Nebulization of corticosteroids to asthmatic children: Large variation in dose inhaled
Abstract
Background and objective
Despite problems associated with assessing the clinical effect and side effects of nebulized corticosteroids, little is known of the amount of drug that is inhaled by children with asthma or how this is affected by different drug formulations. The aim of this study was to test the hypothesis that children with asthma inhale the same proportion of the prescribed dose of nebulized fluticasone, beclomethasone dipropionate (BDP) and flunisolide.
Methods
The amount of nebulized drug that would have been inhaled by asthmatic children was captured on filters between the patient and nebulizer, and the amount contained in particles likely to reach the lung (i.e. <5 μm) is determined.
Results
The children studied would have inhaled 13% of the prescribed dose of fluticasone propionate, 21% of BDP and 25% of flunisolide. However, the percentage of the dose inhaled that was contained in particles <5 μm, and therefore more likely to reach the lungs, was only 5% of the prescribed dose of fluticasone propionate, 8% for BDP and 16% for flunisolide. The inter-subject variation coefficient of the dose inhaled was much greater for suspensions of fluticasone propionate (34%) and BDP (45%) than for suspensions of flunisolide solution (9%).
Conclusions
Our results demonstrate that the prescribed dose may bear little resemblance to the dose delivered from a nebulizer and that the dose inhaled is significantly affected by the drug formulation prescribed.
Abbreviations
-
- BDP
-
- beclomethasone dipropionate
Introduction
In spite of the fact that the use of spacer devices, with or without face masks, is recommended in order to deliver drugs to children with wheezing or asthma, nebulized corticosteroids are still widely used.1, 2 They are also widely used in adults with asthma and chronic obstructive pulmonary disease.3 The amount of drug inhaled from a nebulizer may vary greatly depending on the nebulizer chosen, patients' breathing patterns and how they use the nebulizer.4, 5 It is not commonly appreciated that the drug formulation can affect nebulizer performance.6 Generally, nebulizer systems are sold separately from solutions and suspensions containing active drugs for nebulization, and therefore these formulations are often inhaled via an available nebulizer system rather than via the nebulizer system used during the development of the medicinal product for nebulization itself. However, the differences in the amount of aerosol drug delivered by the various nebulizer systems currently available are significant.7
The physical and chemical characteristics of the preparation and its behaviour during nebulization are factors to be considered when prescribing nebulizer therapy. Not all drugs are appropriate for nebulization. Decrease in water solubility and increase in viscosity mainly produce large particles. This is a significant problem because 99% of the droplets generated are recycled back into the reservoir. Large droplets are entirely recycled, and only fine particles escape the effect of the baffle. This results in the drug failing to exit the nebulizer with the diluent rather than the drug being discharged as respirable particles.8
It has also been shown that the choice of the nebulizer may significantly affect delivery of corticosteroid.7, 9, 10 When a steroid particle from a suspension is nebulized, it is surrounded by a fluid envelope. The resulting aerosol droplet containing the drug is larger, which may reduce its ability to leave the nebulizer or to reach the lower airway. This is not the case for a corticosteroid in solution, when the amount of drug released from the nebulizer in small particles is likely to be significantly greater than that from a steroid suspension. Due to these factors, the prescribed dose may bear little resemblance to the dose of drug inhaled by the patient, and it has been argued that prescribers should have data regarding the amount of drug the patient actually inhales in addition to the prescribed dose.
The aerosolized dose of drug at the mouthpiece of a nebulizer is defined as the emitted dose and reflects the loss of drug during nebulization. This value is less than the nominal dose. This emitted aerosol can be fractionated into fine and coarse. The inhaled dose is equal to the emitted dose. Part of the inhaled dose deposits in the oropharynx and the rest is distributed in the lung on the airway surfaces. The airways filter the inhaled aerosol, depositing particles on the airway surfaces according to droplet size, airflow dynamics and airway anatomy. Inertial impaction is the most important deposition mechanism in the throat, upper and central airways.11 The quantities of particles deposited and the site of deposition determine the efficiency of the drug.
In this study, we investigate the dose that would be inhaled by children with asthma. The interaction of a child with a device potentially has a profound effect on the dose they inhale. The aim of this study was to test the hypothesis that children with asthma inhale the same proportion of the prescribed dose of fluticasone, beclomethasone dipropionate (BDP) and flunisolide from the same nebulizer.
Methods
Nebulisers and drugs
Pari LC Plus nebulizers driven by the Pari TurboBOY N compressor (Pari, Starnberg, Germany) were used for this study. This ‘breath-enhanced, open vent’ nebulizer incorporates a system of valves to increase the gas flow through the nebulizer during inspiration, increasing drug delivery to the patient. The following drug formulations were studied: fluticasone propionate suspension (Flixotide nebules 500 μg/2 mL; GlaxoSmithKline, GlaxoSmithKline S.p.A., Verona, Italy); BDP suspension (0.8 mg/2 mL nebules, Chiesi, Chiesi Farmaceutici S.p.A., Parma, Italy); and Flunisolide solution (30 mg/30 mL solution, Valeas S.p.A., Milan, Italy). The volume nebulized was standardized to a total volume fill of 3 mL as follows: 2 mL of fluticasone propionate 500 μg/2 mL + 0.9% saline to a total of 3 mL; 2 mL of BDP (800 μg) + 0.9% saline to a total of 3 mL; and 0.6 mL of Lunibron (600 μg Flunisolide) + 0.9% saline to a total of 3 mL.
Particle sizing measurements
The particle size distribution of the drug cloud leaving the nebulizer was measured using the Next Generation Pharmaceutical Impactor (Apparatus E- European Pharmacopoeia, Copley Scientific, Nottingham, UK). A vacuum pump was used to maintain a flow of air through the impactor of 15 L/min. The effective cut-off diameters of the impactor stages at 15 L/min had been previously calculated.12 These were 14.1, 8.61, 5.39, 3.30, 2.08, 1.36 and 0.98 μm for stages 1–7, respectively.
To measure particle size, the nebulizer was attached to the throat of the Next Generation Impactor at the other end. The Next Generation Impactor and the throat were pre-cooled before use to minimize evaporative losses.13 The vacuum pump and the compressor were switched on, and the drugs were nebulized for 5 min. Each stage of the Next Generation Impactor was washed with solvent (after the addition of a known amount of suitable internal standard) to ensure that as much drug as possible was recovered for mass balance determinations. Duplicate samples were taken, and the amount of drug present was determined by previously validated internal standard high-performance liquid chromatography methods.9, 10
Patients
Ten children with controlled asthma (mean age: 8.6 years; range 7–10 years) attending our centre were enrolled for the study. Ethical approval for the study was obtained from our centre's local ethical committee, and parents gave written informed consent for the study.
The children inhaled each of the drug preparations for 5 min; the filter was then changed and nebulization continued for a further 5 min (10 min total)—that is, two filters. The drug inhaled was captured by an electrostatic filter pad (Pari GmbH) inserted between the mouthpiece and the patient. This procedure guarantees inhaled drug is captured on the filter without any reaching the patient. Filters were assayed for the relevant drug.
The data from the particle sizing experiments and the amount of drug captured on the filters the children breathed through were combined to give an estimation of the amount of drug contained in various particle size fractions. This estimation is based on the assumption that particles with a mass median aerodynamic diameter of <5 μm are likely to be deposited in the lower airways, while particles <3 μm have a higher probability of penetrating deep into the peripheral airways. The amount of drug deposited on the filter after 10 min of nebulization was multiplied by the mean percentage of drug in particles <5 μm and <3 μm measured using the impactor. Time intervals of 5 and 10 min were employed in this study because we previously observed that for some steroids very little drug was emitted after the first 5 min of nebulization even though the nebulizer was still spluttering and fluid was still present in the nebulizer bowl.9, 10
Results
Amount of drug inhaled by 10 asthmatic children
The amount (mean (standard deviation)) of fluticasone propionate captured on the inspiratory filter after 5 min was 41.5 (18) μg (8.3% nominal dose), which increased significantly (P = 0.01) to 68 (9) μg (13% nominal dose) after 10 min. The variation coefficient of the inhaled dose after 10 min was 34%. The mass median aerodynamic diameter was 5.3 (0.3) μm and the amount of drug in particles <5 μm and <3 μm as a percentage of the drug contained in the aerosol produced was 39.4 (5.6)% and 13.3 (4.4)%, respectively.
The amount (mean (standard deviation)) of BDP captured on the inspiratory filter after 5 min was 83 (39) μg (10.5% nominal dose), which increased significantly (P = 0.01) to 171 (77) μg (21% nominal dose) after 10 min. The variation coefficient of the inhaled dose after 10 min was 45%. The mass median aerodynamic diameter was 5.48 (0.32) μm, and the amount of drug in particles <5 μm and <3 μm as a percentage of the drug contained in the aerosol produced was 36.5 (3.9)% and 11.0 (2.1)%, respectively.
The amount (mean (standard deviation)) of flunisolide captured on the inspiratory filter after 5 min was 114 (24) μg (20% nominal dose), which increased significantly (P = 0.01) to 147 (15) μg (25% nominal dose) after 10 min. The variation coefficient of the inhaled dose after 10 min was 9%. The mass median aerodynamic diameter was 3.36 (0.35) μm, and the amount of drug in particles <5 μm and <3 μm as a percentage of the drug contained in the aerosol produced was 67.8 (5.0)% and 42.9 (5.1)%, respectively.
Discussion
Aerosol deposition in the lung is affected by several factors, including the aerosol-generating system, particle size distribution of the inhaled aerosol, inhalation pattern (e.g. flow rate, volume, breath-holding time), oral or nasal inhalation, properties of the inhaled carrier gas, airflow obstruction, and the type and severity of lung disease. The distribution of target sites and local pharmacokinetics of the drug also affect clinical response.14
Inhaled corticosteroids play a crucial role in the treatment of children with asthma.15 Knowledge of the dose they inhale is crucial for prescribers to determine if lack of clinical effect or the development of side effects are due in part to the method of drug delivery affecting the dose inhaled.16, 17 Our study confirms that the prescribed dose bears little resemblance to the proportion of drug children actually inhale and that this is largely dependent on the formulation of the drug. Following nebulization, the children studied would have inhaled 13% of the prescribed dose of fluticasone propionate, 21% of BDP and 25% of flunisolide. However, the percentage of the dose inhaled that was less than 5 μm, and therefore more likely to reach the lungs, was only 5% of the prescribed dose of fluticasone propionate and 8% for BDP. As predicted, the amount of flunisolide inhaled in particles less than 5 μm was significantly greater at 16%. These differences were magnified further when the amount of drug contained in particles less than 3 μm was compared.
Unexpectedly, the variation in the amount of drug inhaled was much greater between children inhaling the suspensions BDP and fluticasone propionate than for flunisolide (Fig. 1). This has not previously been investigated and suggests that drug dispersion in the nebulizer mixture, drug behaviour during nebulization or the child's breathing pattern through the Pari nebulizer may have a significant effect on the amount of drug delivered from the suspensions and that this is not predictable.
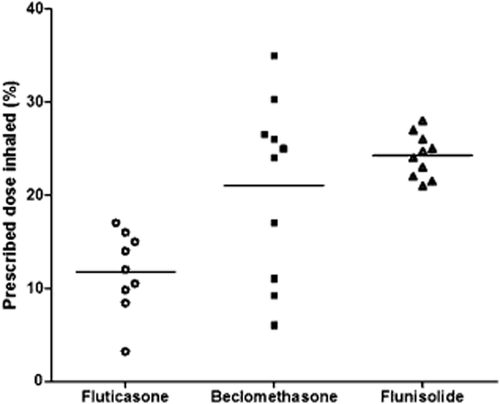
The proportion of the prescribed dose inhaled by children with asthma. Each data point represents the proportion of the prescribed corticosteroid inhaled by a child. Note the wide variation in the dose inhaled by different children for the steroid suspensions beclomethasone dipropionate, and to a lesser extent fluticasone propionate, compared with the steroid solution flunisolide.
These differences may be explained by the different formulations of corticosteroids studied. Corticosteroids available for nebulization may be either in solution, such as flunisolide, or in suspension, such as fluticasone propionate and BDP. This is because of the different water solubility of these drugs. Höegger and Rohdewald rated water solubility of corticosteroids at 37°C as 140 μg/mL for flunisolide, 0.14 μg/mL for fluticasone and 0.13 μg/mL for beclomethasone.18 Most drugs are usually nebulized with the active drug as a solute in water, forming a continuous phase. Suspensions that form two phases are difficult to nebulize. When a particle of drug from a suspension, such as fluticasone propionate and BDP, is nebulized, it is surrounded by an envelope of fluid. The resulting aerosol droplet containing the drug is therefore larger, which may reduce its ability to leave the nebulizer or to reach the lower airway. This is not the case for a corticosteroid in solution, when the drug released from the nebulizer in small particles is likely to be significantly greater than that from a steroid suspension. It is of interest that a now discontinued preparation of BDP resulted in very little drug exiting the nebulizer in particles small enough to reach the lower airways,19 and that this was associated with a poor clinical effect.20, 21 The preparation of the formulation of BDP used in our study was much more concentrated to help ensure adequate drug delivery.
Other factors, such as the choice of nebulizer, may have a profound effect on the dose of nebulized corticosteroids a patient may inhale. For example, in vitro studies have shown that the dose of budesonide that a 10-year-old is likely to inhale from breath-enhanced, open-vent nebulizers is approximately four times greater than that from an open-vent nebulizer and twice greater than that from a conventional nebulizer.22
In summary, our results confirm the need for prescribers, researchers and regulators to have information on both the prescribed dose and the dose patients are likely to inhale from their nebulizer to allow informed interpretation of clinical effect and side effects. However, this may be difficult to predict for the suspension formulations of BDP and fluticasone propionate because of the very wide variation in the proportion of drug inhaled from the nebulizer. Without information on a drug mass median aerodynamic diameter and the percentage of respirable particles released, the inhaled dose remains unknown and the results of clinical trials may be uninformative.
Acknowledgement
This work was supported by a grant from VALEAS Industria Chimica e Farmaceutica, Milan, Italy (Dr M Biraghi).




