Effects of European green crabs (Carcinus maenas) on transplanted Eelgrass (Zostera marina): potential protective measures
Author contributions: KEB, TP, SBG, FE, CAJ-M designed the experiment; KEB provided essential infrastructure; TP, SBG, FE, CAJ-M performed the experiments (supervised and guided by KEB); KEB, TP, SBG, FE, CAJ-M, CVC processed and analyzed the data; TP, SBG, FE, CAJ-M wrote the manuscript with editorial help from KEB, CVC. TP, SBG, FE, CAJ-M share first authorship.
[Correction added on 30 April 2025, after first online publication: M expanded to Mussel banks and Eelgrass changed to eelgrass in the Abstract section.]
Abstract
The global decline of seagrass causes concern regarding the loss of important ecosystem services, such as increased marine biodiversity. Consequently, restoration projects have gained popularity among scientists and local communities. However, for restoration to be successful, certain challenges must be considered and mitigated. One of the known threats against transplanted eelgrass (Zostera marina) is the European green crab (Carcinus maenas), due to grazing and uprooting of transplanted shoots and seeds. While several studies have shown the damaging effects of green crabs in areas where it is invasive, less is known about their impact on eelgrass within its native range in Europe. Here, we investigated four potential protective measures for eelgrass transplants against the European green crab: cages, biodegradable establishment structure elements (BESE elements), stone anchors, and mussel banks. The efficiency of each protective measure was observed over 11 days in indoor aquariums, in which meristem cuts and shoot uprooting were measured variables. The most efficient protective measure was the cage treatment, with an eelgrass survival of 90% after 11 days, compared to the unprotected control treatment which showed a survival of only 37%. Mussel banks and BESE elements led to 60% and 57% survival after 11 days, respectively. Additionally, an investigation into the feeding behavior of the green crab revealed that seeds were the preferred part of the eelgrass plant, with 48% being consumed after 6 days. While our laboratory experiments were small in scale, results highlight the potential for protective measures to mitigate disturbances from biological entities in eelgrass restoration, thereby enhancing the success rate of eelgrass transplantation attempts.
Implications for Practice
- Site selection for eelgrass restoration should consider green crab densities and population dynamics to enhance the survival and growth of eelgrass transplants.
- We suggest implementing targeted protective measures when restoring eelgrass meadows, such as cages, biodegradable establishment structure elements, and mussel banks, in combinations that prevent both uprooting and predation of transplanted shoots.
- Restoration efforts should prioritize protecting transplanted shoots from uprooting and grazing by green crabs to improve transplant resilience and support long-term restoration success.
Introduction
Seagrasses are marine flowering plants, typically found in the soft or sandy bottoms of estuaries, as well as along the coastal margins of tropical, temperate, and subarctic marine waters (McKenzie et al. 2020). Seagrasses possess distinct characteristics compared to other aquatic flowering plants. They flourish in saline environments and typically grow entirely submerged, relying on water currents for pollination and dispersal of their seeds after fertilization (Short & Wyllie-Echeverria 1996).
Seagrass meadows play a crucial role in coastal areas by providing several important ecosystem services. They are particularly efficient in carbon sequestration, with carbon burial rates that make up a significant portion of the total carbon burial in the ocean, and the annual carbon buildup in these meadows is larger than in most terrestrial ecosystems (Duarte et al. 2005; Kennedy et al. 2010). Worldwide, seagrass meadows function as a habitat for a diverse group of marine species, including invertebrates, fish, and other benthic fauna (Baden & Pihl 1984; Orth et al. 2006; Olesen et al. 2024). Over 40 different fish species use seagrass meadows as fish nurseries or as feeding grounds (Pihl et al. 2006; Jayathilake & Costello 2018), and studies indicate that juvenile fish living in seagrass meadows have higher growth and survival rates compared to fish living in alternative habitats (Pihl et al. 2006). Additionally, by creating a vegetation over the soft sand bottoms, seagrass meadows stabilize sediments and reduce water movement (Pihl et al. 2006; Ondiviela et al. 2014). By reducing wave energy and slowing near-bottom currents, they alter the hydrodynamic environment, facilitating sediment deposition within and around the meadow (Short et al. 2007; Hansen & Reidenbach 2012; Ondiviela et al. 2014).
Despite their importance, seagrass meadows are deteriorating globally due to direct and indirect human activities, including pollution and global warming (Borum et al. 2004; Erftemeijer & Lewis III 2006; Palacios & Zimmerman 2007). Several factors contribute to the decline of seagrass meadows, such as coastal constructions, fisheries (mainly bottom trawling), clam digging, boating activities, dredging, and sand mining (Borum et al. 2004; Erftemeijer & Lewis III 2006; Brodersen et al. 2017). However, increased nutrient content primarily derived from agricultural practices poses the biggest threat to seagrass meadows both regionally and internationally (Borum et al. 2004; Waycott et al. 2009; Brodersen & Kühl 2022). Within the last century, nutrient loading has caused a decline of seagrass depths, infiltration, and area distribution of 40–50% (Borum et al. 2004). In Europe, the species that has experienced the highest proportion of declines among seagrass species is eelgrass (Zostera marina), mainly due to coastal eutrophication (Olesen & Sand-Jensen 1994; de Los Santos et al. 2019). The nutrient loading can be traced from urban sewage and agricultural run-off in all marine waters in the Baltic Sea along the coast of Denmark, Sweden, Southern Norway, and Northern Germany (Borum et al. 2004).
To mitigate the loss of natural eelgrass meadows, restoration efforts in northern temperate areas have been attempted (Orth et al. 2020; Lange et al. 2022; Steinfurth et al. 2022). Transplantation and seed broadcasting have proven to be two successful methods contributing to the restoration of ecosystem services, expansion of eelgrass habitats, restoration of fauna communities, and sequestering and retention of nutrients such as carbon, nitrogen, and phosphorus (Davis & Short 1997; Orth et al. 1999; Zhou et al. 2014). However, for seed broadcasting to be successful, it requires factors such as low hydrodynamic stress and low bioturbation activity. In areas where these conditions are not met, restoration efforts have instead shifted toward the transplantation of eelgrass shoots (Kuusemäe et al. 2018; Lange et al. 2022).
Eelgrass transplantation is a technique where shoots are harvested from donor meadows, whereafter they are transplanted to suitable restoration sites (Lange et al. 2022). Eelgrass transplants are often placed in the sediment with an anchoring technique to avoid uprooting and thus increased mortality (Lee & Park 2008). Transplantation without anchoring can be successful, but newly transplanted eelgrasses could potentially be lost within days due to rough weather and hydrodynamic events (Orth et al. 1999; Zhou et al. 2014). Various anchoring techniques have been applied. In a previous study, eelgrass was tied to stones, which were then buried in the sediment (Zhou et al. 2014). This technique was successful, and transplants eventually resembled natural eelgrass beds after becoming established. In Horsens Fjord (Jutland, Denmark) two other anchoring techniques were used (Lange et al. 2022): (a) shoots of eelgrass were bound to nails with wire and buried in sediment and (b) shoots were placed between bent bamboo sticks and buried in sediment. Another recent transplantation method utilizes artificial biodegradable establishment structure elements (BESE) (Temmink et al. 2020; Gagnon et al. 2021). BESE elements mimic the stabilizing effect of eelgrass roots and rhizomes, thereby enhancing sediment stability, which further contributes to eelgrass transplant survival (Christianen et al. 2013; Temmink et al. 2020; Gagnon et al. 2021). Although shoot transplantation has displayed successful outcomes, newly transplanted shoots are unfortunately threatened by biotic and abiotic stress factors like bioturbation, grazing, drifting macroalgae, and hydrodynamics, thereby hindering eelgrass reestablishment (van Katwijk & Hermus 2000; Valdemarsen et al. 2010; Paulo et al. 2019).
One such threat is the European green crab (Carcinus maenas, Linnaeus, 1758). This crab, native to Europe and Northern Africa, is widespread along coastal regions. However, it has gained notoriety as one of the most destructive invasive species globally (Klassen & Locke 2007). The detrimental effect of green crabs on eelgrass meadows has been observed to be mediated by at least two mechanisms: uprooting and grazing, depending on the size/age of the crab. Malyshev and Quij'on (2011) observed in a laboratory experiment the impact of juvenile and adult green crabs on transplanted eelgrass shoots, in which the adult crabs uprooted 10 times more eelgrass shoots than the juvenile crabs after a 6-day period. The grazing effect of the juvenile crabs was much higher than their uprooting effect on the eelgrass shoots. This study clearly showed the different impacts the size/age of the green crabs have on eelgrass, making it an important consideration in site selection. Moreover, a study by Howard et al. (2019) conducted in eelgrass meadows found that male adult green crabs consumed eelgrass rhizomes, which were visually identified in the stomachs of the crabs. The study also showed that removal of aboveground eelgrass biomass occurred more frequently than removal of whole plants.
Although some studies have assessed the negative impacts of green crabs on eelgrass in regions where the crab is invasive, there is less understanding of the issue within its native range in Europe, where they are highly locally abundant. A factor contributing to the high number of green crabs in Danish waters is the decline of its main predator, the Atlantic Cod (Gadus morhua), which has suffered from overfishing throughout many years (Lange et al. 2022; Olesen et al. 2024) and consequently has led to a population boost in the European green crab. Eelgrass restoration studies in Sweden have shown that green crab predation of planted eelgrass seeds is a major source of seed loss, presenting a challenge for restoration efforts (Infantes et al. 2016a, 2016b) and highlighting the need for further studies on the effects of green crabs on eelgrass restoration within its native area.
To address these challenges posed by green crabs, protective measures could be implemented, potentially increasing transplant survival rates. Implementing protective methods such as cage enclosures, net enclosures, bait traps, and artificial biodegradable membranes or structures has proven successful in seagrass transplantation efforts by providing protection from benthic fauna such as lugworms (Arenicola marina) and abiotic factors (Sousa et al. 2017; Temmink et al. 2020; Lange et al. 2022). However, their effectiveness in mitigating the negative impacts of the European green crab has not been thoroughly investigated.
In this study, we investigated how green crab density affects transplanted eelgrass and examined the effect of feeding versus starvation using aquarium experiments. Additionally, we evaluated four different potential protective measures against the European green crab: cages, BESE elements, stone anchors, and mussel banks. Lastly, the feeding behavior of the green crab on eelgrass plant parts was assessed and quantified by a feeding experiment.
Methods
Gathering of Materials and Eelgrass Sampling
Sediment and eelgrass (Zostera marina L.) were sampled at Julebæk Beach (56°03′29.0″N 12°34′38.5″E), Helsingør, Zealand, Denmark on 16 March 2023, for the density and controlled feeding experiment. Plants were harvested from a coastal area with shallow waters, at a depth of approximately 1 m below sea level. For the protective measures experiment, sediment and eelgrass samples were collected at Julebæk Beach on 22 March 2024, and at Amager Beach (55°39′44.4″N 12°38′07.0″E), Zealand, Denmark on 4 April 2024. Eelgrass shoots for the green crab feeding experiment were sampled at Julebæk Beach on 2 May 2024. After collection, eelgrass samples were washed and cleaned of debris and placed in containers with seawater (salinity ≈21) from the sampling site. Sediment was washed in a sieve (mesh size: 1 mm) to remove larger infauna and stored in plastic containers and buckets. Stones used for the protective measures experiment (see below) were collected at Julebæk Beach (Table S1).
Green crabs (Carcinus maenas), used in the density and controlled feeding experiment, were obtained from the National Aquarium of Denmark in Kastrup. All crabs were male, with an average shell size of 5.27 ± 0.18 cm. Green crabs used for the protective measure experiment (mean width ≈5.4 cm; Table S2) were collected from Øresund near the seagrass sampling site in Helsingør. Cages used in the experiment were homemade using wire fencing, while BESE elements, composed of biodegradable potato waste, were purchased from BESE products (BESE, Culemborg, The Netherlands; https://www.bese-products.com) and live Blue mussels (Mytilus edulis) (Table S3) were purchased from a local fish market.
Density and Controlled Feeding Experiment
Aquarium tanks (20 cm × 38 cm) with an area of 0.076 m2 were used to test the effect of green crabs on transplanted eelgrass shoot survival. Fifteen liters of water (salinity ≈20) were added to each tank by mixing 9 L of filter-sterilized seawater (salinity: 33‰) with 6 L of demineralized (DI) water. During the experiment, the salinity was continuously measured and evaporated water was replenished with demineralized water to restore volume and salinity. Room temperature was kept at 15°C, and tank temperatures ranged from 13.5 to 14.3°C. Oxygen levels in the aquariums were kept constant using an air stone connected to a pump. Sand was added and distributed evenly in all tanks to a depth of 1.5 cm. Aquarium tanks were illuminated by a halogen lamp with a light intensity of approximately 125 μmol photons m−2 s−1. The lights were controlled by a timer, mimicking daylight and nighttime in a 14 hours:10 hours light/dark cycle. Photon irradiances were measured using a calibrated irradiance meter (ULM-500, Walz GmbH, Germany) fitted with a submersible spherical micro quantum sensor (US-SQS/L, Walz GmbH, Germany).
Five different aquarium treatments were set up, all containing five shoots of eelgrass (n = 3, total of 15 aquariums), with varying crab densities (i.e. 13 and 26 crabs/m2) and feeding: (1) treatment with one fed crab (C1 + F), (2) treatment with one unfed crab (C1 ÷ F), (3) treatment with two fed crabs (C2 + F), (4) treatment with two unfed crabs (C2 ÷ F), and (5) control treatment (Fig. 1A). The density of 13 crabs/m2 was selected based on a study by Davis et al. (1998), while the other density was chosen to assess potential behavioral effects of keeping two crabs together in the tanks. Since our results did not indicate such behavioral effects, we chose to present the results as the impact of higher density rather than behavioral interactions. Crabs were fed with small dead smelt and Blue mussels. The experiment lasted for 6 days. The effects of green crabs on eelgrass were quantified by counting uprooted shoots and cut leaves. Transplanted eelgrass shoots that were uprooted were counted as not surviving. Measurements were taken after days 1, 3, and 6, whereafter mean values and standard deviations were calculated (mean ± SD, n = 3). Statistical analyses were conducted in R-studio (R version 4.3.1). Bartlett's test was used to assess homogeneity of variance, and when heteroskedasticity was detected, an Aligned Rank Transformation (ART) Analysis of Variance (ANOVA) was performed, followed by a post hoc Dunn's test. ART ANOVA is a nonparametric alternative to a two-way ANOVA that allows for analysis of interactions and main effects when normality and homoscedasticity assumptions are violated (Leys & Schumann 2010; Wobbrock et al. 2011; Durner 2019). It works by first aligning the data to remove effects unrelated to the factor being tested, then ranking the aligned values, and finally applying a traditional ANOVA to the ranked data (Wobbrock et al. 2011). When both normality and homoscedasticity are violated, ART ANOVA proves much more powerful than the traditional ANOVA (Leys & Schumann 2010). The significance level was set at α = 0.05. Survival rates are presented as percentages for clarity.
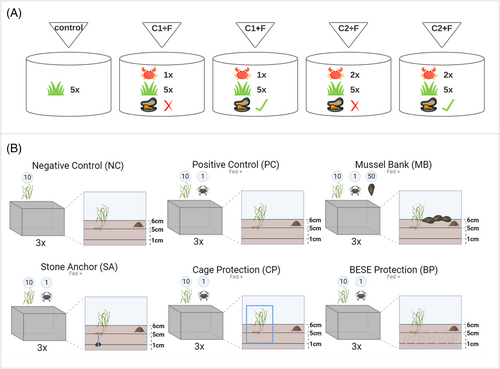
Protective Measures Experiment
Plastic containers (46 cm × 32 cm × 25 cm) were used as indoor aquariums to quantify the effect of adult male green crabs on transplanted eelgrass shoots and to evaluate potential protective measures. The experiment consisted of six different treatments, each with three replicates (Fig. 1B; Fig. S1; n = 3, total of 18 containers). In each aquarium, sediment was layered to a depth of ≈6 cm, and 10 eelgrass shoots were randomly transplanted into the sediment at a depth of 2 cm. Saltwater (salinity ≈22) was added to the aquariums to a height of 15 cm above the sediment surface, and an air stone connected to a pump was provided to ensure sufficient water aeration and movement during the experiment. Water evaporation was minimized by covering the aquariums with lids, and demineralized water was added to restore both volume and salinity when necessary. Temperature and light exposure and intensity conditions were similar to those described in the density and controlled feeding experiment (Tables S4 & S5). Stones used in the stone anchor (SA) treatment were selected based on similar size and weight (Table S1).
In each treatment (except for the negative control), one green crab was introduced (density: 7 crabs/m2). Crabs were initially fed a shrimp at the start of the experiment, with subsequent feeding every 48 hours thereafter.
- MB: 50 mussels were introduced into each aquarium (Table S3) in a clumped distribution.
- SA: Eelgrass shoots were fastened to stones using cotton string, and the stones were buried 4 cm below the shoots (Table S1; Fig. S2b).
- Cage protection (CP): A cage of specified dimensions (average size: 15.33 cm × 30.17 cm × 18.17 cm) was positioned over the eelgrass shoots and buried 4 cm beneath the shoots. The cages were buried to avoid crabs digging underneath and tipping them over, as this was observed during preliminary trials. The mesh size of the cages was 3 × 4.5 cm (Fig. S2a).
- BESE protection (BP): Two BESE element sheets (area: 0.104 m2) were stacked, resulting in a 4 cm high three-dimensional matrix and buried at a depth of 2 cm below the sediment surface. Eelgrass shoots were secured to the upper element sheet using cotton strings.
The experiment lasted 11 days, during which three quantitative measurements were taken in between for each aquarium: (1) number of uprooted shoots, to assess green crab bioturbating effects, (2) count of meristem cuts (rhizome and roots still in sediment without any leaves remaining), to evaluate the crabs grazing effect, and (3) eelgrass shoot survival. Statistical analyses were performed in R-studio (R version 4.3.1). Homogeneity of variance was tested using Bartlett's test, and upon detection of heteroskedasticity, ART ANOVA was applied followed by post hoc Dunn's test. Alpha was set to 0.05. Due to the mortality of crabs in one of the MB treatments, data from this aquarium were excluded from further analysis.
Feeding Behavior Experiment
A secondary experiment was conducted to assess and quantify which part of the eelgrass plant was preferred as a food source (Fig. 2). Plastic containers (20 cm × 38 cm × 25 cm) were used as indoor aquariums. A total of five aquariums (n = 5) were used, each containing one green crab (density: 13 crabs/m2). Five samples of different eelgrass plant parts were introduced to each aquarium: leaves (2–2.5 cm), meristems (1.5–2 cm), rhizomes (1.5–2 cm), roots (2.5–3 cm), and seeds (n = 5; Fig. 2). Five liters of saltwater (salinity ≈20) were added to each aquarium and provided with an air stone connected to an air pump. The water level in the tanks was kept at a height at which the crabs were able to reach the plant parts that floated (water depth ≈5 cm). Green crabs from the protective measures experiment were reused in this experiment and were therefore subjected to 48-hour starvation before the start of the feeding experiment. The experiment lasted 6 days, and the green crabs were not given additional feed during the experiment. Consumption of eelgrass plant parts was recorded throughout the experiment. Data were tested for homogeneity of variance using Bartlett's test, and upon detection of heteroskedasticity, ART ANOVA was applied followed by post hoc Dunn's test. Alpha was set to 0.05. Results are represented as the summed values shown in percentage.
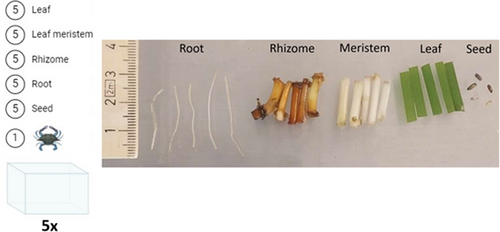
Results
Density and Controlled Feeding
Following the introduction of green crabs to the indoor aquariums, eelgrass leaf cuts and shoot survival were assessed over 6 days (Fig. 3). No damaged eelgrass shoots were observed in the control treatment, confirming that all observed effects resulted from crab activity. The highest level of crab damage was recorded in the aquariums with two crabs (26 crabs/m2), where the survival rate of the transplanted eelgrass shoots dropped to 0% after 3 days (Fig. 3). Both unfed (C2 ÷ F) and fed (C2 + F) crabs caused an average daily loss of 33.33% of transplanted eelgrass shoots. Additionally, these high-density aquariums exhibited the most leaf cuts, with fed crabs causing an average of 10 ± 4 cuts after 6 days, while unfed crabs caused 8 ± 2 cuts (Table S6; Fig. 3).
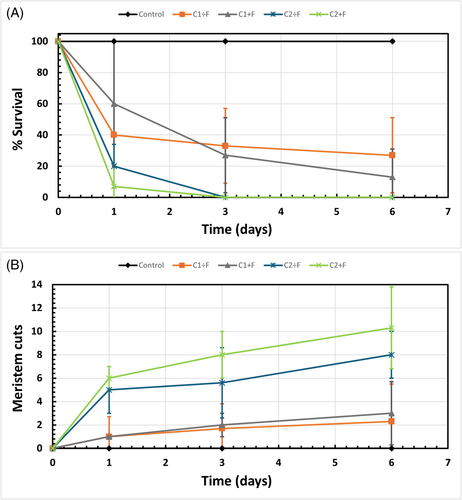
Aquariums containing one crab (13 crabs/m2) exhibited average eelgrass shoot survival rates above 10 % after 6 days (Fig. 3). Unfed (C1 ÷ F) and fed (C1 + F) crabs caused an average daily loss of 12.17 ± 5.17 and 14.5 ± 3.83% transplanted eelgrass shoots, respectively. Fed and unfed crabs caused similar amounts of leaf cut damage, with an average of 3 ± 3 and 2 ± 3, respectively (Table S6; Fig. 3). ART ANOVA results showed significant differences in leaf cut frequency among treatments (F[3,32] = 20.98, p < 0.001) and across time (F[3,32] = 24.55, p < 0.001), with a significant interaction effect (F[9,32] = 2.33, p = 0.0375) (Table S7). Similarly, eelgrass uprooting varied significantly across treatments (F[3,32] = 8.87, p < 0.001) and over time (F[3,32] = 23.93, p < 0.001), though no significant interaction effect was detected (F[9,32] = 1.28, p = 0.285) (Table S7). Post hoc tests showed that high-density aquariums resulted in significantly more uprooting and leaf cuts than low-density aquariums (p < 0.05); however, there was no significant difference in damage between fed and unfed treatments (Table S8).
Protective Measures
In all treatments, loss of transplanted eelgrass shoots was determined, except for the negative control in which no loss was observed (Fig. 4). The greatest loss was observed in the positive control treatment with an average daily loss of 5.76 ± 1.39% transplanted eelgrass shoots. Among the protective measures, the cage treatment was the most effective, with an average daily loss of 0.91 ± 0.91% transplanted eelgrass (Fig. 4), representing an 84% decrease in daily loss rate. The BESE, stone anchor, and mussel bank treatments resulted in average daily losses of 3.94 ± 0.53, 4.55 ± 2.73, and 3.64 ± 1.29%, respectively (Fig. 4), corresponding to a decrease in daily loss rates of 32, 21, and 37%. ART ANOVA showed that eelgrass shoot survival was significantly affected by treatment (F[3,72] = 23.91, p < 0.001), time (F[8,72] = 20.88, p < 0.001), and their interaction (F[24,72] = 3.29, p < 0.001) (Table S9). Post hoc Dunn's tests showed that survival was significantly higher in the BESE (p = 0.0326) and cage (p = 0.0010) treatments compared to the control, whereas the stone anchor treatment (p = 0.4533) did not differ significantly (Table S10).
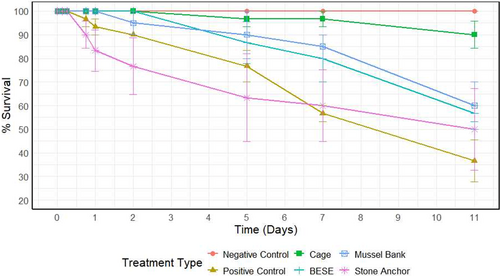
Uprooting was the primary cause of shoot loss in the positive control, with 36.7% of transplanted shoots uprooted after 11 days (Fig. 5A) and uprooting was observed after only 18 hours (0.75 days). In contrast, no uprooting was observed in the cage and BESE treatments (Fig. 5A). The stone anchor treatment expressed the second highest number of uprooting, with 13.3% being uprooted after 11 days (Fig. 5A) while the mussel bank treatment had one uprooted transplanted shoot, but only two replicates were analyzed for this treatment. Therefore, only 5% uprooting was observed after 11 days (Fig. 5A). Eelgrass shoot uprooting was significantly affected by treatment (F[3,72] = 40.95, p < 0.001), time (F[8,72] = 9.60, p < 0.001), and their interaction (F[24,72] = 5.50, p < 0.001) (Table S9). Post hoc comparisons showed that uprooting was significantly lower in the BESE (p < 0.001), cage (p < 0.001), and stone anchor (p = 0.029) treatments compared to the control (Table S10).
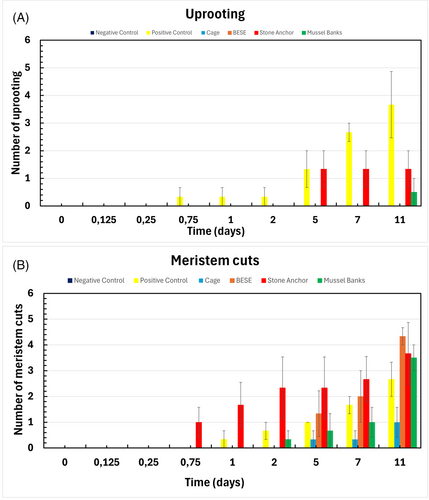
Meristem cuts were observed in all treatments except the negative control (Fig. 5B). The BESE treatment expressed the highest number of meristem cuts with 43.3% out of 30 transplants after 11 days, followed by the stone anchor (36.7%), mussel bank (35%), and positive control treatment (26.7%) (Fig. 5B). The cage treatment showed the lowest occurrence of meristem cuts, with 10% after 11 days (Fig. 5B). For meristem cuts, ART ANOVA results showed significant effects of treatment (F[3,72] = 12.26, p < 0.001), time (F[8,72] = 20.39, p < 0.001), and their interaction (F[24,72] = 2.32, p = 0.0032) (Table S9). However, post hoc tests revealed that only the cage treatment significantly decreased meristem damage (p = 0.0207), whereas BESE (p = 0.2726) and stone anchor (p = 0.1046) treatments did not differ from the positive control (Table S10).
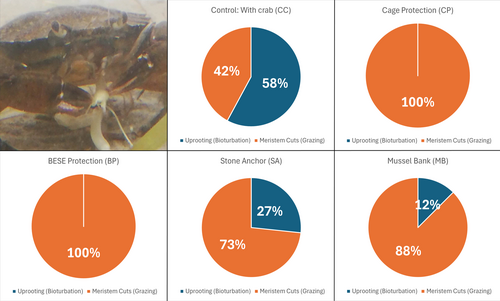
Figure 6 illustrates the causes of shoot transplant loss across the treatments after 11 days. Uprooting was the primary cause of loss in the positive control (58%), indicating that bioturbation by green crabs had a greater impact than grazing when no protective measures were applied (Fig. 6). Conversely, meristem cuts were the dominant cause of shoot transplant loss in all protective treatments, with cage and BESE treatments showing no uprooting at all. In the stone anchor and mussel treatments, meristem cuts remained the primary cause of transplanted shoot loss; however, 27 and 12% of losses were due to uprooting, respectively (Fig. 6).
Feeding Behavior Experiment
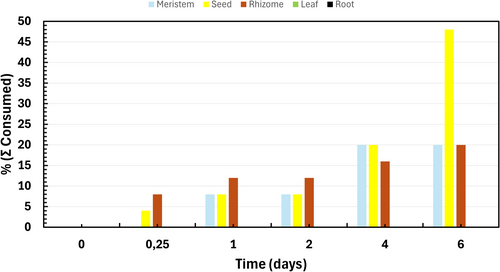
Feeding activity by the green crabs was first observed after 6 hours (Fig. 7). Rhizomes and seeds were the first plant parts to be consumed, whereas meristems were consumed starting after 1 day (Fig. 7). The most consumed plant part was seeds, with 48% consumed after 6 days (Fig. 7). Seeds were consumed in all five aquariums. Around 20% of both rhizomes and meristems were consumed by the green crabs after 6 days (Fig. 7). However, rhizomes and meristems were only consumed by one green crab from one of the five aquariums. Results from the ART ANOVA showed a significant effect of plant part on consumption rates (F[4,140] = 15.21, p < 0.001), while time alone had no significant effect (F[6,140] = 1.50, p = 0.181). However, there was a significant interaction between plant parts and time (F[24,140] = 2.77, p < 0.001) (Table S11). Post hoc Dunn's tests revealed that seeds were significantly more consumed than all other plant parts, with significant differences compared to meristems (p = 0.0076), rhizomes (p = 0.0210), leaves (p < 0.001), and roots (p < 0.001). Rhizomes were consumed significantly more than leaves (p = 0.0258) and roots (p = 0.0258), but not meristems (p = 0.3464). No significant differences were found between meristem and leaf consumption (p = 0.0605) or between root and leaf consumption (p = 0.5) (Table S12).
Discussion
Our findings offer compelling evidence that the presence of green crabs has a detrimental impact on the survival rate of transplanted eelgrass, with higher crab densities exacerbating these effects.
The cage treatment was the most effective method for protecting eelgrass from green crab bioturbation and grazing. Both this method and the use of BESE elements completely prevented uprooting of transplanted eelgrass shoots. Green crabs preferentially consumed eelgrass seeds over rhizome and meristem tissues, while completely avoiding leaves and roots.
Effect of Green Crabs on Transplanted Eelgrass
In our aquarium experiments, the presence of adult male green crabs at a density of 7 crabs/m2 resulted in the average survival of 37% of transplanted eelgrass shoots after 11 days. Similar results have been observed by Davis et al. (1998), in which a treatment with the same density of 7 crabs/m2 damaged 26% of transplanted eelgrass shoots after 7 days. Another in situ study (Howard et al. 2019) observed that eelgrass bed enclosures with a density of 5.6 crabs/m2 resulted in a 73–81% greater loss of eelgrass shoots compared to the control treatment after 4 weeks. These findings indicate that green crabs pose a critical threat to eelgrass restoration efforts.
Understanding whether green crabs directly damage eelgrass through grazing or indirectly through bioturbation is crucial for developing effective strategies to mitigate the damage they inflict upon eelgrass transplants. In this study, we observed that 58% of the loss of transplanted eelgrass shoots in the positive control treatment was due to bioturbation, with 42% resulting from grazing. However, since the crabs were confined to small containers which might affect their behavior (e.g. increased digging and burrowing), the results should be viewed with some caution. Previous studies indicate that adult male green crabs uproot 10 times more eelgrass shoots than juvenile male green crabs (Malyshev & Quij'on 2011). Conversely, eelgrass biomass loss caused by juvenile green crabs was primarily attributed to grazing rather than uprooting. Thus, selecting a protective method against either uprooting or grazing should be based on the size and age of the green crabs.
Green crab densities vary widely across different regions, with reports ranging from 0.0001 to 12.4 crabs/m2 in various marine environments (Menge 1983; Davis et al. 1998; Young et al. 1999). Our results indicate that higher crab densities lead to an increased damage to transplanted eelgrass shoots. Notably, by doubling the density of green crabs, the average daily loss of transplanted eelgrass shoots is increased in similar proportions. Consequently, restoration projects conducted in areas with high green crab densities may be less effective and would potentially benefit from the implementation of protective measures. Given that several factors, including crab density, size, and sex, have an influence on the damage to transplanted eelgrass, accounting for both crab density and green crab population dynamics would improve site selection for restoration projects. The results of our experiment must be taken with some caution as several studies have demonstrated that the confinement of predators such as the green crab may alter its behavior (Hulberg & Oliver 1980; Hall et al. 1990). However, our results are consistent with previous literature (Davis et al. 1998; Howard et al. 2019), which highlights the profound damaging effects of green crabs on seagrass transplants.
Effect of Investigated Protective Measures
The cage treatment was the most effective protective measure in terms of protecting transplanted shoots against uprooting and meristem cuts from green crabs, with a 90% survival of transplanted shoots after 11 days. A restoration study conducted in New Hampshire, United States, showed a similar trend with decreased disruption of transplanted shoots by green crabs using fences equipped with crab traps as a protection strategy against bioturbating organisms (Davis & Short 1997). The fences also protected against other benthic fauna such as horseshoe crabs, indicating that this method is effective in preventing transplanted eelgrass from being uprooted by bioturbation. However, in our experiment, meristem cuts were detected, and a green crab was observed entering one of the cages through the holes of the mesh. Hence, modifications to mesh size may further improve protection by completely excluding smaller crabs. Moreover, upscaling this method for large-scale restoration projects might present some challenges and extra costs. Secure anchoring is needed to keep the cages in place during rough weather, and regular cleaning of the cages is required to prevent fouling, as heavy fouling would reduce seagrass light availability.
The BESE treatment demonstrated protection against green crabs with a survival of 57% after 11 days, completely preventing uprooting of transplanted eelgrass shoots. Previous studies have investigated the effect of BESE elements on sediment stabilization and their protection against hydrodynamics (Temmink et al. 2020; Gagnon et al. 2021). The BESE elements reduced sediment movement, which may help enhance the survival of transplanted shoots (Moksnes et al. 2018). Along with our result on uprooting, this suggests that BESE elements can effectively protect against bioturbation and preserve sediment stability for the transplanted shoots. Similar to other protective structures, such as cages or fences, BESE elements can prevent crabs from digging under and into the transplanted eelgrass shoots (Davis et al. 1998; Schotanus et al. 2020; Gagnon et al. 2021). While BESE elements improved survival rates and provided protection against bioturbation, they were less effective against grazing, which remained a significant cause of meristem damage. Previous studies indicate that shoot cuts on transplanted eelgrass result in poor survival rates (Davis et al. 1998), suggesting that BESE elements alone cannot fully protect transplanted shoots against green crabs. Therefore, combining this method with additional protective measures might prove advantageous in future restoration efforts.
The mussel bank treatment expressed low levels of uprooted eelgrass shoots with 5% uprooting after 11 days, while 23% of the shoots were lost to meristem cuts. The average transplanted shoot survival was 60% after 11 days. Former studies indicate that green crabs predate on Blue mussels and soft-shell clams (Elner 1981; Wong 2013; Christie et al. 2020). In our study, the presence of mussel banks may have led to reduced eelgrass grazing by green crabs, potentially securing a higher relative survival of transplanted eelgrass shoots as the crabs might have focused their predation on mussels instead. Furthermore, less sediment disruption was observed in the mussel bank aquariums, suggesting lower bioturbating behavior. A recent study conducted in Horsens Fjord (Jutland, Denmark) showed that bait traps with mussels effectively protected transplanted eelgrass along with net enclosures (Lange et al. 2022), demonstrating the potential of mussels as a protection measure against green crabs. However, extrapolating their results to our findings should be done with caution, as we did not directly investigate feeding activity on the mussel banks in our study.
Green Crab Feeding Behavior
The aim of the feeding experiment was to assess whether green crabs favored certain parts of the eelgrass plant. Results showed that eelgrass seeds were the most consumed part, with 48% consumed after 6 days. The preference for seeds may be due to their higher nutritional value, as a previous study found mature seeds to contain 51% starch (Irving et al. 1988). Other crab species such as Ovalipes ocellatus and Pagurus longicarpus have also been observed to graze on eelgrass seeds (Wigand & Coolidge Churchill 1988). Furthermore, studies in Sweden indicate that green crab predation of planted eelgrass seeds is a significant source of seed loss, with field cage experiments showing that one enclosed green crab caused an average seed loss of 73% over a 1-week period (Infantes et al. 2016b). This, coupled with the results of our study, suggests that green crab predation on eelgrass seeds could potentially lower the success rate of seed-based restoration efforts. Moreover, the consumption of seeds by the green crabs could lower eelgrass sexual reproduction, potentially leading to decreased genetic diversity and diminished resilience to environmental disturbance of the eelgrass meadows. A recent study conducted in the United Kingdom found that planting seeds in hessian bags helped protect them from predation by green crabs (Unsworth et al. 2024). While this technique was not investigated in our study, their findings suggest its potential to improve seed-based restoration success by reducing seed predation and physical disturbances, and future research may benefit from investigating this approach further.
In our study, 20% of rhizomes and meristems were consumed after 6 days. Eelgrass rhizomes have previously been found in the stomachs of green crabs (Howard et al. 2019), and ingestion of meristem tissue has also been observed by Malyshev and Quij'on (2011), as well as in the density and controlled feeding experiment of this study. Given that crustaceans possess the necessary enzymes to digest plants such as eelgrass (Linton & Greenaway 2007), it is plausible that eelgrass can be part of the diet of green crabs. Leaves and roots were not consumed in our experiment, indicating a clear avoidance of these plant parts, consistent with previous findings where grazing was observed on meristems but not on leaves (Malyshev & Quij'on 2011). This avoidance could be due to the higher fiber content in leaves and roots, making them less favorable as they are harder to digest.
To conclude, our findings show that green crabs can significantly decrease the survival rate of transplanted eelgrass shoots by directly damaging the base of the shoots and, most detrimentally, uprooting the eelgrass transplants. Green crab densities and population dynamics should therefore be considered when selecting sites for future eelgrass restoration projects.
The feeding behavior of green crabs showed that they exhibit a preference for eelgrass seeds, followed by rhizome and meristem tissue, with no consumption of leaves and roots. Using protective measures to prevent the crabs from feeding on eelgrass seeds could help mitigate negative impacts that this might have, such as reduced eelgrass sexual reproduction which may lead to decreased genetic diversity.
Protective measures such as cages, BESE elements, stone anchors, and mussel banks demonstrated an increase in the survival of transplanted eelgrass shoots subjected to high green crab densities, with cages and BESE elements completely preventing eelgrass uprooting. By implementing a combination of different protective measures in future eelgrass restoration projects, such as cages or mussel banks alongside BESE elements, the transplanted shoots might experience less grazing and uprooting, thus increasing the potential for growth, expansion, and survival. While our study presents a laboratory-based assessment of different protective measures against green crabs, testing this in situ in future field studies will be crucial to further assess their effectiveness and feasibility for future restoration projects. In particular, given the promising results of BESE elements, we recommend future in situ studies to further evaluate their effectiveness in alleviating bioturbation stress on transplanted eelgrass.
Acknowledgments
This research project was funded by a grant from Danmarks Frie Forskningsfond (Independent Research Fund Denmark, KEB: 3164-00153B). We thank The Øresund Aquarium (Øresundsakvariet, Denmark) for providing the green crabs for the protective measures and feeding experiment. The data that support the findings of this study are available from the corresponding author upon reasonable request.




