On integrity in immunity during ontogeny or how thymic regulatory T cells work
Abstract
The Standard model of T cell recognition asserts that T cell receptor (TCR) specificities are positively and negatively selected during ontogeny in the thymus and that peripheral T cell repertoire has mild self-major histocompatibility complex (MHC) reactivity, known as MHC restriction of foreign antigen. Thus, the TCR must bind both a restrictive molecule (MHC allele) and a peptide reclining in its groove (pMHC ligand) in order to transmit signal into a T cell. The Standard and Cohn's Tritope models suggest contradictory roles for complementarity-determining regions (CDRs) of the TCRs. Here, I discuss both concepts and propose a different solution to ontogenetic mechanism for TCR-MHC–conserved interaction. I suggest that double (CD4+CD8+)-positive (DP) developing thymocytes compete with their αβTCRs for binding to self-pMHC on cortical thymic epithelial cells (cTECs) that present a selected set of tissue-restricted antigens. The competition between DPs involves TCR editing and secondary rearrangements, similar to germinal-centre B cell somatic hypermutation. These processes would generate cells with higher TCR affinity for self-pMHC, facilitating sufficiently long binding to cTECs to become thymic T regulatory cells (tTregs). Furthermore, CD4+ Foxp3+ tTregs can be generated by mTECs via Aire-dependent and Aire-independent pathways, and additionally on thymic bone marrow–derived APCs including thymic Aire-expressing B cells. Thymic Tregs differ from the induced peripheral Tregs, which comprise the negative feedback loop to restrain immune responses. The implication of thymocytes’ competition for the highest binding to self-pMHC is the co-evolution of species-specific αβTCR V regions with MHC alleles.
1 THE COMMENT ON COHN'S TRITOPE MODEL AND THE CASE FOR ALLELE-SPECIFIC MAJOR HISTOCOMPATIBILITY COMPLEX RECOGNITION BY THE T CELL RECEPTOR
The generally accepted view of T cell recognition of its ligand—“antigenic peptide within MHC molecule” (pMHC)—is derived from a combination of germline selection of T cell receptor (TCR) variable (V) regions with intrinsic affinity for MHC generally1 and somatic selection for TCRs with best fit for self-MHC (the Standard model). Therefore, thymic selection skews the random T cell repertoire in favour of TCRs that can bind self-MHC alleles together with endogenous peptides derived from self-antigens.
Thymocytes with mild TCR binding to MHC alleles are allowed to mature (positively selected), whereas developing T cells bearing TCR with the highest binding to MHC molecules are deselected (otherwise autoimmunity would develop).
The problem lies in the notion that evolution does not favour those TCR alleles that would not bind MHC. Thus, non–MHC-binding TCR V regions would be lost in the course of phylogeny. Hence, we lack the explanation on how the TCR V regions were being preserved during natural selection.
Along with this in mind, Cohn's Tritope model2, 3 suggests that TCR-MHC interactions are germline-determined (and thus allele-specific) and these can explain better how TCR Vs could be evolutionarily selected. This model postulates that each species has its own set of alleles that can interconnect. The greater the evolutionary distance between the species, the lesser the interconnectivity between TCR and MHC alleles.
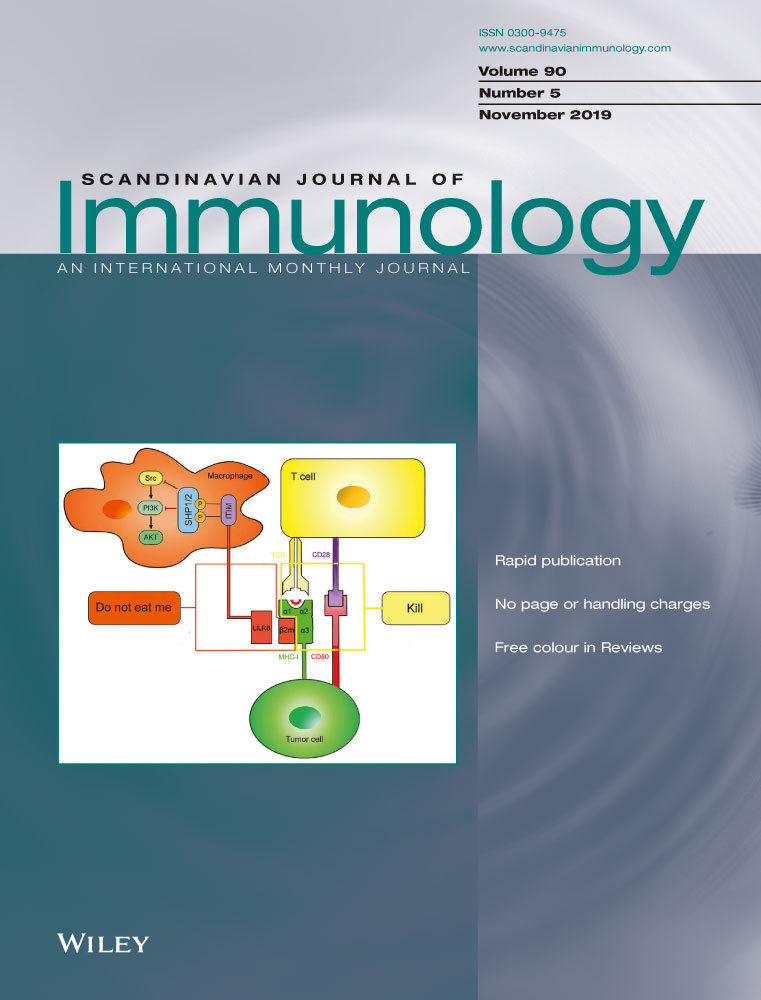
In detail, Cohn suggests that six “areas” on the αβTCR V-region surface comprise complementarity-determining region (CDR) 1, CDR2 and CDR3 parts of both TCR V alpha and V beta chains, which could bind the molecular surface of MHC plus peptide molecules. The Tritope model groups them in three paratopes (Figure 1), two of which can bind an allele of the MHC and one that binds the peptide.4 The “paratope” that can bind peptide in the groove of the MHC consists of CDR3 region of both alpha and beta TCR V regions. Bretscher5 has criticized Cohn's “framework” of T cell recognition, and the Standard model1, 6-8 points out that no area on the TCR V-region surface could be isolated that will only bind the MHC allele or the peptide, and that all six “topes” in theory are influenced by an interaction between any given peptide with a certain MHC molecule (Figure 1 and Table 1).
| Observation | Explanations | ||
|---|---|---|---|
| Standard model | Tritope model | Integrity/dynamic model (this study) | |
| Mild anti–self-pMHC αβTCR reactivity in peripheral T cells | Outcome of the positive selection of DP thymocytes | Yes, by entraining (deactivating other paired chain) | Yes, by competition for high-affinity binding to cTEC (to generate tTregs) |
| CDR1 and CDR2 of TCR Vs bind MHC alleles of the species | Yes, but always in a meld with CDR3 | Yes, exclusively, that is self | Yes; initially exclusive, then in a meld with CDR3 |
| TCR Vs CDR3 binds peptide reclining in the MHC molecule | Yes, but always in a meld with CDR1 and CDR2 | Yes, exclusively, that is non-self or neutral self | Yes; initially exclusive, then in a meld with CDR1 and CDR2 |
| Probability (an estimate) in selecting TCR V alleles that bind to the MHC alleles of the same species in the course of natural selection | Low: non- or mild reactivity would be deselected evolutionarily | High: this is classical self- and non–self-argument: self-MHC/ self-TCR V [CDR1 + 2] interactions are naturally selected | High: the initial DP cell repertoire resembles the Tritope model |
The contradiction arising from the Standard and Tritope models has been experimentally investigated in TCR-transgenic mouse models, and the results were divided as follows: A third of published references is in agreement with the Tritope view,9-11 whereas two thirds of manuscripts support the Standard model.12-17
Here, I would like to suggest the solution by proposing the model that includes dynamic changes in TCR V regions during cortical thymic development of TCR self-specificities. It allows T cells to have high-affinity TCRs for self-pMHC and consecutively mutate away, except not to lower the TCR-pMHC affinity, but to increase it in order to compete for prolonged binding to cortical thymic epithelial cell (cTEC), which would in turn allow the winners to differentiate further (see below and legends to Figures 2 and 3).
The Tritope model when viewed with a static principle is supported by 1/3 of evidence.9-11 However, if we assume a dynamic stepwise model, we would be able to explain the additional 2/3 of evidence.12-17 The dynamic model might be an improvement on the Standard model that could now clarify the three lines of evidence9-11 supporting the Tritope model (Table 1).
2 THE DYNAMIC MODEL OF TCR V-REGION SELECTION IN THE THYMUS
Despite the fact that the TCRs are generated by random V-(D)-J rearrangements, the initial repertoire must be mainly anti–self-pMHC, because TCR V regions seem to have coevolved with MHC alleles. This is observed in elegant experiments with chimeric TCRs containing various vertebrates’ V regions, showing that they could have the ability to recognize mouse MHC class II molecules, suggesting evolutionary conserved binding to MHC.1 Furthermore, TCR V-domain can have alternate conformations when interacting with MHC I as opposed to binding to MHC II molecules.18
Thymic selection of T cell receptor V regions involves processes that lead to: (a) the detection of self-peptides, and later non–self-peptides; (b) restriction to self-MHC; and (c) natural selection for recognition of MHC alleles within a species. It has been generally accepted that selection of thymocytes involves positive and negative outcomes (ie the Standard model): negative for either the high-affinity/avidity binding to self-pMHC or no binding at all, and positive for the mild (intermediate) reactivity to self-pMHC (Table 1).
Here, I propose that developing T cells are positively selected—except not for the mild binding, but for the highest affinity binding to self-pMHC molecules in thymic cortex, thereby competing among each other to generate natural thymic T regulatory cells (tTregs), which, according to the Integrity model, serve the purpose of keeping the integrity of an organism against immunologic self-destruction or horror autotoxicus (Table 1).
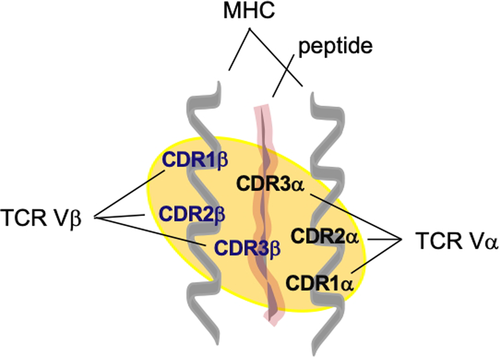
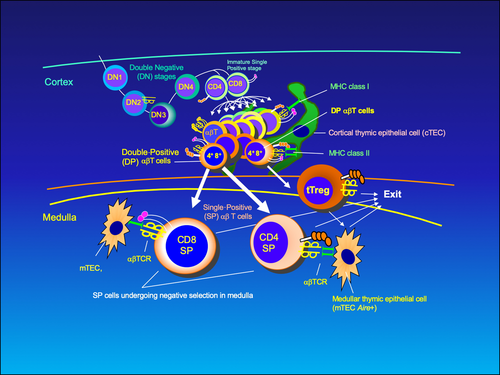
I suggest the following scenario during the cortical phase of T cell development (Figure 2). Double-positive (DP; CD4+CD8+) thymocytes that express αβTCRs should have intrinsic affinity for self-pMHC. The TCR/self-pMHC signal would allow them to proliferate in the cortex and generate a range of progeny that would, firstly, compete for higher affinity/avidity binding (>10−6 M and above) to self-pMHC on cTECs.
During the competition, thymocytes would strive to increase the affinity of initial TCR binding. This is supposedly driven by TCR editing mechanisms like additional rearrangements of the TCRs (ie V-Jα, or even V-D-Jβ) and somatic hypermutation of N-region sequences (via TdT during these additional rearrangements), of which the latter seems to be the most important mechanism.19 This process resembles the BCR aspect in germinal-centre B cell receptor V-region hypermutation that increases affinity for the antigen, giving selective advantage for survival of B cells with the highest affinity for antigen bound on follicular dendritic cells.20 However, I suggest that this would happen only to an immature stage of developing T cells. Therefore, editing of pre-existing T cell specificity would only occur with secondary rearrangement, unlike somatic hypermutation in B cells that acts post-rearrangement.
Cortical thymic epithelial cells and medullary TECs (mTECs) develop from a common precursor with distinct phenotype (epithelial cell adhesion molecule [EpCam]+, Ulex europaeus agglutinin-1 [UEA1]−, Ly51+, PLET1+, MHCIIhi).21 Further, cTECs are positive for the expression of Ly51 on cell surface, but not UEA-1 (Ly51+ UEA-1−), while mTECs are Ly51-UEA-1+. Developmental pathways of both thymic epithelial cells include immature, mature and terminal stages, each with distinctive molecular markers. Thus, we can distinguish mature cTECs phenotype (CD40+ β5thi CD205+ MHCII+) from that of mTECs (CD40+, CD80/86hi, MHCIIhi) in the mouse. Additionally, only mTECs express autoimmune regulator encoded by the Aire gene and tissue-restricted antigens (TRAs), which can ectopically express a defined number of diverse self-antigens.22 Human cTECs supposedly also display a limited set of tissue antigens in a form of cortical/self-peptides on their MHC class I and class II molecules, perhaps as a minuscule representation of antigens important for each tissue's mechanical structure and integrity, which we could call the integrity homunculus. On the other hand, mTECs display a different set of body's antigens (using Aire or Fezf2),22, 23 and we could call such representation AIRE/FEZF2 homunculus. Apart from thymic epithelial cells, haematopoietic bone marrow–derived cells also contribute to T cell development. They include thymic B cells at the cortico-medullary junction that can also express Aire and thus present their idiotypes, as another set of self-peptide-MHC ligands to developing T cells.24 All these cells are important for central tolerance, and it is tempting to speculate that they might have different roles (see Further discussion below). Moreover, in the periphery, there are bone marrow–derived unique antigen-presenting cells that can express Aire and be able to impart tolerance like “extrathymic Aire-expressing cells” (eTACs) with an interesting phenotype (MHCIIhi, CD80lo, CD86lo, EpCAMhi, CD45lo)25 and “group 3 innate lymphoid cell” (ILC3)-like population (with high levels of MHCII and co-stimulatory molecules),26 but their roles will not be discussed here.
The DP T cells that bind with mild affinity to cTEC-expressed ligands (pMHC) are positively selected in sense that when they transiently downregulate one of the co-receptors (with which they have been selected), they could migrate to medulla (Figures 2 and 3B). (This is akin to positive selection of the Standard model).
However, those DPs with intermediate- to high-affinity TCR keep rearranging further their α-chains and eventually might end up in having even higher affinity TCRs (Figure 3A). This population of DP thymocytes that has the affinity around 10−6 to 10−7 M could be negatively selected in the cortex (Figure 3C). This is supposedly due to competition for survival (Figure 3A), and winners; namely, the DP clones that see self-peptide-MHC the longest would become cortical tTregs. The recent finding that tTregs have high functional avidity cognate interactions27 supports the high-affinity interaction part of the hypothesis. Keiback et al describe tTregs specific for antigenic peptide-MHC ligand (derived from self-antigen, myelin-oligodendrocyte glycoprotein) that are important for tolerance preventing experimental encephalomyelitis (a model for multiple sclerosis) in mice.27 Modigliani et al28 previously suggested the high-avidity interaction of developing thymocytes with self-pMHC thymic ligands for the generation of regulatory T cells. In contrast, in the paper by Pennington et al,29 the DN2-stage thymocyte, expressing a preTCR (consisting of a rearranged β-chain and pre-Tα), was envisaged to become a Treg by skipping positive and negative selections.
Furthermore, the winners of the competition, having the highest avidity for the pMHC ligand, might downregulate the other (unused) co-receptor. Theoretically, this opens the possibility for the generation of CD8+ tTregs. However, because only CD4+ tTregs have been described so far, further discussions will be centred on CD4+ population.
T cell development involves migration of single-positive (SP) T cells to medulla (Figure 2). It is possible that CD4+ tTregs, selected as described above, would also migrate to medulla before exiting the thymus. For conventional T cells that were positively selected in the cortex, the consecutive selection in medulla is a negative one (central tolerance), where SP T cells are tested against a different and more diversified set of self-pMHC ligands (Figure 3). The process deletes most of conventional T cell clones that are autoreactive. The evidence shows the existence of at least two regulatory T cell populations generated in the thymus that homes in the periphery. There is a perinatal wave of CD4+ Foxp3+ tTregs, produced by interaction with Aire-related TRA-derived pMHC ligands on mTECs. The other Treg population is distinct, because it is Aire-independent. Both, however, stably persist in adults.30 The former population constitutes central tolerance to a defined set of self-tissue antigens.30, 31 It protects an organism from development of multiorgan autoimmune disease such as autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy observed in individuals who have a mutation in the gene encoding Aire. The other tTreg population is Aire-independent, and its generation might include bone marrow–derived thymic APCs such as plasmacytoid dendritic cells (DCs), conventional DCs and B cells.30, 32 Recently, using transcriptomics, Owen et al showed two distinct progenitor tTreg populations: CD25+ Treg progenitor (TregP) that employs negative-selection developmental programme, and Foxp3lo TregP that co-opted positive-selection programme33 (Figure 2 legend). It is tempting to speculate that at least one of these progenitors stems from the selection on cTECs as proposed here.
3 THE ROLE OF TCR EDITING
Receptor editing is the observation that a proportion of antigen-responsive cells expressing a functional antigen receptor undergo secondary rearrangements of their V genes.34, 35 In the case of the TCR, it might mainly refer to T cell development during DP stage in the cortex, and so far concerns primarily Vα. Receptor editing has been suggested as cell-saving device.
The editing process (only secondary J rearrangements) has theoretically been calculated to decrease self-specificity in only 3%-4% of the whole population of cells, and probably most of those would lose self-restriction capability, provided a standard, single αβTCR model is taken as a starting point for analyses.36 Similarly, under the Tritope model such editing would also change only 3%-4% of specificities. However, unlike the Standard model, in the Tritope model, editing would tend to retain self-restriction to MHC of an individual, and it would only destroy one alloreactive capability exchanging it with another. Cohn surmises that TCR editing as a potential diversifier from anti–self-pMHC to anti–non-self-repertoire affects only a minority of thymocytes, suggesting that editing is neither of harm nor of value to the species.36
I disagree with Cohn's conclusion for two reasons: (a) N-region diversity, shown previously as the major mechanism in skewing T cell repertoire from initial anti-self to anti–non-self,19 has been left out of calculations, and (b) the type of the selection is different in the thymic cortex under the Tritope, Standard and Integrity models (escape from deletion in the former two vs competition for reward in the latter), and this might affect variability too (Table 2). It is thus reasonable to suggest that N-region hypermutation during “positive selection” of thymocytes can boost editing variability higher than previously estimated.36
| Observations/Predictions | Explanations | ||
|---|---|---|---|
| Standard model (Unitope) | Tritope model | Integrity/dynamic, Multitope model | |
| higher number of successful TCR α and β pairings | Yes; preferential TCR chain pairing, to generate lower affinities for self-pMHC and avoid apoptosis | Yes; to diversify the T cell repertoire | Yes, to generate TCR pairs with higher affinities for self-pMHC and avoid apoptosis |
| TCR Vs rearranged to the furthest Js | Yes (observed for J αs) | Irrelevant | Yes |
| intensive N-region diversity | Unknown | Irrelevant | Yes and relevant |
| Probability (guesstimate) in selecting TCR V alleles that bind to the MHC alleles of the same species in the course of evolution | Low: that is, generating low-affinity binding would be naturally deselected although the Standard model claims it is selected | High: provided entraining is proven as a mechanism for deactivation of TCR recognition | High: due to selection of the TCR partner to join in competing for the highest affinity binding |
The editing process in T cells (including N-region variability on secondary rearrangements) would be downregulated in thymic emigrants, perhaps as a sign of their maturity and competence to exit thymus. It might be inactivated for the rest of their lives, which is plausible, as otherwise autoimmunity would develop.
This whole scenario might explain the generation of the mild affinity of peripheral T cells’ TCR towards self-MHC alleles.
4 FUNCTIONAL ASPECTS
Invading parasites and micro-organisms might avoid recognition by T cells if they mutate away to mimic self-antigens of the host. Then, negative selection and deleting all anti–self-reactivities might allow a niche for survival of pathogens and parasites and would compromise the defensive function of the adaptive immune system. Vrisekoop and Forsdyke suggested that anti-foreign T cell repertoire might be developed by being initially positively selected on near-self in order to prevent such avoidance.37-39 The dynamic model (with the Integrity hypothesis) presented here is consistent with such a notion.
The SP (CD4 or CD8) αβ T cells that constitute peripheral repertoire have an affinity for the self-pMHC lower by several orders of magnitude than those encountered in the initial (preselection) repertoire. This reactivity according to the Standard model would keep them alive in times of desperation when their numbers become low, as seen in homeostatic proliferation. According to the Integrity model, such interactions might be sufficient to keep the organization of the immune system in a state of vigilance (to be elaborated elsewhere), that is constant watch for pathogenic stimuli through alertness to sentinels of the innate immunity.40
Recently, a report that CD4 T cell tolerance to tissue antigens is mediated by thymically derived antigen-specific Tregs is consistent with the dynamic competitive model of thymic TCR selection presented here.41 Furthermore, in the Integrity model, tTregs are peripheral forerunners of central tolerance and thus protectors from autoimmunity. They are also ultimate keepers of organism's integrity seen as tissue and organ stability that allow their normal functioning and integration of various cells and tissues into one whole, which is needed for such purpose. This mechanism (via tTregs), within the adaptive immunity, can give asylum to potential commensals in the gut or perhaps other areas within our bodies.40, 42, 43 The Integrity model is partially based on the Danger model.44
5 THYMIC TREGS AND INTEGRITY OF AN ORGANISM
The tTregs would keep integrity of tissues in the following scenario. The foci within cTECs where tTregs are being selected for high-affinity binding to self-pMHC ligands include epitopes of the hypothetical “integrity” proteins. These are unknown as yet (to be discussed elsewhere, in preparation), but could be envisaged as proteins that function in cell-cell contacts and cell-extracellular matrix (ECM) bindings or are specific for each tissue function and organization. It is supposed that tTregs would be able to home in a target tissue “monitoring” the area and preventing triggering of effector T cells by several mechanisms. For example, they could interact during the initial phases of the immune responses by decreasing the numbers of activated T cells in the draining lymph nodes or intervening at mucosal surfaces where low MHC class II molecule expression on plasma cells in lamina propria of the gut could provide sites for suppression of CD4 or CD8 effectors. It is envisaged that at least a three-cell interaction might be needed for integrity check in the tissues. Macrophages, DCs and B cells are candidates for such interactions.
6 THE MECHANISM OF tTREG FUNCTION
According to the Integrity concept, tTregs could be activated with three signals, as outlined previously.40, 43 In short, signal 1 and signal 2 represent TCR-pMHC ligand interaction and co-stimulation, respectively. Signal 3 is the disruption of integrity signal. It has two components: structural and functional. Here is the major difference between the Danger and Integrity models; namely, the Danger model44 has only one component of signal 3 (and, instead, uses “signal 0” for its description).
Signal 3 drives signal 2, which could be positive as well as negative.40 (The Danger model employs conceptually only positive signal 2.44) The innate immune cells, macrophages and DCs in disrupted tissues can sense the exposed self-antigens (some of which are also expressed on cTECs, as integrity homunculus), and they constitute structural (mechanical) damage component of signal 3. As a result, these putative important-for-organism-integrity molecules are phagocytized, processed and presented by the peripheral APC. Thymic Tregs seeded in most compartments of the body would become activated by such APCs, and exert their guardian function as protectors against autoimmunity as well as against dissolution of the organization of a body (keeper of integrity). Allegedly, an attack led by conventional T cells on targets carrying these epitopes would disintegrate the particular tissue and/or organ.
The function of tTregs is depicted in Figure 4A. They would recognize integrity antigens as self-pMHC ligands on APCs (for which they have been selected in the thymic cortex) and would compete with conventional T cells for the same epitope, effectively suppressing clonal response to this particular self-antigen. The anti–self-integrity conventional T cells might be generated in the course of fighting an infection, or a side effect of many T cell responses. The frequency of such cells is theoretically low, as most of anti–self-reactivity is depleted by the negative selection in thymus. The self-reactive CD4+ tTregs could inhibit licensing of autoreactive conventional CD8+ T cells on the same APC by bystander inhibition (Figure 4B), and prevent their activation. Thus, tTregs would constitute the last checkpoint of self-tolerance, essentially preventing or stopping autoreactivity.
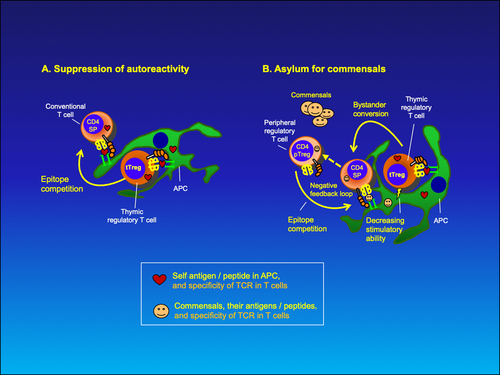
Recent review45 on antigen specificity of Treg development in the thymus and Treg function in the periphery lists evidence that supports the Integrity concept about thymic-derived Tregs being protectors against autoimmunity.
7 THE MECHANISM OF ACTION OF THYMIC TREGS ENCOUNTERS WITH BENEFICENT COMMENSALS
Natural tTregs can also protect beneficent commensals from the host immune attack. This might depend on a functional component of the third signal, which would be a benefit-sensing feature. For example, vitamin K–producing bacteria would be sensed (by picking up beneficent vitamin K–dependent enzyme activities), and a beneficial signal 3 by innate immune cells or stromal cells would hinder DCs (with phagocytized commensals’ antigens, since bacteria would have broken and damaged the barrier) to migrate to the draining LNs and to activate anti-commensal response. However, some mature DCs might sneak through this protection measure and eventually generate anti-commensal T cell response. Such conventional T cells would be stopped by tTregs (specific for integrity antigens) either during their activation or as effectors on tissue APCs.
Do peripheral (p)Tregs (with CD4+, Foxp3+, CD25+ phenotype) generated from conventional T cells play a role in commensal protection?
I advocate that there are functional differences between peripheral Tregs (pTregs) and tTregs. Apart from the TCR affinity for the ligand, mild in pTregs and (intermediate to) high in tTregs, their main disparity might be in the character of their suppression; namely, pTregs inhibit the activation of conventional T cells interacting with the same antigen-presenting cell (epitope competition), or prevent quiescent T cells from binding to APCs by sustaining APCs in a less stimulatory state (a short-range bystander suppression). Recent evidence describes pTregs as the IL-2–dependent negative feedback loop that has a role in ending the immune response, by downregulating ongoing conventional T cell response to the same antigen.46 Unfortunately, the generation of such pTregs is still unclear.
On the other hand, tTregs would similarly suppress effector T cells by epitope competition, but the scenario might change if and when a beneficent commensal would be encountered. In the course of activation of tTregs, the frequency of tTregs competing for the same epitope on APCs with conventional T cells might increase. I imagine that such increase at some level would impair non-specifically other ongoing activation processes in vicinity resulting in bystander inhibition. In addition, I propose that tTregs would convert conventional T cells into pTregs (or perhaps accelerate their appearance) that, for example, react against beneficent commensal's antigenic peptide/MHC ligand (Figure 4B).
8 THYMIC TREGS AND COMMENSAL-TURNED PATHOGEN
What happens if the commensal becomes a pathogen? Switch into the pathogenic state would also change the functional component of signal 3. This could involve alterations either in signal 1 or signal 2. For example, a destructive functional signal 3 might resemble a pathogen activity that could hypothetically cause secretion and activation of transglutaminase 2 enzyme (important for ECM assembly), which, in turn, could transamidate or deamidate a number of peptides and proteins (for a review, see Lorand and Graham47) such that they would lose their beneficial roles. In addition, deamidated self-integrity peptides presented by the APC to tTregs would fail to activate the latter, because there would be none around specific for this combination of self-deamidated-pMHC ligand. Hence, without signal 1, tTregs would not be activated and such a state would then allow a robust anti-pathogenic response. Alternatively, a change from beneficial to destructive signal 3 component would influence the switch of signal 2 from positive to negative for tTregs. The latter results with diminished suppression exerted by tTregs, thereby commencing the activation of the anti-pathogen T cell responses. Here, the Integrity model seems to include co-stimulation/co-inhibition features of the model proposed by Sinclair and Anderson,48 which is a different concept in itself.
9 THE LINK BETWEEN THE GENERATION OF THYMIC TREGS IN THE THYMUS AND BENEFICENT COMMENSALS (OR LEARNING WHOM TO GIVE ASYLUM)
The asylum function would be controlled by tTregs and not pTregs, although the latter would be instrumental in the suppression of the responses as negative feedback loop of the conventional T cell immune responses. The asylum or “tolerance and protection against the host immune cell attack” would preserve beneficent commensals. For example, commensals could be bacteria, which could penetrate mucosa, but would neither poison the host, nor damage structural cell-cell or cell-ECM interactions to the level that would lead to large disintegration of tissues.
- Commensals might mimic self-integrity structural components. They would be beneficent if pathogens, which exert their pathogenicity by destroying cell-cell or cell-ECM connections, would have the mirror image of (and could bind) integrity proteins. Thus, commensals that resemble self-integrity antigens could neutralize (outcompete) attack by such pathogens. The components of commensals that mimic conformation of self-integrity proteins could have a proportion of its peptides similar to integrity homunculus. Therefore, tTregs, which are selected for binding to integrity homunculus-pMHC ligands in the thymic cortex, might recognize commensal's peptides on peripheral APCs and inhibit adaptive immune cell attack on beneficent, integrity-mimic commensal population. In this scenario, pathogens could overcome defences by mimicking self-integrity structures and exploit tTregs to avoid immune cell attack, perhaps leading to chronic infectious disease.
- It is possible that commensals might be shaped as a mirror-image form of hypothetical integrity components of the host and thus be able to bind to them. As mentioned above, pathogens could sometimes evolve a strategy to avoid attack by the host immune cells by mimicking host (self-) structures. In such a case, one possible solution for the defence of the host would be to employ detrimental autodestructive immune response in order to get rid of the pathogen (provided the host survives) or, instead, keep beneficent commensals in asylum, anticipating they would outcompete pathogens or perhaps use both strategies (but with a transient autodestruction phase).
Hence, let us consider the last option in the host infected with self-mimicking pathogen. I suggest that self-proteins (as peptides) resembling “mirror images” of beneficent commensals would be present in the thymus to generate tTregs specific for them. The molecules present in the host that are capable of binding mirror image of self-mimicking-pathogen–derived product are immunoglobulin (Ig) V regions. Therefore, Ig V-region idiotypes (Id), derived from host B cells passing through the thymus (and expressing Aire),24 could be a source of Id-specific self-peptides in the thymus, and used for the generation of tTregs to prevent host T and B cells in attacking commensals.
Thus, the protection of beneficent commensals would be learned by the immune system. I suggest that the learning process (other than the 2-week perinatal Aire-dependent window of tolerance in mice by tTregs) is implemented either with Aire-related or with Aire-independent mechanism using B cells passing through the thymus (at the cortico-medullary junction). B cell idiotypes of antibodies specific for beneficent commensals, initially produced against them in the conventional immune response against foreign antigen, would be expressed as Aire-dependent TRAs that could be either transferred to cTECs or expressed on B cells as pMHC ligands, and consequently used for the generation of tTregs. Therefore, the Id produced (in the example, specific for anti–self-mimicking antigen) would be involved in the protection of commensals by generating tTregs.
10 THE PROTECTION BY TTREGS (ASYLUM) IS MORE THAN TOLERANCE
Because of the above-mentioned scenario, beneficent commensals resembling anti–self-mimicking antigens could attenuate the attack of self-mimicking pathogens by outcompeting them for habitat in gut flora, in the future. This protective (asylum) function of the immune system is arguably more than just tolerance, the latter being perhaps just “careless” non-reactivity. Asylum would have similar benefits for the host immune defence as the selection of the repertoire on near-self, proposed recently by Germain group39 and Forsdyke.37, 38
The Integrity model provides an explanation how the asylum function could be achieved (to be discussed in more detail elsewhere). The explanation at some point might resemble in part the Id/anti-Id suppression network model described by Coutinho et al,49 Modigliani et al28 and Stewart and Coutinho,50 but differs from it in conceptual workings of the immune system, especially in its mechanisms of activation and regulation.
In short, Coutinho et al28, 49, 50 discussed development of central and peripheral tolerance mechanisms of “natural tolerance”, and they define it as non-reactivity of the immune system to organs and tissues. They based their analyses on the experimental system using thymic transplantation in birds, which pre-dates discovery of Aire-driven TRA expression on mTECs and haematopoietic APCs in the thymus and in the periphery. Coutinho et al suggested two types of tolerance development: dominant and recessive, both provided by the thymus and the periphery. Dominant tolerance (defined as the ability of transplanted thymic epithelium to impart tolerance to other tissues of the donor) was associated with the generation of suppressive regulatory T cells, and they postulate a homunculus of self-antigens being present in the thymus to positively select regulatory cells.49 Haematopoietic cells (originating in periphery) would also have similar dominant tolerizing ability, if transplanted into the thymus. In the periphery, the thymic regulatory T cells supposedly could educate conventional T cells to become regulatory ones. The regulatory T cells would inhibit APC functions. The ratio between regulatory T/APC and conventional T/APC numbers would control the T cell activation and the initiation of the immune responses against both self- and non–self-antigens. The anti–self-response would be possible, as the authors propose existence of “physiologic autoreactivity”. Recessive tolerance was linked to deletion of T cell clones specific for self-antigens both in the thymus and in the periphery. Coutinho et al state that T cell repertoire for dominant tolerance is different from those of conventional T cells, and perhaps only in part overlapping with each other.28, 49
In this study, I also connect dominant tolerance with tTreg appearance selected on integrity self-antigen homunculus in the thymus. Similarly, tTreg repertoire would be different from conventional T cells’ one, and also differently distributed among individuals of a species. Here, I propose that tTregs’ repertoire has higher TCR affinities for self-ligands (self-pMHC) than that of conventional T cells. Modigliani et al28 proposed rules for positive and negative selection of thymocytes and suggested that higher avidity for self-pMHC ligands would select regulatory T cells, but without implying TCR editing, which is the important difference discussed here; namely, Modigliani et al28 define avidity as a product of affinity of the TCR by the number of copies of its ligand, perhaps improved by the higher rates of internalization and recycling of TCRs. In contrast, here I suggest that higher avidity to select tTregs includes the TCR editing, which can increase its affinity, besides the binding of a co-receptor to the pMHC ligand.
Furthermore, the major difference between the two models lies in the way of proposed immune system function; namely, Coutinho et al suggest a Jerneian-type suppressive network that drives both B and T cell actions. The Suppressive network model proposes BCR idiotype/anti-idiotype mutual interactivity that balances suppression vs activation of B cells, and a similar network for T cells.28, 49, 50 I find it unclear under which conditions a non–self-(foreign) antigen could stimulate anti-foreign response and when it would generate a suppressive (regulatory) one.
On the other hand, with the Integrity model,40, 42, 43 I suggest a three-signal principle (with six possibilities of signalling: positive and negative signals 1-3) for the activation of conventional quiescent anti-foreign T and B cells, and tTregs. The Integrity model is based in part on the Danger model by Matzinger44 that has only three signalling possibilities (signals 0, 1 and 2).
Similarities between the Suppressive network28, 49, 50 and the Integrity40, 42, 43 models are the dominance of the proposed tolerance mechanisms and the ideas of representation of self-homunculus in the thymus. The differences are in the assembly of self-homunculus ligands, in the mechanism of the generation of thymic regulatory T cell repertoire, and in the mechanisms of action of immunocytes, including tTregs and pTregs in the periphery of the immune system.
11 THE DYNAMIC MODEL PREDICTIONS
Other explanations and predictions of the dynamic competition model in the thymus include (a) a much higher number of successful TCR α and β pairings, (b) higher level of Vs being rearranged to the furthest Js in the genetic areas and (c) intensive N-region diversity (Table 2). Using elegant besides extensive experimental system, recent findings support the former two aspects: enlarged Vα and β chain pairing ability and preferentially distal Jα segment rearrangements.8 However, Marrack's and Kappler's explanation for the great bias towards distal Jαs was evolutionarily less compatible as the other two models; namely, increased time available for rearrangements for the thymocytes in single TCRβ transgenic animals to “search” for a suitable alpha chain partner cannot account for natural selection of the TCR V-MHC pairings within a species (Table 2). There is an alternative explanation. It could be that both findings were signs of the competition for the high-affinity binding to self-pMHC in order to lock on targets suitable for differentiation into tTregs. Perhaps a similar outcome (preferentially distal Jβ rearrangements) might be found for the TCR beta chains in a different experimental setting.
The proposed constancy (unchangeability) of somatically generated diversity of the TCR in mature T cells would ensure two hallmarks of immunity: immunologically competent T cell repertoire and conservation of TCR V-region alleles coupled to MHC alleles of a species, which would otherwise be lost in the course of phylogeny.