human | Forearm | 17 | 36 | 1.5 |
| 2. | Pig | Back | 26 | 66 | 3.4 |
| 3. | Pig | Ear | 10 | 50 | 1.3 |
| 4. | Mouse | Back | 5 | 13 | 0.8 |
3 PENETRATION THROUGH SKIN
The factors responsible for measuring the efficacy of TDDS include physicochemical characteristics of the drug and the type of the formulation. Whereas, the efficiency of treatment depends on the penetration of drug through the target layers of the skin at effective concentrations. (Table 2).
| Sr no. | Drug molecule characteristics | Description | Reference |
|---|---|---|---|
| 1 | Drug molecule size and molecular weight |
|
21, 39, 76 |
| 3 | Lipophilicity |
|
28 |
| 4 | Hydrogen-bonding groups |
|
28, 77 |
| 5 | Solubility |
|
78, 79 |
| 6 | Ionization of drug molecule |
|
80 |
| 7 | Stereochemistry and steric interaction |
|
28, 81, 82 |
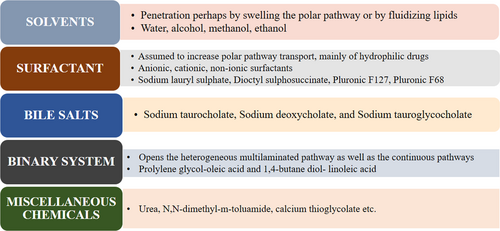
Effective penetration of drug molecule plays an important role in TDDS. There are various routes of penetration of drug into skin. The micro and macromolecules can enter into the skin through three distinct pathways: (a) The intercellular pathway, which is through the lipid matrix occupying the intercellular spaces of the keratinocytes hence most favor by lipophilic molecules, (b) the transcellular (intracellular) pathway, which is through the corneocytes, and (c) trans appendageal pathway, which is across hair follicles, sebaceous glands, and sweat glands.24, 25 After application of topical formulation drug molecule come in contact with skin surface, at the skin surface, molecules come across cellular debris, microorganisms, sebum, and other materials, which negligibly affect permeation.26, 27 For the intracellular route, drugs pass directly through the cells of the stratum corneum, whereas in the intercellular route, they diffuse around the cells in a tortuous manner.25, 28 Intracellular route is difficult to move since it has to reach each cell's lipophilic membrane first, then the cell's hydrophilic core, and then back out of the lipophilic membrane hence it is preferable by lipophilic as well as hydrophilic molecules depending on their polarity.29 The intracellular route also requires some hydrophilic character to carry through the inside of the corneocyte, but incidentally enough the intercellular route is another polar path of penetration. The appendageal pathway involved in the absorption of liposomes, nanoparticles, and cyclodextrin-inclusion complexes as per the studies reported by the different preachers.30, 31 One long-standing approach to increasing the range of drugs that can be delivered effectively through dermal route was the use of penetration enhancers, chemicals that interact with skin constituents to promote the flow of drugs.32 Various mechanical and chemical ways are studied by numerous researchers which overcomes the skin barriers and manipulate these pathways for successful delivery of drug molecule across the skin.33-35 In Figure 1, various class of chemical penetration enhancers along with their most probably used examples are mentioned.36 The ideal chemical enhancers should be non-toxic, non-irritating and should allow therapeutic agents into the body while preventing the loss of endogenous material from the body. Skin models are used for evaluation of the activity of the penetration enhancers, in order to increase the efficiency of transdermal formulations containing penetration enhancers.37
4 METHODS FOR EVALUATING PENETRATION OF DRUG INTO SKIN
A number of drugs are given by transdermal route to target deeper dermal, subcutaneous, and muscle layers.38 For such drugs, it is important to determine the drug level within the skin and understand the penetration behavior into deep dermal tissue layers in order to evaluate the dermal bioavailability or assess the bioequivalence between different formulations for which a gold standard lie in vivo human skin should be peffered.39, 40 This is not always feasible, though, because of the high cost of clinical trials and questions about the introduction of drugs or products with potentially harmful effects. Therefore, other techniques are used to obtain the desired information.41 One of these techniques is the use of in vitro penetration and permeation models. Moreover, one of the in vitro-in vivo correlations for topical preparations can be obtained by measuring the drug release from formulation and in vivo measurement of drug concentration in the SC or dermatopharmacokinetic (DPK) subject.42 Many researchers concluded that in vitro measurements can be used to predict absorption in vivo, if properly conducted. The Organization for Economic Cooperation and Development (OECD) published several issues about this topic, including the Guidance Notes on Dermal Absorption (No. 156),15 Test Guidelines 427 (in vivo methods)43 and 428 (in vitro methods).38 The European medicine agency also provided the guidelines for the evaluation of topical products.16, 44 These documents provide guidelines on and explanations of how to perform dermal absorption assays, but they do not adequately control the measurements. Certain invasive techniques such as indirect radiochemical methods, surface scrapping, sampling of hair, and suction blister sampling, which are too non-specific or too traumatic, are generally not preferred for routine use in humans.45 However, few feasible methods, most commonly used for the pharmacokinetic assessment of topically formulations, are discussed below.
5 CLASSIFICATION OF METHODS
- In vitro techniques
- Franz diffusion cell
- In vivo techniques
- Ex vivo
- Tape stripping technique
- Franz diffusion cell
- In-vivo
- Skin biopsy
- Suction Blister
- Micro dialysis
- Others
- Confocal laser microscopy
- Ex vivo
5.1 In vitro techniques
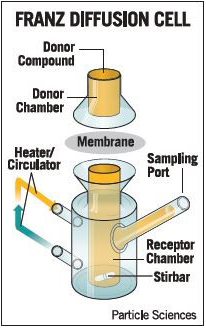
5.1.1 Franz diffusion cell
Franz diffusion cell is generally used to assess skin permeability, check drug and formulation relationship, and evaluation of transdermal formulations.9, 46 (Figure 2). The apparatus consists of a donor and receptor compartment between which the animal model membrane is fixed in a way such that the stratum corneum is facing the donor compartment where the formulation to be examined is applied, while the dermis (full thickness skin) is touching receptor compartment.47 The permeation rate of the drug from the donor compartment through the skin into the receptor is determined by measuring the amount of drug permitted over time, for example, high-performance liquid chromatography (HPLC) as an analytical method.26 This method is normally used with excised human or animal skin. Numerous animal models have been suggested as a substitute for human skin, including primate, porcine, mouse, rat, guinea pig, and snake skins.16, 48 However, when biological skin is not readily available, synthetic membranes are employed.49 In 1987, the FDA published a report on the important factors to be considered, including membrane type (dermatomized skin or heat-separated epidermis?), receptor fluid, cell design (static or flow-through?), application (finite or infinite dose?), and temperature.50 In 1989, Aly et al carried out three phases of research for topical antifungal, first by research pig skin and human body skin in side-by-side comparison and by demonstrating that cyclopyroxolamine 1 percent, in two separate vehicles, lotion, and cream, similarly penetrated all stratum corneum layers in both skin types and inhibited the growth of T. mentagrophytes and Candida albicans.50, 51The synthetic membranes employed in Franz cell drug diffusion studies have two functions: simulation of the skin and quality control.52 Animal models and cell membranes equivalent to human skin are used. Table 3 provides a summary of the specifications, advantages, and limitations of alternative skin animal membranes commonly used in TDD research.53
| Sr no. | Model | Specifications | Disadvantages |
|---|---|---|---|
| A | Animal models used for Ex vitro studies | ||
| 1 | Rats, rodents skin83 |
The problem of fur can be eliminated using hairless animals |
More permeable than human skin |
| 2 | Porcine/ Pigs ear skin5, 22, 58 |
Permeability is close to human skin |
Difficult to obtain High cost |
| 3 | Monkeys skin72 |
|
|
| 4 | Snake skin7 |
structure, composition, lipid content, and water permeability |
the lack of hair follicles could influence drug permeability |
| B | Artificial reconstructed skin models used for In Vitro studies | ||
| 1 | Reconstructed epidermis84, 85 |
|
Relatively weak barrier especially for lipophilic drugs |
| 2 | Reconstructed full thickness skin86 |
|
Absence of the vascular network may provide false barrier nature |
| C | Lamellar matrix21, 26, 87 |
Permeation studies showed good barrier nature |
The lipid composition may be dissimilar to SC lipids. Need further investigations with various drugs |
| D | Stratum corneum model57 |
Provides reproducible and consistent results with a distinction between the barriers mimicking compromised and healthy skin |
Rarely use due to high cost |
Advantages
- It is use as gold standard for DPK evaluation
- No continuous sample collecting is required as it can be automated
- Requires low amount of drug required for analysis
Disadvantages
- Differences between research labors
- Different types of cells are required depending on the type of formulation release kinetics
- Use of instrument requires practice as it is not user-friendly
5.2 In vivo techniques
5.2.1 Ex vivo
Tape stripping technique
Tape stripping is a well-established method to investigate the skin penetration, local bioavailability, or dermatopharmacokinetics (DPK) of topically applied substances whose target is the underlying viable tissues.54, 55 Frequently porcine ear skin is used as a substitute for human skin in dermatological research and is useful for tape stripping experiments where the penetration of active substances into the uppermost skin layers is investigated.56 In this method, formulation to be tested is applied topically and the cell layers of the stratum corneum are sequentially removed from the respective skin area by using adhesive films.7 Choice of adhesive tape, pressure required to be applied to the adhesive films, and velocity of their removal from the skin are important factors.7 The tape strips contain corneocytes and the corresponding amount of the penetrated formulation, which can be determined by classical analytical chemical methods. The drug concentration on each tape is determined individually and penetration profiles may be derived, as a function of depth within the SC layer.54 Also drug or excipients level in the skin can be determined either in the removed tape strips, or directly in the tape stripped skin.57 The data thus obtained could be used for comparison of the penetration of different formulations to assess the bioequivalence.8 The FDA draft guidance indicates the assessment of both drug uptake into and drug elimination from the stratum corneum by tape stripping technique. The data thus obtained could be used for comparison of the penetration of different formulations to assess the bioequivalence.58 In addition, the method can be employed both in vivo and in vitro, and in humans and animals such as pigs, rats, guinea pigs, and mice.54 Moreover, it must be kept in mind that the successive application and removal should be carried out from the same treated skin area. In addition, there are several factors which could eventually alter the quantity of stratum corneum removed in each adhesive tape strips. These include size of the corneocytes, number of cell layers, and thickness of SC, age, and composition and amount of lipids (depending on the anatomical site). Several methods have been proposed to quantify the amount of stratum corneum removed by individual tape stripping. These include weighing, spectroscopic, and microscopic measurements.
Advantages
- This method is simple, inexpensive, efficient, quick, and relatively non-invasive approach to assess the quality and efficacy of drugs, excipients, cosmetics in the skin following the topical dermatological application.59
- In general, the variation in the tape stripping method is considerable as the number of tape strips used in removing the stratum corneum varies with the type of tape, pressure applied during application, and force in removal.7
- It is stand-alone technique thus mainly helpful to assess the local bioavailability of drugs whose target site is the stratum corneum itself.
Disadvantages
- Velocity of removing this tape can be varied which leads to error in the evaluation data.
- The major disadvantage is with the detection of material by using this method which includes interference with chemical absorbing in the wavelength range of coenocytes absorbance at wavelength 430 nm.
5.2.2 In vivo
By 2013, the EU dedicated itself to removing animal testing for cosmetics, thereby giving greater prominence to human in vivo studies, particularly those that can be performed non-invasively.60 Hence, various imaging techniques like confocal microscopy, tomography, fluorescence studies received much attention by the research scientist.
Skin biopsy
Skin biopsy technique is generally differentiated according to the area used for the biopsy sample like if it is the level of the dermis (shave biopsy) or through to the sub-cutis (punch biopsy). These methods are invasive and generally performed under local anesthesia. Skin biopsy has not attracted very much attention as routine approach for tissue sampling and analysis of post-drug application in transdermal delivery system.55 Despite of many attempts to reduce the tissue trauma, this method is not remotely acceptable61, 62 and its use in the foreseeable future will be restricted to animal and in vitro studies.
Advantages
- This method offers a snapshot of drug disposition in the different skin layers.
Disadvantages
Suction Blister
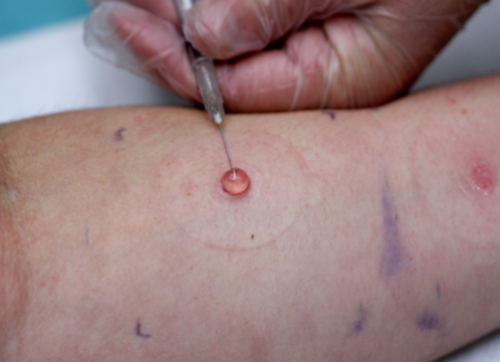
Applying a partial negative pressure to the skin disrupts the epidermal-dermal junction and forms a blister which fills progressively with interstitial fluid and serum.63, 64 (Figure 3) This liquid offers a pharmacokinetic compartment, in which a previously applied drug can be sampled with a hypodermic needle and quantified; if multiple blisters are raised (as is possible with certain commercially available devices), then a concentration-time profile of the drug in the skin can be obtained.55 This approach is quite invasive and causes obvious scarring and is difficult to do over relatively small areas of skin. The technique can be used to compare topical formulations in a reasonably objective way, but the potential binding of the drug to skin tissue, especially for very lipophilic species, may mean that very low levels are present, if at all, in the blister fluid.65, 66
Advantages
- Method is easy to perform
- Low chance of rejection of results obtained from this method40
Disadvantages
- Too invasive a technique for practical and routine application55
Microdialysis
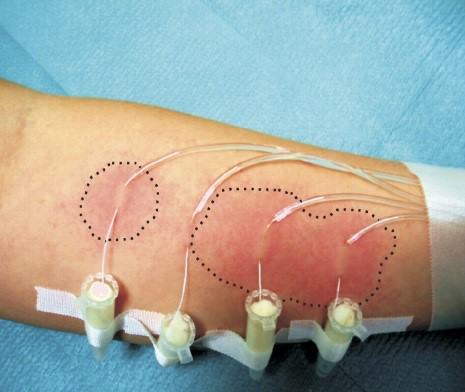
Microdialysis technique has been introduced to dermatological research as a valuable in vivo tool that is minimally invasive and enables assessment of full local pharmacokinetic profiles of percutaneous drug penetration from each sampling site (Figure 4).67 Microdialysis is based on the passive diffusion of compounds down a concentration gradient across a semipermeable membrane forming a thin hollow tube (typically a few tenths of a millimeter in diameter), which at least, in theory functionally represents a permeable blood vessel.55 The main key step for this technique is the insertion of a small microdialysis catheter made up of dialysis membrane and referred as microdialysis probe into the tissue of interest. The microdialysis probe is designed to mimic a blood capillary and consists of a shaft with a semipermeable hollow fiber membrane at its tip, which is connected to inlet and outlet tubing. It is connected with in- and outlet tubes, which is constantly perfused on the inside while the outside is in direct contact with the medium of interest.9, 67 Once inserted into the tissue or (body)fluid of interest, small solutes can cross the semipermeable membrane by passive diffusion. The direction of the analyte flow is depend on the respective concentration gradient and allows the usage of microdialysis probes as sampling as well as delivery tools.68 The sample solution leaving the probe (dialysate) is collected at certain time intervals for analysis.69, 70 Relative recovery of test substance describes the efficacy of this method as it is nothing but the ratio between the concentration of a substance in the dialysate and the true extracellular concentration.42 This method is used with certain non-invasive bioengineering methods like transepidermal water loss (TEWL), ultrasound technique to observe barrier function and functional status of the skin.70 Skin barrier function shows impact on penetration61 measurement of this parameter leads to a valuable addition in penetration studies through human skin.71 The technique effectively helps in valuable investigation of penetration of drugs and other substances through the skin.
Advantages
- This method can give very detailed chronological pharmacokinetic data, and simultaneously several sampling sites can be considered in the same volunteer.72
- The semipermeable membrane used prevents cells, cellular debris, and proteins from entering into the dialysate. Thus due to the lack of protein in the dialysate, a sample clean-up prior to analysis is not needed and enzymatic degradation is not a concern.73
- Insertion of the probe to particular location of the selected tissue helps in evaluation of extracellular concentration gradients due to transporter activity or other factors, such as perfusion differences. Thus, it is been suggested as the most appropriate technique to be used for tissue distribution studies.
Disadvantages
- Use of microdialysis probe may can alter tissue morphology resulting in disturbed microcirculation, rate of metabolism, or integrity of physiological barriers.
- Need of sensitive analytical method to detect small concentrations of analyte.40
5.2.3 Other techniques
Confocal laser scanning microscopy (CLSM)
Confocal laser scanning microscopy is widely used for checking spatial drug distribution of the principle drug inside the tissue by using fluorescence phenomenon.12 The technique works on the principle of point illumination and detection which is the ability to differentiate between the light originating from different planes of the specimen. The confocal microscope can image thick samples with high resolution have enabled their use in studying skin penetration of various molecules and delivery systems. CLSM is useful in analyzing localization of different nanoparticles, lipid microparticles, noisome, liposomes etc in specific skin tissue samples. Various studies have been carried out using CLSM to understand the transport of hydrophilic and hydrophobic drugs into the skin using these drug carriers that is, niosomes, liposomes, nanoparticles, etc Technique has been applied in vivo and in vitro for studying the skin structure without physically cutting tissue or to assess the effects of physical and chemical enhancers on skin permeability.74, 75
Advantages
- Non-invasive technique and clear high defined images obtained from microscope help in assessing drug penetration into skin
- High-resolution images with depth sensitivity can be obtained by using CLSM
Disadvantages
- Qualitative analysis is possible with this technique, not quantitative.
- Images are also limited to determined points of the skin at determined times, and the images do not represent the dynamic process of skin permeation for prolonged times.
- Limited fluorescence markers are available for these studies.
6 CONCLUSION
These evaluation methods and models with all their limitations and advantages provide promising tool for studying dermatopharmacokinetics of various transdermal formulations. The evaluation methods help in understanding the penetration mechanism of molecules in the skin which ultimately help in evaluation of molecules which lead to skin irritation. These evaluation methods and model set up a new approach in developing topical pharmaceutical formulations for various skin infection disorders.
ACKNOWLEDGEMENT
We would also like to thank Institute of Chemical Technology, Matunga, Mumbai, India for their continuous support.
CONFLICT OF INTEREST
The authors reported no potential conflict of interest.




