Oral immunization induces a novel CXCR6+β7+ intraepithelial lymphocyte subset predominating in the small intestine
Abstract
Intestinal T cells form a central part of the front-line defence against foreign organisms and need to be situated in the mucosa where infection occurs. It is well accepted that immunization by a mucosal route favours localization of antigen-specific effector T cells in the mucosal epithelium, while systemic immunization does not. The aim of the study is to determine how homing receptors are specifically involved in retaining effector T cells in the small intestine after oral immunization. We here demonstrate that the chemokine receptor CXCR6, integrins β7 and CD29 contribute differentially to the epithelial retention phenotype of CD8+ T cells in the small intestine of mice. CD8+ intraepithelial lymphocytes (IELs) of unvaccinated mice are predominantly β7 single positives, and subcutaneous immunization-induced antigen-specific CD8+ effector IELs are mainly composed of CXCR6+, CD29+ and CXCR6+CD29+ cells. Strikingly, the majority of oral immunization-induced antigen-specific CD8+ effector IELs exhibit a distinct, tissue-specific CXCR6+β7+ double-positive phenotype, cytotoxic potential and enhanced intraepithelial localization. Transfer of antigen-specific CD8+ T cells preactivated with certain immuno-stimuli (such as monophosphoryl lipid A) results in increased accumulation of donor IELs with the CXCR6+β7+ phenotype. As β7 exclusively paired with αE on IELs, our results strongly suggest that CXCR6 may cooperate with the heterodimer αEβ7 to preferentially retain intestinally induced effector IELs in the epithelium. The identification of this novel IEL phenotype has significant implications for the development of vaccines and therapeutic strategies to enhance gut immunity.
1 INTRODUCTION
T cells are an essential component in providing host protective immunity and maintaining immune homeostasis in the intestine.1-3 It has become increasingly recognized that route of immunization has a profound impact on the localization of vaccine-induced antigen-specific T cells, and by extension their ability to form protective mucosal immunity at the intestinal mucosae. Enteric immunization offers significant advantages over parenteral immunization in the induction of robust antigen-specific CD8+ effector IEL responses against various pathogens.4-8 These IELs acquire effector memory phenotypes soon after entering the intestine,7 and they do not recirculate but rather are retained in the epithelium for as long as months with no need of stimulation by antigen or gut flora.9, 10 The enhanced intestinal responses are highly associated with increased numbers of functional effector IELs that persist in the epithelium.
Localization of IELs is first accomplished through a migration process involving certain homing receptors in response to local chemokine gradients and cell-cell/cell-matrix adhesion.11, 12 It is well established that the chemokine receptor 9 (CCR9) and the heterodimeric integrin α4β7 mediate the entry of T cells from the blood to the small intestine lamina propria where their ligands CCL25 and mucosal vascular addressin cell adhesion molecule 1 (MAdCAM-1), respectively, are constitutively expressed.13-20 Dendritic cells (DCs) in the lamina propria and mesenteric lymph nodes (MLNs), but not in the spleen, particularly drive the expression of α4β7 and CCR9 on T cells through synthesizing all-trans retinoic acid derived from vitamin A.21-23 Once inside the lamina propria, T cells start downregulating CCR9 and α4β7 while differentiating into resident memory T cells.9 Some of them are induced to express αEβ7 and migrate to the epithelium in response to CCL25, thereby retaining themselves within the intraepithelial compartment through the interaction of αEβ7 with E-cadherin expressed on epithelial cells.14, 24, 25 However, studies have suggested that the αEβ7-E-cadherin interaction does not seem to be the only mechanism responsible for retention of intraepithelial effector T cells.7, 26
A few other homing receptors may potentially be involved in intraepithelial retention of IELs in the small intestine. The chemokine receptors CXCR6 and CX3CR1 are found on a low proportion of IELs, and their respective ligands CXCL16 and CX3CL1 are readily detectable in epithelial cells.27-29 They are upregulated by inflammatory cytokines IL-1β and TNF-α and are likely to directly retain IELs in the epithelium. Levels of other chemokine receptors, CCR2, CCR5, CCR6 and CXCR3, are also elevated during inflammation,17, 30-34 but investigations have largely centred on understanding of their roles in localization of lamina propria lymphocytes. Much less is currently known about how antigen-specific effector IELs are effectively retained in the epithelial layer after enteric immunization. In this study, we examined a range of homing receptors on small intestinal IELs in order to determine what homing receptor phenotype would result from oral vaccination. We discovered that the majority of vaccine-induced antigen-specific CD8+ IELs exhibit a distinct CXCR6+β7+ phenotype and such phenotype is highly associated with enhanced intestinal localization and cytotoxic potential.
2 MATERIALS AND METHODS
2.1 Animals and reagents
C57BL/6 mice with 6-8 weeks of age were obtained from Institute of Laboratory Animal Sciences (CAMS&PUMC) unless otherwise indicated. All experimental protocols were approved by the Institute of Materia Medica Animal Authorities.
Antibodies for flow cytometry were purchased from BioLegend, eBioscience or BD Biosciences. Fibroblast-stimulating lipopeptide (FSL-1), polyinosinic-polycytidylic acid (poly(I:C)) and monophosphoryl lipid A (MPLA) were purchased from Invivogen. CpG 1826 oligonucleotide and SIINFEKL peptide were synthesized by Sangon Biotech (Shanghai, China) and Chinese Peptide Company (Hangzhou, Zhejiang, China), respectively. All were free of endotoxin and protein contamination. The MHC tetramers (tet) loaded with SIINFEKL peptide were obtained from the NIH Tetramer Core Facility. Detailed information of reagents and consumables used in this study is provided in Table S1.
2.1.1 Segmentation of the small intestine
Prior to cell isolation, the small intestine was divided into a total of six subsegments (ss1‒6) nearly equal in length to each other. In some experiments, the small intestine was divided into three pieces corresponding to ss1‒2, ss3‒4 and ss5‒6, respectively.
2.1.2 Isolation of intestinal lymphocytes
Peyer's patches and visible lymphoid aggregates were removed from the small intestine before processing for isolation of IELs. Each subsegment was cut into a few pieces respectively, stirred at 37°C with Ca2+- and Mg2+-free HBSS containing 10% FBS, 15 mmol/L HEPES, 1 mmol/L EDTA, 2 mmol/L dithiothreitol. Epithelial cells were collected and the remaining tissue was discarded. A discontinuous density Percoll (GE healthcare) gradient of 44% and 67% was used to enrich IELs centrifuged at 900 g for 30 min at room temperature without brakes. The IELs were recovered from the interface.
Absolute number of IELs was expressed as number per 50 mm2 area by dividing cell number of a subsegment by surface area of the intestinal wall (mm2) and multiplying by 50. An intestinal tube with 50mm2 surface area is roughly 10 mm long.
2.1.3 Vaccination
The ∆actA rLM-OVA strain of Listeria monocytogenes (rLM-OVA) expressing OVA protein was incubated to mid-logarithmic phase in BHI broth (BD, Sparks, MD, USA) supplemented with streptomycin and erythromycin (Sangon Biotech, Shanghai, China) and aliquoted and stored at −80°C before use. All mice were subjected to 5 h fasting prior to vaccination. For subcutaneous (s.c.) vaccination, a dose of 1 × 104 CFU was injected into each hind footpad. For per os (p.o.) administration, mice received 1 × 109 CFU of rLM-OVA by oral gavage shortly after sodium bicarbonate treatment. Two inoculations were given 1 w apart and body weight was monitored daily. Infected mice recovered from weight loss within 1 w. Samples were collected 2 w after the first inoculation.
2.1.4 Activation of OT-I cells with DCs
Bone marrow-derived DCs were cultured as described previously.35 Briefly, bone marrow cells were isolated and cultured at 5 × 106 cells/mL in complete RPMI 1640 supplemented with 2-mercaptoethanol and GM-CSF (20 ng/mL, Peprotech). Half of the media was removed and replaced with fresh media containing GM-CSF every 2-3 d. At d5, 5 × 105/mL DCs were pulsed with SIINFEKL peptide (10 nmol/L), FSL-1 (0.1 µg/mL), poly(I:C) (20 µg/mL), and CpG 1826 (3 µg/mL) for 24 hours. In some experiments, DCs were pulsed with peptide and either MPLA (0.05 µg/mL) or FPC (the combination of FSL-1, poly(I:C) and CpG 1826) dosed at 0.1, 25 and 2 μg/mL, respectively. OVA-specific CD8+ T cells isolated from OT-I mouse spleen were cocultured with DCs at a ratio of 5:1 in IL-2-containing medium for 3 d before adoptive transfer.
2.1.5 T cell stimulation with anti-CD3/CD28 antibodies
Splenocytes from wild-type C57BL/6 mice were plated at 2 × 106/mL in complete RPMI 1640 medium supplemented with HEPES (10 mmol/L), sodium pyruvate (1 mmol/L), 2-mercaptoethanol (55 μmol/L), IL-2 (5 ng/mL) in the presence of anti-CD3 (2 µg/mL, 145-2C11, BioLegend) and anti-CD28 (1 µg/mL, 37.51, eBioscience) mAbs. Cells were harvested on day 3 for transfer.
2.1.6 Tetramer staining and flow cytometry
For the detection of OVA-specific CD8+ T cells, freshly isolated or antigen-stimulated cells were incubated with H-2Kb-SIINFEKL tetramers (tet) obtained from NIH Tetramer Core Facility for 30 min at 37°C. Cells were then stained with anti-CD8α mAb for a further 30 min at 37°C. After washing, cells were resuspended in PBS for flow cytometry analysis. Sample data were acquired on a FACSVerse (BD, Sunnyvale, CA, USA) and analysed with FlowJo software (TreeStar Inc, Ashland, OR, USA). MFI values were obtained based on the populations positively stained with the tested antibody.
2.1.7 In vitro stimulation and intracellular cytokine staining
Freshly isolated cells were rested for overnight followed by tetramer staining. Cells (2 × 106 per well in 200 μL culture medium) were then stimulated with the SIINFEKL peptide at 100 nmol/L in the presence of 1 μg/mL of brefeldin A at 37°C. Anti-CD107a mAb was also added at the beginning of the stimulation. After 6 hours of incubation, cells were stained with surface markers followed by fixation and permeabilization using the intracellular fixation/permeabilization buffer prior to intracellular staining of IFN-γ.
2.1.8 Adoptive transfer
A total of 2 × 107 CD45.2+ activated wild-type or OT-I splenocytes were transferred intravenously to CD45.1+ congenic wild-type mice. At 3 d after transfer, lymphocytes were isolated for flow cytometry analysis.
2.1.9 Statistics
Comparisons between groups were analysed by Student's t test. Comparisons among means of more than two groups were determined by two-way ANOVA with post hoc Tukey's test. Statistical analysis was performed using GraphPad Prism 8. Significance was considered as *P < .05, **P < .01, ***P < .001.
3 RESULTS
3.1 Immunization results in differential changes in the expression of homing receptors on vaccine-induced antigen-specific CD8+ IELs
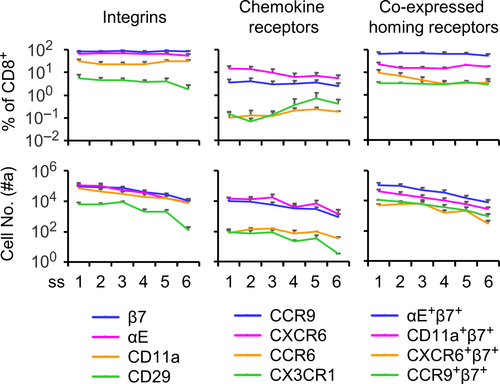
We first determined homing receptor expression on CD8+ IELs of naïve mice (a full list of antibodies is given in Table S1 and the gating strategy shown in Figure S1A). IELs were obtained from six surgically prepared subsegments of the small intestine. In contrast to CD4+ IELs, which represented only a small percentage (< 6%), CD8+ IELs accounted for more than half of the total IELs in all intestinal subsegments (Figure S1B). The majority (60% ~ 80%) of CD8+ IELs expressed β7 (forming heterodimer with αE, data not shown) and less than 30% were CD11a (αL integrin) positive (Figure 1). CD29 (β1 integrin), CCR9 and CXCR6 were each found in a much lower frequency (3% ~ 10%), whereas other tested homing receptors including CCR6 and CX3CR1 were nearly undetectable (Figure 1 and data not shown). Additionally, there were a small proportion of CD8+ IELs that co-expressed CD11a+β7+ (~20%), CXCR6+β7+ (~10%) and CCR9+β7+ (~4%) (Figure 1). The results indicate that in the steady state, small intestinal CD8+ IELs characteristically express the heterodimer αEβ7. To address whether the vaccine-induced CD8+ IELs produce a different phenotype, we vaccinated mice either per os (p.o.) or subcutaneously (s.c.) with recombinant Listeria monocytogenes expressing ovalbumin (rLM-OVA) to prime OVA-specific CD8+ effector IELs, which can be identified by tetramer (tet) staining. We then investigated homing receptor-expressing cells on the tet+CD8+ IEL population. Following p.o. or s.c immunization, the frequency of β7+ cells (formation of αEβ7 heterodimers on these cells confirmed; data not shown) was significantly decreased while the frequency of CXCR6+ and CD29+ cells was markedly increased in tet+CD8+ IELs (Figure 2A). Compared to unvaccinated mice, CCR9+ cells, which were mostly positive for β7, were also increased following p.o. vaccination (Figure 2B). Notably, CD11a was expressed by almost all tet+CD8+ IELs (Figure 2B). Collectively, the data indicate that immunization by p.o. and s.c. routes leads to differential changes in the expression of homing receptors on vaccine-induced antigen-specific CD8+ IELs. As CD11a expression overlapped with tetramer positivity and CCR9 was essentially co-expressed with β7 in a small proportion of β7+tet+CD8+ IELs (Figure 2C), the former two receptors appeared not to be ideal for the phenotype analysis to reveal expression pattern changes. Indeed, expression of CXCR6 and CD29 is inversely related to that of β7 and the receptors are not expressed by all tet+CD8+ IELs, leaving more possibilities for further analysis; thus, we focused our subsequent investigations on these three molecules.
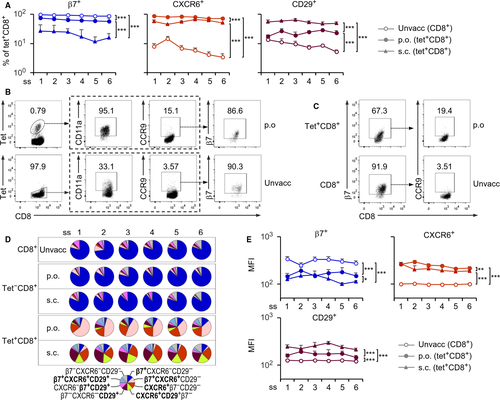
For better visualization, a pie chart was used to illustrate the frequency of eight phenotypically different subsets, including single-, double- and triple-positives as well as triple-negative cells (Figure 2D). Before vaccination, unprimed CD8+ IELs in all intestinal subsegments were predominantly β7 single-positive cells (70 ~ 80%). Following either p.o. or s.c. vaccination, tet−CD8+ IELs resembled their unprimed counterpart, showing the β7 predominance ‘unvaccinated pattern’ (Figure 2D). This observation was confirmed by a relatively unchanged mean fluorescence intensity (MFI) of the homing receptor expression compared to unvaccinated controls (Figure S2), indicating that tet−CD8+ IELs have an unaltered phenotype.
Tet+CD8+ IELs, however, exhibited significant phenotypic changes following vaccination by either route. The β7+ single-positive population decreased markedly to less than 10% regardless of the route of immunization (Figure 2D). In p.o. vaccinated mice, β7+CXCR6+(CD29−) double-positive cells (35 ~ 60%) were the predominant population, in addition to a small population of CXCR6+(β7−CD29−) single-positive cells (15 ~ 25%). In s.c. vaccinated mice, no single predominant population but three major ones (one double-positive and two single-positive) were observed: CD29+CXCR6+(β7−), CD29+(β7−CXCR6−) and CXCR6+(β7−CD29−), each of which accounted for 15 ~ 30% (Figure 2D). The former two subsets were indeed detectable in p.o. vaccinated mice but at a much low percentage (2 ~ 5%). The phenotypic changes were further verified through MFI analysis, which showed downregulation of β7 and upregulation of both CXCR6 and CD29 (Figure 2E). Thus, immunization leads to dramatic reciprocal changes in expression of the three homing receptors, leading to distinct signature patterns for IELs that can distinguish different routes of vaccination.
3.2 CXCR6+β7+CD8+ effector IELs are small intestine-specific and exert cytotoxic potential
To determine whether the p.o. pattern was specific for intestinal tissue, MLNs, spleen, lung and liver were harvested from vaccinated mice and tet+CD8+ T cells were analysed for expression of β7, CXCR6 and CD29. It was shown that tet+CD8+ T cells in these tissues all underwent significant phenotypic changes compared to unprimed and tet−CD8+ T cells, especially in the spleen, lung and liver, where CD29+ single-positive cells became the predominant subset, accounting for 55 ~ 80% (Figure S3). The pattern of tet+CD8+ MLN cells harvested from p.o. immunized mice was generally similar to the s.c. pattern of tet+CD8+ IEL except that β7+ single-positive cells comprise a significant proportion (25%).Therefore, these data indicate that the p.o. pattern is unique to the small intestine and the emergence of the CXCR6+β7+ double-positive population reflects a distinct signature of the pattern.
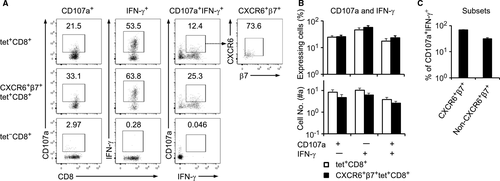
We proceeded to determine the cytotoxic potential of the CXCR6+β7+ double-positive subset of IELs obtained from the p.o. vaccinated mice. Cells (2 × 106) were stimulated with the OVA peptide SIINFEKL and then stained for CD107a and IFN-γ expression for flow cytometry analysis, which was based on 2 × 105 CD8+ IELs. Tet+CD8+ IELs, but not tet−CD8+ IELs, expressed CD107a and IFN-γ, and among the CD107a+IFN-γ+ bifunctional cells, more than 70% were CXCR6+β7+ cells (Figure 3A–C). A slightly increased frequency of IFN-γ expression and CD107a/IFN-γ co-expression was found in the CXCR6+β7+ subset compared to the general tet+CD8+ population, although the differences were not statistically significant. Nonetheless, the results suggest that CXCR6+β7+ cells are the main line of the CD8+ IEL-mediated cytotoxic response in the small intestine.
3.3 The CXCR6+β7+ phenotype is associated with enhanced small intestinal localization
We wondered whether the CXCR6+β7+ phenotype is associated with increased intestinal localization of vaccine-induced antigen-specific effector IELs. Our analysis demonstrated that the percentage of tet+ cells among CD8+ IELs and the number of tet+CD8+ IELs were significantly higher in p.o. than in s.c. vaccinated mice (Figure 4A). In addition, antigen-specific CD8+ T cells induced by p.o. vaccination declined significantly towards the distal end in the lower half of the small intestine. There was a significant positive correlation between the percentage of CXCR6+β7+ cells and the number of tet+CD8+ IELs (Pearson correlation coefficient, r = 0.6136, P = .0001) (Figure 4B). However, no association was observed between the percentage of either CXCR6+- or β7+-single-positive cells and the number of tet+CD8+ IELs. Thus, antigen-specific CD8+ effector T cells bearing the CXCR6+β7+ phenotype display enhanced localization in the small intestinal epithelium.
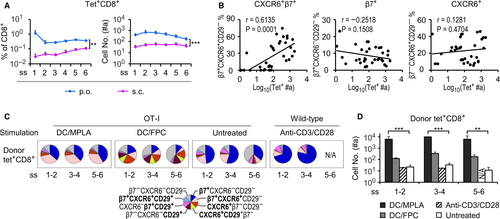
Having identified a positive association between CXCR6+β7+ IELs and enhanced small intestinal localization, we wished to verify the results by performing adoptive transfer experiments to investigate intestinal localization of in vitro primed T cells. Splenocytes isolated from OT-I CD45.2+ mice were stimulated with bone marrow-derived DCs, which had been pulsed with the OVA peptide SIINFEKL and activated with the toll-like receptor agonists, either monophosphoryl lipid A (MPLA, DC/MPLA) or the combination of FSL-1, poly(I:C) and CpG oligodeoxynucleotides (abbreviated as FPC, DC/FPC), as described elsewhere.36, 37 Cells were cocultured at a DC:T ratio of 1:5 for 3 d followed by iv injection of 2 × 107 OT-I cells into naïve wild-type CD45.1+ mice. Some wild-type splenocytes from CD45.2+ mice were stimulated with anti-CD3/CD28 monoclonal antibodies (2 × 106 cells/mL in culture containing 2 μg/mL of anti-CD3 and 1 μg/mL of anti-CD28) also for 3 day before adoptive transfer of 2 × 107 cells per mouse. Donor cells (either OT-I or wild-type origin) in the IEL compartment were recovered from recipient mice 3 d after transfer and analysed for their phenotype and localization efficiency.
Transfer of DC/MPLA-activated OT-I splenocytes resulted in a higher percentage of CXCR6+β7+ cells within the tet+CD8+ donor population than transfer of DC/FPC-activated OT-I splenocytes (Figure 4C). DC/FPC-primed OT-I donor cells had a phenotype somewhat reminiscent of the s.c. pattern (containing primarily the three subsets CXCR6+, CD29+ and CXCR6+CD29+), whereas anti-CD3/CD28-activated tet+CD8+ wild-type donor IELs still displayed the unvaccinated pattern, which mainly consists of β7+ (Figure 4C). Results further showed that DC/MPLA was more effective than DC/FPC in increasing the number of adoptively transferred tet+CD8+ OT-I donor cells in the epithelial compartment (Figure 4D). Wild-type T cells activated with anti-CD3/CD28 costimulation, however, showed no improvement in the intestinal localization. In addition, no significant differences were found among subsegments in the number of donor cells recovered from the IEL compartment. Thus, T cells activated by MPLA are able to acquire the CXCR6+β7+ cell phenotype and have enhanced capability for their localization in the small intestinal epithelium compared to the adjuvants tested or the TCR/CD28 costimulation.
4 DISCUSSION
Intestinal IELs with their special capacity to permeate the epithelium provide the front-line defence through executing cytotoxic function.38, 39 Expression of the gut specific homing receptor αEβ7 allows IELs to be located within epithelium through interaction with E-cadherin on enterocytes. Further evidence suggests that αEβ7 is not the only homing receptor involved in the retention of effector CD8+ IELs.7, 15 Our findings demonstrated that CXCR6 was upregulated after immunization and co-expressed with β7 on oral but not subcutaneous vaccine-induced antigen-specific CD8+ IELs, leading to a specific IEL subset with a distinct CXCR6+β7+ double-positive phenotype. This novel subset proved to be the predominant population of the cytotoxic IELs and highly associated with enhanced intestinal localization. Because this double-positive population was barely detected in non-intestinal tissues, it is reasonable to suppose that enteric immunization imprints the CXCR6+β7+ phenotype on antigen-specific conventional CD8+ IELs.
Selective expression of CXCR6 as shown in this study may represent a potential mechanism through which the CXCR6-CXCL16 axis plays an essential role in retaining enterically-induced effector T cells with expression of β7. Given the observation of low number of CXCR6+ single-positive effector IELs after parenteral immunization, it could be argued that the adhesive contact between αEβ7 and E-cadherin strengthens the CXCR6-CXCL16-mediated retention through improved tissue specialization. This notion is also supported by low number of CXCR6+CD29+ IELs following both parenteral and enteric immunization, the latter of which may elicit this subset through induction of systemic immune responses.5, 40, 41 A possible explanation for the low retention of CXCR6+ and CXCR6+CD29+ cells could be due to formation of loose attachment to the epithelium,42 but it merits further investigation. Thus, our results suggest that CXCR6 and αEβ7 may cooperate in the retention of effector IELs within the intestinal epithelium.
Antigen-primed conventional CD8+ IELs can acquire a substantial capacity to perform cytotoxic function. We demonstrated that the majority of effector IELs triggered to display degranulation and cytokine production were CXCR6+β7+ cells. This is largely consistent with previous studies showing that IFN-γ-producing cells are seen more in CXCR6+ than in CXCR6−CD8+ T cells.43 The CXCR6+β7+ double-positive cells with cytotoxic potential, as evidenced by the ability to express both IFN-γ and CD107a, may share some phenotypic and functional characteristics with other lymphocytes within the intestinal epithelial compartment. As a major type of IELs, γδ T cells exhibit a diverse array of effector functions and have been shown to be mostly αEβ7+ with a low proportion of CXCR6+ cells.25, 44 This CXCR6lo phenotype is probably because γδ IELs are more tissue-resident and may use other mechanisms for local distribution.45 It has been reported that a subpopulation of αE+NKp44+ intraepithelial type 1 innate lymphoid cells are also CXCR6+ and express IFN-γ and CD107a in response to phorbol ester.46 However, whether these IEL subsets display a significantly increased expression of CXCR6 upon stimulation remains to be elucidated.
Another important finding of the study was to demonstrate that MPLA stimulation is more effective than FPC stimulation in inducing the CXCR6+β7+ phenotype in vaccine antigen-specific CD8+ IELs, and resulted in more than 10 times higher IEL retention. It is worthy to note that the both MPLA- and FPC-induced homing receptor patterns largely resembled the p.o. and s.c. patterns, respectively, which may explain the significant differences in localization efficiency between two treatments. In addition, MPLA- and FPC-induced homing receptor patterns had a higher proportion of β7+ single-positive cells than the p.o. pattern, presumably due to the functional heterogeneity of the in vitro-generated bone marrow-derived DCs that stimulate variable T cell responses.47 Future studies of therapeutic modalities will require optimization of the in vitro stimulation conditions.
It is worthy of notice that the intraepithelial retention in the lower half of the small intestine of vaccinated mice declined towards the distal end and was overall less efficient than in the upper half. Similar observations were made in previous reports.48, 49 As discovered in this study, the lower retention efficiency in the distal part of the small intestine should result from the lower proportion of the CXCR6+β7+ subset. However, retention of transferred MPLA-preactivated T cells in the recipients was almost as efficient in the distal as in the proximal small intestine, and an equivalently high proportion of CXCR6+β7+ subset was seen in these segments. It might imply that cell transfer could potentially improve the lower retention in the distal small intestine. Further research is warranted to elucidate the underlying mechanisms.
In conclusion, our study reveals a novel conventional IEL effector subset characterized by a distinct CXCR6+β7+ phenotype that is closely associated with enhanced intraepithelial localization after enteric but not parenteral immunization. Certain immunostimulatory agents may specifically induce T cells to express the CXCR6+β7+ phenotype, allowing better retention within the intestinal epithelial layer. More research is needed to extend our understanding and determine how best to exploit the findings in future design of preventive or therapeutic vaccines as well as cellular immunotherapy.
ACKNOWLEDGMENT
This work was supported in part by National Natural Science Foundation of China (81871784), Beijing Natural Science Foundation (5131002), and CAMS Major Collaborative Innovation Project (2016-I2M-1-011). We acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of MHC class I tetramers. The authors thank Rong Xiao and Tengsen Gao for their technical assistance.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHORS CONTRIBUTION
QZ, JBL, JJL and MYL performed data analysis and interpretation. JJL, MYL and JBL conducted the cell isolation, flow cytometry and administration, and CXG, LZZ and MHL were involved in some experiments. JBL and JJL participated in manuscript writing, and QZ oversaw the overall execution of the projects and gave final approval of the version to be submitted.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.