Paediatric and adult bronchiectasis: Diagnosis, disease burden and prognosis
ABSTRACT
Bronchiectasis is a chronic, debilitating disease with increasing worldwide prevalence and burden. Accurate and early diagnosis is essential for both its management and prognosis. This review will discuss the diagnosis of bronchiectasis, the international burden of the disease and its current prognosis.
INTRODUCTION
Bronchiectasis is a chronic, debilitating disease characterized by permanent dilatation of the bronchi.1 The airways are continuously inflamed and infected causing the characteristic features of the disease, namely persistent cough, sputum and recurrent infective episodes.2 The worldwide prevalence and impact of the disease has become increasingly recognized over the past few decades, with studies demonstrating not only the clinical heterogeneity of bronchiectasis but also the differences in terms of gender, ethnicity and age. Bronchiectasis is more common and typically more severe with increasing age.3 Over the age of 18 years, bronchiectasis becomes more common in females and it is recognized that in all age groups females have a poorer prognosis.4 Ethnicity is also important with Seitz et al. reporting that among those who had at least one computerized tomography (CT) scan confirming bronchiectasis, Asians had a 2.5- and 3.9-fold higher period prevalence compared with whites and blacks suggesting ethnic variations even in the United States among patients with bronchiectasis.5
DIAGNOSIS
Adults
Symptoms and history taking
The diagnosis of bronchiectasis is usually suspected in adults who regularly cough up large volumes of sputum, or in individuals who present with recurrent chest infections.
Cough is present in 90.2–96% of patients and sputum expectoration is reported in approximately 75% of patients, with a mean (SD) daily volume of 38 ± 34 mL at diagnosis.6, 7 Sputum is often discoloured, with a yellow or green purulence reflecting airway inflammation and infection.8, 9 Other common symptoms include breathlessness, haemoptysis, chest pain and wheeze (Table 1). Less commonly, tiredness and difficulty with concentration ensue.
Diagnosis is often delayed as symptoms are synonymous with other respiratory conditions such as chronic obstructive pulmonary disease (COPD) and asthma. Chronic cough is one of the most common reasons for patients to present to primary care.10 Chronic sputum production is also common; in one study, 2 patients per week in a practice of 10 000 presented with persistent lower respiratory tract infection symptoms in spite of antibiotic treatment, and among those over over-third had a diagnosis of bronchiectasis.11
There are a number of different causes of bronchiectasis or diseases associated with bronchiectasis which need to be considered when making a diagnosis. Such co-morbid disease may be related to the cause of the bronchiectasis or indeed be a consequence, but it is important to establish an aetiology for bronchiectasis as early as possible as management of some underlying conditions may alter prognosis.12, 13 Therefore, a comprehensive current and previous medical history should be considered including detail of childhood infections and respiratory symptoms, family history of bronchiectasis or other lung diseases, smoking history, rhinitis, gastro-oesophageal reflux and systemic inflammatory symptoms such as rashes, muscle pain and joint problems. Table 2 includes the more common aetiologies and co-morbidities.
| Aetiology | Suggested initial investigations | Expected abnormal findings |
|---|---|---|
| Immunodeficiency and chronic Infection | Serum Ig | |
| CVID | IgG, IgA and IgM Baseline specific antibodies to polysaccharide capsules of Streptococcus pneumoniae |
Deficiencies as per condition Low baseline antibody response |
| HIV | HIV antibody |
Positive antibodies |
| Human T-lymphotrophic virus | ||
| Post-transplantation (particularly haematological or lung) | ||
| Malignancy | ||
| Airway disease | ||
| Asthma | History and spirometry Methacholine challenge testing |
Normal or obstructive pattern with reversibility Hyperresponsiveness to methacholine |
| ABPA | Total IgE; Aspergillus-specific IgE antibodies; thoracic imaging |
Elevated total IgE >5000 IU/mL; elevated serum IgE specifically to Aspergillus or cutaneous wheal >3 mm to Aspergillus; new radiological infiltrates |
| COPD | History and spirometry | Irreversible obstructive pattern and history |
A1AT deficiency |
A1AT level; A1AT genotype | Deficient level of A1AT; detection of specific gene mutations (e.g. S, Z and M) |
| Rhinosinusitis | History History and examination |
Nasal discharge/congestion; nasal drip; facial discomfort; hypo- or a-nosomia; nasal polyps |
Post-infectious |
||
| Pneumonia, tuberculosis, pertussis, viral infections (measles, influenza and adenovirus) | History Imaging |
Positive history for previous infection Radiological evidence (e.g. granulomas) |
| Aspiration | ||
| Gastro-oesophageal reflux disease/recurrent aspiration | History | Reflux, dysphagia, vocal cord dysfunction all supporting features |
| Foreign body inhalation | Bronchoscopy (if suggestive history/single lobe bronchiectasis) | Foreign body in airways |
| Impaired mucociliary clearance | ||
| Cystic fibrosis | Supporting history; sweat chloride testing and subsequent cytogenetics | Supporting clinical features include: younger age, family history, history of malabsorption and history of infertility Sweat chloride >30 mmol/L requires further assessment |
Primary ciliary dyskinesia (and Kartagener's syndrome) |
Supporting history, nasal nitric oxide, ciliary frequency/pattern from brushings | Supporting history of upper and lower respiratory infections, infertility, situs inversus. Nasal nitric oxide levels are low. Reduced ciliary beat frequency and pattern |
| Structural lung disease | ||
| Interstitial lung disease | History, imaging and serum markers as appropriate | Classical radiological findings specific to the condition |
| Bronchiolitis | ||
| Sarcoidosis | ||
| Systemic inflammatory diseases | ||
| Rheumatoid arthritis | History, rheumatoid factor, anti-CCP, imaging |
Positive supporting history and examination, elevated rheumatoid factor, elevated anti-CCP, classical changes on joint imaging |
| Connective tissue diseases | History and relevant autoantibody screen |
History positive for connective tissue disorders. Positive diagnostic criteria as relevant to the suspected diagnosis |
| Inflammatory bowel disease | History, GI opinion and specialist investigations (e.g. colonoscopy) |
- A1AT, alpha-1 antitrypsin; ABPA, allergic bronchopulmonary aspergillosis; CCP, cyclic citrullinated peptide; COPD, chronic obstructive pulmonary disease; CVID, common variable immune deficiency; GI, gastroenterology; HIV, human immunodeficiency virus; Ig, immunoglobulin.
Finally, the impact of bronchiectasis on health-related quality of life (HRQL) is well recognized.14-16 Anxiety and depression are common, with depression in 21.1–34% and anxiety present in 39.8–55% and should be screened for at presentation.17, 18
Signs
The most common signs in adults include crackles on auscultation, present in 69.9–73% at the time of diagnosis, most commonly in the lower zones.6, 7 Crackles are typically present on expiration and may be reduced in intensity temporarily by coughing. Wheeze is less common (21–34% at presentation) and clubbing is also a recognized although infrequent finding (2–3%).6, 7 Often, physical examination is normal.
Investigations
In adults, confirmation of the diagnosis is made radiologically, ideally when the patient is clinically stable using high-resolution computerized tomography (HRCT).19 The defining feature essential for diagnosis is the presence of bronchial dilatation.20 Volumetric computerized tomography (CT) has improved sensitivity with more frequent identification of bronchiectasis and interobserver agreement is also significantly better for the diagnosis of bronchiectasis compared with incremental or interspersed slices but may involve greater radiation doses.21 Thin section (<1 mm) slices acquired using a high spatial frequency reconstruction algorithm should be used.22 Bronchial dilatation is evidenced by the internal diameter of the bronchial lumen measuring greater than that of the adjacent artery (bronchioarterial ratio > 1) (Fig. 1). Additional features suggestive of bronchial dilatation include a lack of tapering and airway visibility within 1 cm of the costal pleural surface or touching mediastinal pleura.23 Other classic radiological findings associated with bronchiectasis include bronchial wall thickening, interlobular septal thickening, emphysema and a mosaic attenuation pattern due to small airway disease.23-25 Although the utility of CT scan in identifying a cause of bronchiectasis is thought to be of limited value particularly on an individual patient basis, attention should be paid to potential radiological evidence of causative features of bronchiectasis, such as bronchomalacia, tracheobronchomegaly and lung sequestration.26
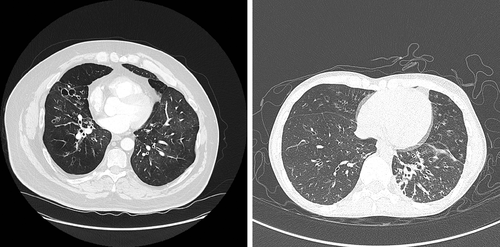
HRCT changes characteristic of bronchiectasis may also be associated with other diseases, such as part of airway remodelling in asthma. When the clinical picture of cough, sputum and infections is not present, then a radiological diagnosis only of bronchiectasis may be considered.
Additional investigations at the time of presentation should include screening tests for some of the more common aetiologies or associated co-morbidities as well as relevant information to assist with future disease monitoring (Table 3). More detailed screening may take place in specialist units such as measurement of baseline specific antibody capsular polysaccharides of Streptococcus pneumoniae; for those with low levels, immunization with 23-valent polysaccharide vaccine is undertaken with repeat testing to assess for an appropriate response. Other more specialized investigations may be necessary to fully explore the potential aetiology for bronchiectasis and should be guided by both history and clinical examination, for example, sweat chloride testing for suspected cystic fibrosis (CF).
| Investigation | Role | Findings |
|---|---|---|
| Chest radiograph | Disease assessment | Signs of bronchiectasis ± aetiology Exclude differential diagnoses |
| HRCT chest scan | Diagnosis | Evidence of bronchial dilatation greater than adjacent artery and any other supporting or diagnostic features |
| Spirometry | Disease assessment | Presence of airflow obstruction ± reversibility |
| Total IgE | Disease assessment | Elevated (for ABPA >5000 IU/L) |
| Sensitization to Aspergillus fumigatus | Disease assessment | Elevated serum IgE specific to Aspergillus or positive skin prick test |
| IgG, IgA and IgM | Disease assessment | Deficient levels suggestive for immunodeficiency syndromes |
| Sputum bacteriology | Disease assessment | Supporting evidence for chronic airways infection and Mycobacterial infection |
- ABPA, allergic bronchopulmonary aspergillosis; HRCT, high-resolution computerized tomography; Ig, immunoglobulin.
Paediatric
Symptoms and history taking
Bronchiectasis should be suspected when a child presents with a recurrent or persistent ‘wet’ cough (i.e. >6 weeks’ duration).27 A study of 93 children with persistent cough and bronchiectasis found that the duration of the cough correlated with severity markers for bronchiectasis.28 Children with episodes of recurrent pneumonia, recurrent prolonged bacterial bronchitis or a cough that is not responsive to a prolonged (more than 4 weeks) course of antibiotics should also be considered for an underlying diagnosis of bronchiectasis.29-31 In addition, children investigated for asthma in whom symptoms do not improve with treatment should be investigated for bronchiectasis, especially those presenting with cough variant asthma. A history of infection with Bordetella pertussis, adenovirus or Mycobacterium tuberculosis should also raise suspicion for an underlying diagnosis of bronchiectasis.
A recent study of 34 paediatric patients (mean age: 13.69 ± 4.67 years) diagnosed with bronchiectasis found cough to be a presenting feature in 98% with sputum present in 76.5%. Other presenting features reported included haemoptysis (14.7%) and clubbing (14.7%).32
Exploring co-morbidities such as malnutrition, gastro-oesophageal reflux disease, sleep-related disorders, features suggestive of underlying immunodeficiency syndromes (severe and persistent recurrent infections), features suggestive of underlying primary ciliary dyskinesia (rhinitis, respiratory distress of unknown cause and severe chronic serous otitis media) and other features suggestive of CF (including, but not limited to, diarrhoea, failure to thrive and electrolyte disorder) should also prompt consideration of an underlying diagnosis of bronchiectasis.
Signs
Classical signs of established disease include clubbing, cyanosis, chest deformity and hyperinflation, but these signs are not always present. A significant sign is the presence of inspiratory crackles and wheeze is much less common.
Radiology
In addition to both characteristic symptoms and signs, chest imaging is used. HRCT scans demonstrating abnormal bronchial dilatation confirms diagnosis, although there is debate as to the correct bronchial-arterial cut-off ratio for diagnosis in paediatrics, particularly given that appearances will change with age.33, 34 The diagnosis of bronchiectasis in paediatrics currently remains both clinical and radiological.
DISEASE BURDEN
Disease burden can be thought of in a number of different ways. For patients, it is about symptom burden and the effect on their HRQL. From a healthcare perspective, it is about healthcare utilization and cost. From an epidemiological perspective, it is about understanding who has the disease and how that is changing over time. Truly assessing incidence and prevalence at a population level is difficult given that the current disease definition requires both clinical symptoms and HRCT changes. In recent years, there have been registries established such as EMBARC and the US registry.35, 36 Whilst registries are not able to provide information on disease epidemiology, they are useful for deep phenotyping a sub-select group of individuals and a platform from which to recruit individuals to trials.
In a clinical setting, making a diagnosis of bronchiectasis should be relatively straightforward. How that diagnosis is recorded in medical notes, although perhaps not thought about in as much detail is equally as important. Most of the epidemiological studies on disease burden do not come from collection of data from new cohorts, but from existing data, relying on coding which may have been done from a clinical or billing standpoint. In addition, sources of data and coding strategies differ by country as do selection criteria for more specific study designs. Previous studies have used insurance data, hospitalization diagnosis data and primary care data as sources to determine population burden (Table 4).3, 5, 37-40
| Coding | Author | Country | Prevalence | Time period |
|---|---|---|---|---|
| ICD (secondary care) | Weycker37 | USA | 52/100 000 adults | 1999–2001 |
| ICD (secondary care) | Seitz5, 38 | US | 370/10 000 over 65 | 2000/2007 |
| ICD (secondary care) | Ringshausen39 | Germany | 67/100 000 adults | 2013 |
| Read (primary care) | Quint3 | UK | 566.1/100 000 in women and 485.5/100 000 in men | 2013 |
| ICD (primary care) | Monteagudo40 | Catalonia, Spain | 35/10 000 | 2012 |
Irrespective of the source, one common theme seems to be the steady increasing prevalence and incidence of the disease, likely attributable to an ageing population, and increased access to CT scans.
The burden of bronchiectasis may be worsened by overlap with other common respiratory diseases, in particular COPD. Bronchiectasis and COPD share common symptoms which may contribute to diagnostic delay which in turn can worsen disease severity and impede optimal management.41 Treatments used for one disease (e.g. inhaled corticosteroids in COPD) are not recommended for patients with bronchiectasis and their use may in fact increase the incidence of atypical infection in patients with bronchiectasis. Whilst the overlap of asthma with bronchiectasis is not recognized as frequently, again it is not inconceivable that it exists and that it would worsen disease trajectory for similar reasons to bronchiectasis and COPD overlap. In one study in China, patients with bronchiectasis and asthma were found to exacerbate more than those with bronchiectasis alone.42 As these overlap syndromes become more recognized, we may in turn see an additional healthcare cost, increased mortality and decreased patient quality of life.
Adults
Two studies in the United States have found the prevalence to be increasing over time and in older age groups, a finding that has been replicated in Germany, albeit at a smaller magnitude.5, 37, 39 In keeping with this being a true bronchiectasis diagnosis, other studies around the same time periods have seen an increase in hospitalization associated with bronchiectasis, suggesting the disease burden is increasing and bronchiectasis is not just a chance finding on CT.
Not only has secondary care data suggested an increased burden, a study in the UK using primary care data found a year-on-year increase in incidence and prevalence from 2004 to 2013 again mirroring the secondary cares studies with the highest incidence and prevalence being in the older population.3 Similar findings were seen in a Catalonian study (Fig. 2).40
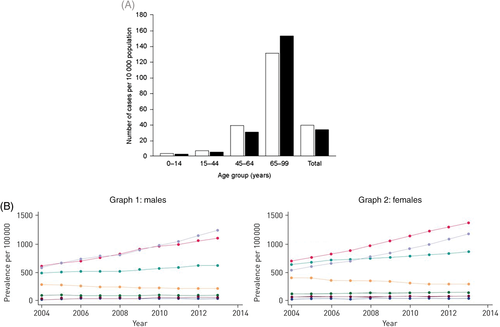
 , women;
, women;  , men) and (B) the UK from 2004 to 2013, graph 1 males and graph 2 females (age (years):
, men) and (B) the UK from 2004 to 2013, graph 1 males and graph 2 females (age (years):  , 18–29;
, 18–29;  , 30–39;
, 30–39;  , 40–49;
, 40–49;  , 50–59;
, 50–59;  , 60–69;
, 60–69;  , 70–79;
, 70–79;  , ≥80). Reprinted from Monteagudo et al.,40 with permission.
, ≥80). Reprinted from Monteagudo et al.,40 with permission.PAEDIATRIC
There is a paucity of data around the burden of non-CF bronchiectasis in children. This is predominantly because radiological confirmation is needed and accessibility to CT is often limited. It is thought to be a major health issue for indigenous populations in New Zealand (Maori and Pacific Islanders), Australia (aboriginal) and United States (native Alaskans), with the self-perpetuating cycle between frequent exacerbations and disease progression contributing to the burden.43 More recently, however, it has been found to be an issue in affluent societies even in non-indigenous settings.44 There are no data from low-income countries, where the prevalence is thought to be highest. Limited data from high-income countries are summarized below (Table 5).45-48 However, it is apparent that burden is linked to socio-economic status with the highest hospital admissions seen in socially disadvantaged populations.
| Country | Dates | Prevalence | Population |
|---|---|---|---|
| Finland45 | 1983–1992 | 0.5/100 000 | Incidence in hospitalized children in Finland |
| Australia46 | 2002 | 1470/100 000 | Aboriginal children under 15 |
| USA47 | Early 2000s | 1600/100 000 | Alaskan native children |
| NZ48 | 1998–2000 | 1/6000 | Auckland tertiary referral centre |
| UK49 | 2005–2007 | 0.2/100 000 | Multicentre registry across UK |
| Ireland50 | 1996–2006 | 2.3/100 000 | Irish children |
| United Arab Emirates51 | 1994–1995 | 13.3/100 000 | Paediatric hospital clinic in UAE |
| NZ | 2009–2013 | 15/100 000 | Children living in poverty in NZ, multi-ethnic |
| Fiji52 | 1985–1989 | 7/100 000 | People native to Fiji |
| USA5 | 2000–2007 | 1106 cases per 100 000 people | 5% Sample of the Medicare outpatient claims database. 8-Year period prevalence reported |
- NZ, New Zealand.
ECONOMIC BURDEN
Of course, the economic burden of bronchiectasis cannot be ignored but is often looked at within the context of respiratory disease as a whole and not an individual disease. There are no studies for paediatric disease, and those for adult disease are summarized in Table 6.37, 53-56
| Study | Country of origin | Year | Findings |
|---|---|---|---|
| de la Rosa53 | Spain | 2013 | Mean annual cost per patient Euro 4672 |
| Sanchez-Munoz54 | Spain | 2004/2013 | Mean annual cost per patient Euro 3515 in 2013 |
| Weycker37 | USA | 1999–2001 | Total healthcare costs per year for bronchiectasis patient $5681 more than matched controls |
| Joish55 | USA | 2005–2009 | Increased average and respiratory-related cost per patient versus control of USD 2319 (95% CI: 1872–2745) and USD 1607 (95% CI: 1406–1809), respectively |
Blanchette56 |
USA | 2007–2013 | Pre and post Pseudomonas |
PROGNOSIS
There is little in the literature regarding mortality in bronchiectasis. Certainly, mortality is greater than in the general population, with the average age of death lower than in the general population. In the paper by Quint et al., they found that for women the age-adjusted mortality rate for the bronchiectasis population was 1437.7 per 100 000 and for the general population 635.9 per 100 000; (comparative mortality figure of 2.26). In men, the age-adjusted mortality rate for the bronchiectasis population was 1914.6 per 100 000 and for the general population 895.2 per 100 000; (comparative mortality figure of 2.14), indicating that mortality for both men and women with bronchiectasis is more than twice the mortality in the general population, independent of age differences between the two populations.3
Prognosis is disease severity and co-morbidity dependent and in a tertiary referral centre in the UK, male gender, age, respiratory function and Pseudomonas aeruginosa were all found to be independently associated with mortality.57 No study has looked at differences in prognosis with different aetiologies as numbers have been too small. There are a number of scoring systems now available that may aid in prognosis which are summarized below.
ADULTS
Bronchiectasis Severity Index
The Bronchiectasis Severity Index (BSI) was developed in the UK using data from a prospective cohort study that had 4 years of follow-up.58 This cohort had strict inclusion criteria and the score developed assigns points based on nine variables including age, BMI, forced expiratory volume in 1 s (FEV1) % predicted, previous hospital admission, number of exacerbations in the previous year, MRC breathlessness score, pseudomonas colonization, colonization with other organisms and radiological severity; thus, stratifying patients into mild, moderate and severe (http://www.bronchiectasisseverity.com/15-2/). Whilst useful and an important step forward, it still requires validation in other cohorts and countries. The score generated is categorized into mild (score of 0–4) with a 4-year mortality risk of 0–5.3% and a 4-year hospitalization risk of 0–9.2%; moderate (score of 5–8) with a 4-year mortality risk of 4–11.3% and a 4-year hospitalization risk of 9.9–19.4%; and severe (score of ≥9) with a 4-year mortality risk of 9.9–29.2% and a 4-year hospitalization risk of 41.2–80.4% .
Bronchiectasis Aetiology Comorbidity Index
The Bronchiectasis Aetiology Comorbidity Index (BACI) was developed in 2016, following on from the BSI, in an international cohort of nearly 1000 patients.13 In this study, the impact of co-morbidities on 5-year mortality and 4-year hospitalization was studied and a cumulative score developed grouping patients into low (score of 0, with a 5-year mortality of 3.5% and a 4-year hospitalization of 11.7%); intermediate (score 1–5 with a 5-year mortality of 11.7% and a 4-year hospitalization of 14.8%) and high (score ≥ 6 with a 5-year mortality of 34.9% and a 4-year hospitalization of 36%). The co-morbidities found to be most important in contributing to the score in descending order of importance are metastatic or haematological malignancy, COPD, cognitive impairment, inflammatory bowel disease, chronic liver disease, iron deficiency anaemia, peripheral vascular disease, diabetes mellitus, asthma, pulmonary hypertension and ischaemic heart disease. In essence, the BACI strengthens the BSI and it is suggested the two are used together.
FACED and eFACED scores
The FACED score is another method for predicting 5-year mortality derived from a multicentre Spanish cohort of over 800 patients.59 It comprises FEV1 % predicted, age, colonization with P. aeruginosa, radiological extension of bronchiectasis (lobes) and modified MRC breathlessness scale. One important thing to note about this score is that the calculations were performed at initial diagnosis and it has not been validated for use when following up existing patients.
The FACED score has subsequently been updated with the eFACED in 1470 patients to predict exacerbations in addition to mortality.60 In this system again, patients are grouped into mild (score 0–3), moderate (score 4–6) and severe (score 7–9). The area under the receiver operator curve (ROC) for all-cause 5-year mortality is 0.87, and respiratory mortality 0.86.
Comparing the scoring systems
In a paper by Costa et al., BSI and FACED were compared in a population of patients with bronchiectasis in Portugal.61 According to FACED, only approximately 12% of individuals had severe bronchiectasis as opposed to one-third by BSI, suggesting that although the variables in both scores overlap, they do not correlate well, thus suggesting they are measuring different things. In a study by McDonnell et al. including over 1600 European patients with bronchiectasis, a similar scoring distribution was seen.62 In essence, the aim of FACED is to predict mortality, and BSI may be better used to assess severity. Neither one should be used to guide treatment decisions.
CT scoring systems
A recent simplified CT scoring system to help assess clinical disease severity has been developed, the Bronchiectasis Radiologically Indexed CT Score (BRICS).63 This score was developed in a UK cohort of 184 patients with clinical evidence of bronchiectasis suspected to be either post-infective or idiopathic in nature and externally validated in 302 patients. The score uses bronchial dilatation and the number of bronchopulmonary segments with emphysema and ranges from 0 to 5 (1 being mild disease; 2–3 moderate disease and >3 severe disease). The area under the ROC for FEV1 % predicted is 0.79; 0.71 for sputum purulence and 0.75 for hospital admissions per year.
Paediatric
There are currently no scoring systems available for children. In terms of exploring prognosis in the paediatric population, a number of single centre studies exist looking at mortality. In one study in England in Wales from 2001 to 2007, of the 5745 deaths attributed to bronchiectasis, only 12 were in the 0–14 year age group.64 Mortality was found to be higher in a single centre in New Zealand between 1991 and 2006.65
FUTURE
It is clear that establishing a diagnosis of bronchiectasis early can allow both monitoring and intervention which may ultimately have prognostic implications. However, the importance of the highly heterogeneous nature of bronchiectasis is becoming increasingly acknowledged.66 To date, there has been only one consistent clinical subgroup identified as having similar features and prognosis in bronchiectasis, that those colonized with P. aeruginosa have a greater rate of lung function decline, a poorer HRQL and more frequent exacerbations.67, 68 However, recent randomized controlled trials of interventions have failed to meet their primary endpoints and it has been speculated that one of the reasons for this is differences within the defined study populations.69 A greater understanding of disease pathogenesis and aetiology is necessary and is emerging; in the future, this will need to be incorporated at diagnosis to allow more accurate disease phenotyping with the aim of stratifying patients to individualized treatment plans, to help reduce disease burden, improve prognosis and plan healthcare delivery.
The Authors
Dr J.Q., MSc, PhD, FHEA, FRCP, is currently Reader in Respiratory Epidemiology at the National Heart and Lung Institute (NHLI), Imperial College London, and an Honorary Consultant at the Royal Brompton Hospital. She leads a clinical epidemiology research group focusing on respiratory and cardiovascular disease. Her work centres largely on the use of electronic health records to study COPD bronchiectasis and asthma. Dr M.P.S., MB ChB, MD, FRCP(Ed), is an Assistant Professor in the Pulmonary Division in the University of Alberta, Edmonton, Canada. She directs the Bronchiectasis Clinic and also conducts translational research in the condition. She has previously conducted several studies in bronchiectasis, including the role of nebulized gentamicin and regular physiotherapy. She is also a member of the BTS Bronchiectasis Guideline Group, co-authoring the 2019 Guideline.
Abbreviations
-
- A1AT
-
- alpha-1 antitrypsin
-
- ABPA
-
- allergic bronchopulmonary aspergillosis
-
- BACI
-
- Bronchiectasis Aetiology Comorbidity Index
-
- BMI
-
- body mass index
-
- BSI
-
- Bronchiectasis Severity Index
-
- CCP
-
- cyclic citrullinated peptide
-
- CF
-
- cystic fibrosis
-
- CT
-
- computerized tomography
-
- FACED
-
- (F: forced expiratory volume in 1 s [FEV1]; A: age; C: chronic colonization by Pseudomonas aeruginosa [PA], E: radiological extension [number of pulmonary lobes affected], and D: dyspnea)
-
- FEV1
-
- forced expiratory volume in 1 s
-
- HIV
-
- human immunodeficiency virus
-
- HRQL
-
- health-related quality of life
-
- MRC
-
- Medical Research Council
-
- NZ
-
- New Zealand
-
- ROC
-
- receiver operator curve