MiR-183 delivery attenuates murine lupus nephritis-related injuries via targeting mTOR
Funding information
This research was supported by a grant from the fund of National Science Foundation of China (81700585).
Abstract
MicroRNAs (miRNAs) play a vital role in the occurrence and development of many human diseases, including systemic lupus erythematosus (SLE). SLE is an autoimmune disease characterized by the production of autoantibodies against nuclear antigens and multiorgan involvement. Study of miRNAs involved in SLE provides new insights into the pathogenesis of SLE and might lead to the identification of new therapeutic interventions. The aim of this study was to investigate the effect of miR-183 injection on the progression of SLE by using MRL/lpr mouse model. The expression levels of miR-183 and mTOR mRNA were detected by quantitative real-time PCR assay. The effect of miR-183 on the course of spontaneous disease progression in the MRL/lpr mice was examined by intraperitoneal injection of miR-183 into mice and followed by monitoring lifespan, anti-dsDNA antibody levels, urinary albumin levels, blood urea nitrogen (BUN) levels, and Tregs and Th17 cell population. We found that miR-183 injection resulted in reduction of anti-DNA antibody and immune complex component levels, restoration of Tregs and Th17 cell population and prolongation of survival. Our findings suggest that miR-183 injection may serve as an effective therapeutic treatment for delaying or easing pathologic features of SLE.
1 INTRODUCTION
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that can cause damage in multiple organs, including the joints, skin, kidneys and central nervous system, through the production of various autoantibodies and immune complexes. The development of lupus nephritis (LN), which leads to the severe breakdown of immune tolerance and organ dysfunction is one of the most important factors influencing the prognosis in SLE.1 A number of factors contribute to the development of LN, including genetic mutation, hormonal imbalance, environmental challenging and presence of drugs.2, 3 Although the precise pathogenesis of LN is not completely understood, numerous clinical and animal studies have demonstrated that B cells, proinflammatory Th17 cells and regulatory T cells (Tregs) play a critical role in the pathogenesis of LN.4-6
Rapamycin, a potent immunosuppressive agent and mTOR inhibitor, has been demonstrated to have strong activity in inhibition of allograft rejection in animal models of transplantation.7, 8 Rapamycin was shown to be a potent inhibitor of S6K1 kinase activation and an important mediator of PI3 kinase signalling. Rapamycin forms a complex with 12-kd FK-506 binding protein (FKBP12) and rapamycin-FKBP12 complex binds and specifically acts as an inhibitor of mammalian target of rapamycin (mTOR).9, 10 In lupus-prone MRL/lpr mice, rapamycin has been shown to prevent the typical rise in anti-double-stranded DNA antibody and urinary albumin levels, restore T cell mitogen-stimulated splenocyte proliferation and interleukin-2 production, and prolong survival of MRL/lpr mice.11-13 Rapamycin has been used safely and effectively to treat renal transplant rejection since 1999,14 thus targeting mTOR might be a useful therapeutic method for SLE treatment.
The mTOR protein, which is inhibited by Rapamycin, can also be inhibited by miRNAs. miRNAs are small stretches of noncoding RNAs comprising approximately 22 nucleotides in length. They bind to the 3′-untranslated region (3′-UTR) of target mRNA, which leads to a decrease in the production of the protein translated by the target mRNAs.15 miRNAs have been established as a master regulator of cellular processes and are implicated in the pathogenesis of several human diseases including autoimmune diseases.16 Disruption of miRNA biogenesis or function impacts the development and function of different types of immune cells (eg B cells, Th17 cells, Treg cells and macrophages) leading to the immune tolerance breakdown and eventually the development of autoimmune-related disorders such as SLE or LN.17, 18 Since abnormal expression of miRNAs is a key component of human disease pathogenesis, there is growing interest in the manipulation of miRNA expression to normal levels by miRNAs injection.19 miRNAs-based therapeutic approaches are being considered as promising therapeutic strategies as miRNAs work by highly specific binding to the complementary site on the mRNA target(s).20, 21 For example, overexpression of downregulated miR-26a in hepatocellular carcinoma (HCC) in mouse liver resulted in inhibition of cancer proliferation and initiation of apoptosis.22 Subsequently, in another study, the downregulation of miR-34a level was increased by delivering artificial miR-34a with NOV340 liposome in an orthotopic model of HCC. Restoration of miR-34a levels resulted in significant tumour size reduction and prolonged survival.23
In the present study, we found that the expression levels of miR-183 were downregulated in renal tissues from LN patients and mTOR is a direct target of miR-183. We aimed to explore the possibility of development of miR-183-based therapeutic treatment for easing pathologic features of lupus using MRL/lpr mouse model.
2 MATERIALS AND METHODS
2.1 Clinical information
From June 2017 to July 2018, a total of 20 patients who were diagnosed with LN in Liaocheng People's Hospital were recruited in this study. There were 9 males and 11 females in this cohort, aged at 37.6 ± 15.8 years. No immune suppressant, immune modulator or steroid hormone has been used within 3 months. No one was complicated with malignant tumour, acute/chronic infection or other autoimmune diseases. SLE disease activity index (SLEDAI) was employed based on clinical manifestation and laboratory tests. Those patients with higher than 5 points were regarded as in the active phase (average score = 10.7 ± 4.1). Pathological samples were collected from renal tissues of LN patients. Another 20 normal renal tissue samples were collected from age/sex-matched patients who received renal tumour resection, but without primary glomerulonephritis, diabetes and hypertension. Renal tissue samples were snap frozen with liquid nitrogen and stored at −80°C for subsequent experiments. Informed consent was derived from each participant before participation, and the procedures were approved by the human ethics committee of Liaocheng People's Hospital.
2.2 Reverse transcription and quantitative real-time PCR
The expression levels of miR-183-3p and mTOR mRNA were analysed using High-Capacity cDNA Reverse Transcription Kit and TaqMan® miRNA assays (Applied Biosystem) following the manufacturer's recommendation. The probe ID number for hsa-miR-183-3p in the TaqMan® assays is 002270. U6 was used as a reference transcript for normalization. The expression levels of mTOR mRNA were measured by qPCR with ABI Step One Plus Detection System. The probe ID number for mTOR in the TaqMan® assays is Hs00234508_m1. 18S was used as a reference transcript for normalization. All reactions were performed in duplicate.
2.3 Mice
Female MRL/lpr mice were purchased from SLAC (Shanghai, China). All experiments were approved by the ethical committee of Liaocheng People's Hospital and were performed in line with the National Institute of Health Guide for Care and Use of Animals. The sex- and age-matched C57BL/6 mice as normal control group (Ctrl) were used as normal control. Meanwhile, two additional groups of 12-week-old female MRL/lpr mice (15 mice per group) were used to compare the survival from 12 to 28 weeks of age.
2.4 Lymphocytes and CD4+ T cell isolation
Renal tissues of MRL/lpr mice were cut into 1-2-mm pieces and incubated in RPMI 1640 containing 1% foetal calf serum (Invitrogen), 1 mM, dithiothreitol and 5 mM EDTA for 20 minutes at 37°C. Tissue pieces were then digested with Liberase TL and DNase I (0.125 U/mL) in RPMI 1640 while shaking. The resulting tissue suspension was passed through a 100-μm cell strainer, centrifuged and resuspended in 40% Percoll and underlayed with 80% Percoll. After centrifugation at 600 g for 20 minutes, lymphocytes were recovered from the 40/80% interface and resuspended in complete medium.
The CD4+ T cell was isolated from lymphocytes using the Miltenyi MACs Kit according to manufacturer's instructions. CD4+ T purified using the Miltenyi system are ≥96% pure.
2.5 Lymphocytes staining
Total lymphocytes from mice were suspended in phosphate-buffered saline (PBS) containing anti-FcgRII/III mAb (BD Biosciences), stained at 4°C with peridinin-chlorophyll (PerCP)-conjugated anti-CD4 (BD Biosciences) and allophycocyanin (APC)-H7 conjugated anti-CD8 antibodies (BD Biosciences) for 30 minutes. After washed in PBS, cells were further stained for 30 minutes at 4°C with anti-IL-17A-FITC (Mabtech) or combinations of anti-CD25 and anti-Foxp3. Cell population were analysed by BD FACSCAlibur 2 Laser, 4 Color Flow Cytometer.
2.6 miR-183 mimic and antibodies
MiR-183 mimic (miR-183) and miRNA control (miRNA-control) were all purchased from GenePharma Co., Ltd. MRL/lpr mice received intraperitoneal injection with either miR-183 mimic (183 group) or miRNA-control (LN group) at a dose of 1 nmol per mouse twice per week from 12 to 28 weeks of age. Antibodies against C3, lgG, p-mTOR, mTOR, p-P70S6K and P70S6K were purchased from Cell Signaling Technology.
2.7 Blood urea nitrogen detection
Blood urea nitrogen (BUN) level was detected by a commercial auto-analyser (Beckman Coulter). Urine samples from experimental mice were collected every 2 weeks using metabolic cages. Urinary protein levels were semi-quantified by Multistix 10SG reagent strips (Bayer Healthcare).
2.8 Immune complex deposition detection
The frozen kidney sections were blocked with 10% foetal bovine serum and then stained with FITC-conjugated rabbit anti-mouse IgG (Santa Cruz) or FITC-conjugated goat IgG fraction to mouse complement C3 (Cedarlane). Fluorescence intensity was calculated and scored from 0 to 3 (0, no fluorescence; 0.5, trace, just detectable above background; 1, fluorescence scattered and light; 2, bright but not diffuse; and 3, bright and diffuse).
2.9 Anti-dsDNA ELISA
An anti-dsDNA ELISA Kit (Laibio) was used to quantitate serum antibodies that bind to double-stranded DNA. The assay was performed according to the manufacturer's instructions. The quantity of anti-dsDNA antibodies was determined by comparison against a standard curve.
2.10 Statistical analysis
The results were analysed using the SPSS 13 statistical software. Quantitative variables were analysed using Student's t test between two groups or one-way analysis among multiple groups. The differences were considered significant at P < .05. The correlations between miR-183 and mTOR mRNA expression levels were analysed using Spearman rank correlation test. The differences were considered significant at P < .05.
3 RESULTS
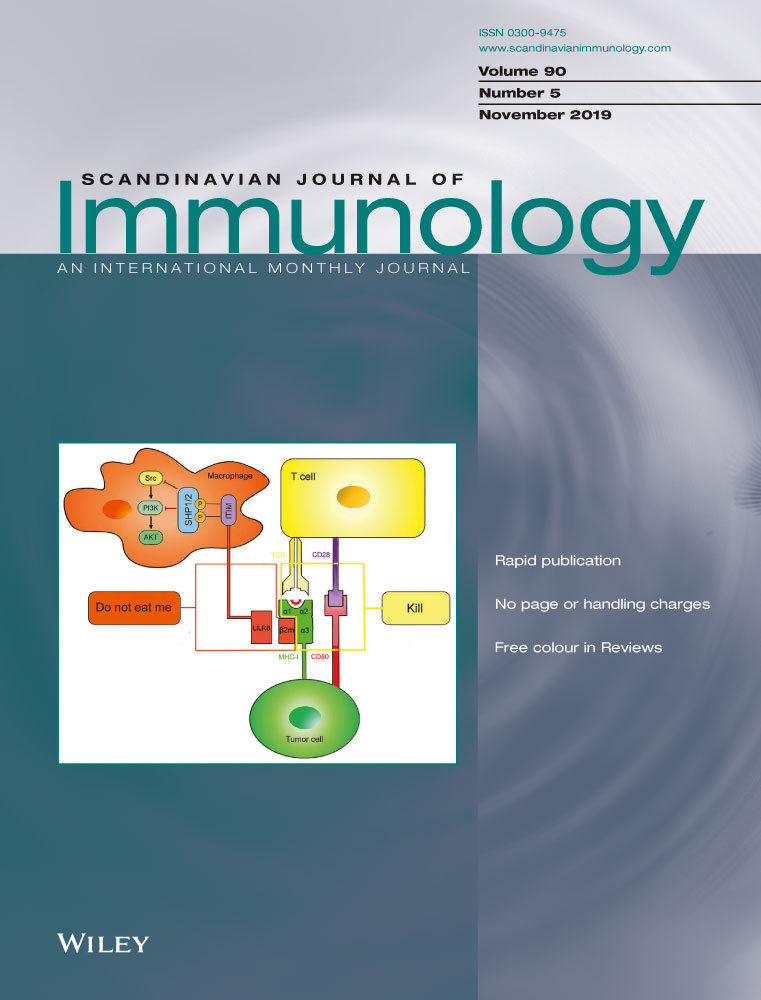
3.1 MiR-183 was downregulated in LN patients and mice
Previous studies have shown that mTOR, a key regulator of metabolic activity, plays a very important role in SLE disease pathogenesis and mTOR is a direct target of miR-183 in neuroblastic cells.24 To investigate the association of the expression levels of mTOR and miR-183, a total of 40 renal tissues with 20 from LN patients and 20 from healthy donors were collected for this study. Total RNA from each sample was extracted and the expression levels of mTOR mRNA and miR-183 were analysed by TaqMan real-time PCR. The results demonstrated that the mTOR mRNA levels were significantly upregulated; while miR-183 levels were markedly downregulated, in renal tissues from LN patients compared with that from healthy donors (Figure 1A,B). Spearman's rank correlation analysis showed that the expression levels of mTOR and miR-183 in 20 renal tissues from LN patients were inversely correlated, suggesting that miR-183 may target mTOR expression in LN patients (Figure 1C). Furthermore, downregulation of miR-183 and upregulation of mTOR were identified in kidney samples from MRL/lpr mice when compared to normal control mice (Ctrl) (Figure 1D,E), and the expression levels of mTOR and miR-183 in 15 renal tissues from MRL/lpr mice were inversely correlated (Figure 1F).
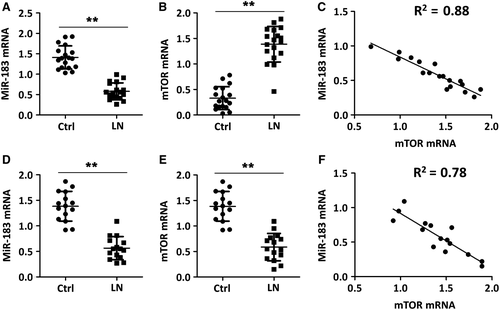
3.2 Urinary albumin levels and blood urea nitrogen (BUN) levels
Next, we aimed to compare the difference of urinary albumin levels and BUN levels between MRL/lpr mice and Ctrl. There were no significant differences in the urinary albumin levels among all the mice at the start point of the study. When the interaction between treatment and time was evaluated within groups, we observed that the urinary albumin values were significantly higher in MRL/lpr mice than that in Ctrl. The urinary albumin values were decreased in MRL/lpr mice receiving miR-183 injection treatment compared with that in MRL/lpr mice receiving miRNA-control injection treatment (LN group) (Figure 2A). Similarly, the BUN levels in MRL/lpr mice were about 3.5-folds higher than that in Ctrl. The BUN levels were decreased by almost 50% in MRL/lpr mice after receiving miR-183 injection treatment when compared with that in MRL/lpr mice receiving miRNA-control injection treatment (LN group) (Figure 2B). The results clearly showed that miR-183 injection decreased urinary albumin levels and BUN levels in MRL/lpr mice.
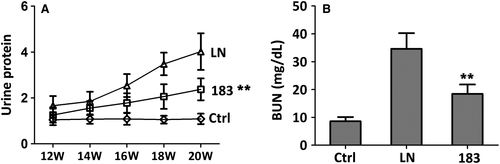
3.3 Anti-dsDNA antibodies and renal deposition of immune complex
As shown in Figure 3A,B, anti-DNA antibody (IgG) concentration and immune complex component levels (anti-C3 and anti-IgG) were significantly decreased in MRL/lpr mice after miR-183 injection treatment compared to miRNA-control treatment (LN group) (Figure 3A). The fluorescence intensity of C3 and IgG was also decreased in miR-183 treatment groups compared to LN group (Figure 3B). These results demonstrated that miR-183 injection can effectively reduce anti-dsDNA antibodies and renal deposition of immune complex in MRL/lpr mice.
3.4 Survival evaluation
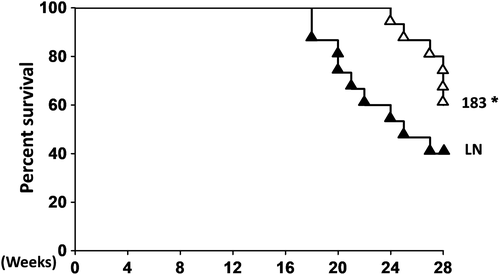
We observed that the miRNA-control-injection MRL/lpr mice (n = 15) started to die at the age of 17 weeks and only 40% of them were still alive at age of 30 weeks. However, miR-183-injection MRL/lpr mice (n = 15) started to die at age of 22 weeks. At the age of 30 weeks, 40% of miRNA-control-injection mice (LN group) were alive and 60% of miR-183-injection mice were alive (Figure 4). These data strongly suggested that miR-183 injection treatment significantly prolonged the survival rate of MRL/lpr mice compared with miRNA-control LN groups.
3.5 Tregs and Th17 cell population
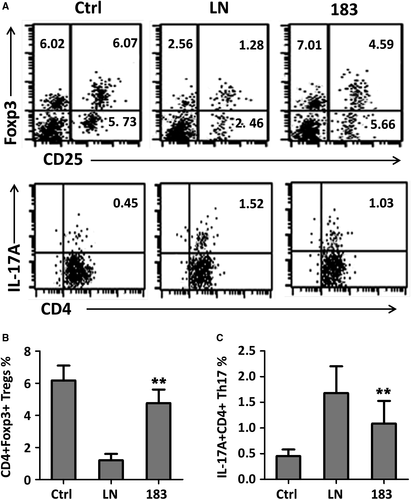
It was reported that impaired function of Tregs and/or increased Th17 cell population leads to a failure in immune tolerance and triggers autoimmunity. Total lymphocytes from kidneys of Ctrl, miRNA-control-injection MRL/lpr mice (LN group) and miR-183 injection MRL/lpr mice were isolated at the end of experiments, and the Tregs and Th17 cell population were analysed by flow cytometry assay. Gating strategy for all three groups could be found in Figure S1. Treg cells were evaluated as CD4+CD25+ lymphocytes expressing FoxP3, and Th17 cells were defined as CD4+IL-17A+ lymphocytes. The results showed that the CD4+CD25+Foxp3+ Treg cell population were significantly decreased, whereas CD4+IL-17+ Th17 cell population were increased, in miRNA-control-injection MRL/lpr mice (LN group) compared with that in Ctrl (Figure 5A,B). MiR-183 injection treatment resulted in upregulation of the number of Treg cells and downregulation of the number of Th17 cells, leading to partial restoration of Tregs and Th17 cell population.
3.6 MiR-183 targets mTOR
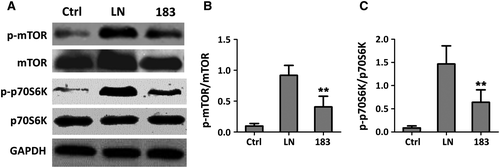
P70S6 kinase (p70S6K), a mitogen-activated serine/threonine protein kinase that is required for cell growth and G1 cell cycle progression, is a downstream target of mTOR signalling pathway. Overactivation of mTOR/p70s6k signalling pathway has been reported in murine LN.25, 26 Next, we aimed to confirm that miR-183 regulates mTOR/p70s6k signalling pathway through targeting mTOR expression. To reach this goal, CD 4+ T cells were isolated from renal tissues of MRL/lpr mice with miR-183 mimics or miRNA-control (LN group) treatment as well as Ctrl group. The CD 4+ T cells from these three groups were subjected to Western blotting assay. Indeed, we observed that both p-mTOR and total mTOR protein levels were significantly decreased upon miR-183 mimic transfection compared to miRNA-control (LN group). The p-p70s6k and total-p70s6k protein levels also decreased in miR-183 overexpression cells (Figure 6A,C). These data suggested that miR-183 inhibited mTOR and p70s6k expression and further inhibits mTOR/p70s6k signalling pathway activation (Figure S2).
4 DISCUSSION
Developing more effective treatment for lupus remains a priority in the field. Several independent experiments have demonstrated that rapamycin intervention to very young MRL/lpr mice can reduce many lupus pathologies present in these mice.11-13 Rapamycin treatment resulted in normalization of T cell mitogen-stimulated splenocyte proliferation and IL-2 production, decreasing spleen and lymph node size, reducing inflammation of lung, liver and kidney, inhibition of anti-dsDNA antibodies production and prolongation of survival rate.
Rapamycin's primary role as an mTOR inhibitor sparked us to question what other regulators of mTOR may have therapeutic benefits for SLE patients. We found that miR-183 is a strong regulator of mTOR. In fact, aberrant expression of miR-183 has been reported in a variety of human diseases and extensive research evidence suggests that miR-183 plays a diverse role in the development and progression of human disease.27 For example, on the one hand, miR-183 functions as an oncogene through promotion of cell proliferation, tumour invasion and chemo-resistance by inhibition of programmed cell death 4 (PDCD4) in gastric cancer and pancreatic cancer,28, 29 but on the other hand, miR-183 exerts inhibitory effect on tumour invasion and progression via suppressing Zinc finger E-box-binding homeobox 1 (ZEB1) and matrix metallopeptidase 9 (MMP-9) in lung cancer and cervical cancer,30, 31 respectively. Although upregulation of miR-183 has been reported in MRL/lpr mice,32 surprisingly, we found that miR-183 levels were markedly downregulated in renal tissues from LN patients and miR-183 levels were inversely correlated with mTOR mRNA levels. We further confirmed that miR-183 specifically targets mTOR and inhibits mTOR/p70s6k signalling pathway activation.
These three lines of evidence, downregulation of miR-183 in LN patients, specific targeting of mTOR by miR-183, and the reduction of LN progression upon treatment with rapamycin, promoted us to propose a hypothesis that miR-183 injection may reduce SLE progression via targeting mTOR in MRL/lpr mice. Consistent with the results of rapamycin treatment, we found that miR-183 injection treatment can effectively reduce anti-DNA antibody and immune complex component levels, restore Tregs and Th17 cell population and prolong survival rate of MRL/lpr mice.
5 CONCLUSION
Recently, miRNAs have emerged as therapeutic options for the treatment of disease. The advantages of miRNA-based treatment are obvious. Firstly, miRNAs can regulate a broad set of genes simultaneously.33 Secondly, miRNAs can also affect tissue microenvironment through modulation of different type of cells and unlike cancer drugs, miRNAs usually does not have cytotoxicity to normal cells.34 Thirdly, miRNAs showed reduced immune response and low toxicity when compared with lentivirus- or protein-based gene therapy.35 Our results demonstrated for the first time that miR-183 injection treatment may serve as an effective therapeutic treatment for reducing pathologic features of SLE patients.
CONFLICTS OF INTEREST
All authors declare that they have no conflict of interest.