Soil amendments impact root-associated fungal communities of balsam poplar on a phosphogypsum reclamation site
Author contributions: MAN developed the experimental design; KBB did all the laboratory work; MR established the research sites, collected the samples, provided data on the site and treatments; KBB, AD analyzed data; MAN conceptualized and supervised all the work; KBB, AD, MAN wrote, reviewed and edited the manuscript.
Abstract
During reclamation organisms like fungi directly interact at the soil–plant interface and can potentially impact plant growth, health, and overall revegetation success. Therefore, a study was conducted to determine how topsoil amendments (mycorrhizae, biochar, compost, manure) influence root-associated fungal communities and growth of fast growing Populus balsamifera trees on a phosphogypsum (PG) reclamation site in Alberta, Canada. Tree height, diameter, biomass, and diversity of Ascomycota and Basidiomycota were assessed using Illumina MiSeq sequencing targeting the fungal internal transcribed spacer region 1 (ITS1) and ITS2. Topsoil amendments and root location (topsoil vs. PG within a treatment), greatly affected the root fungal community. Basidiomycota preferentially grew in PG, and had a high treatment specific root fungal community with highest diversity of ectomycorrhizal fungi and basidiomycete yeast in biochar PG and compost PG. Populus balsamifera roots were associated with a high diversity of Ascomycota, found equally in roots in topsoil and PG, with a trend towards higher diversity and abundance in PG. The core genus found across all samples was Fusarium, a potential plant pathogen. All topsoil amendments strongly reduced abundance of Fusarium and other Ascomycota in poplar roots. Biochar and compost reduced number and abundance of potential fungal pathogens and increased number of potential plant growth promoting Basidiomycota, indicating favorable growth conditions for P. balsamifera on a phosphygypsum reclamation site by application of these amendments. This study indicates compost and biochar can be effective soil amendments to support P. balsamifera establishment and growth on a PG reclamation site.
Implications for Practice
- Layering of reclamation soil may increase niche space for different fungi and enhance fungal diversity that could be favorable for Populus balsamifera.
- Phosphogypsum (PG) provided favorable conditions for Basidiomycota including important ectomycorrhizal fungi and plant growth promoting yeasts.
- Amendments can facilitate fast growing woody P. balsamifera growth on a PG reclamation site.
- Use of compost and biochar as amendments can reduce potential fungal pathogens and enhance Basidiomycota which may be supportive for P. balsamifera on a PG reclamation site.
Introduction
Soil and root fungi play key roles in ecosystem functioning, with importance in pedogenesis, decomposition, nutrient cycling and modification of plant growth and disease suppression (Bardgett & van der Putten 2014; Taylor et al. 2016). Root-associated fungal communities provide critical links between above and below ground processes for plants in terrestrial habitats (Bonito et al. 2014; Dhar et al. 2018), and ecosystem stability and maintenance rely on biodiversity and functional dynamics of these organisms (Danielsen et al. 2012; Boldt-Burisch & Naeth 2019). However, we only know details of the roles in plant growth and ecosystem stability of a portion of the great diversity of root-associated fungi (Joubert & Doty 2018).
Fungal diversity is reflected in the high number of taxa, morphologies, habitats and life strategies (Badotti et al. 2017). Ascomycota is the largest and most diverse phylum of extant fungi which encompasses more than 33,000 named species and a vast number of undescribed fungi, Basidiomycota is the second largest phylum with more than 31,000 known living species, (Watkinson et al. 2016; Badotti et al. 2017). Both phyla comprise a large variety of species associated with roots. Root microbiomes are highly diverse and can include species engaged in mutualistic symbiosis, saprophytic antagonists causing plants diseases and/or fungi that can be either, depending on environmental conditions (Taylor et al. 2016; Velez et al. 2017). Many of the Basidiomycota are ectomycorrhizal, and are invaluable to most trees and forest ecosystems (Janowski et al. 2019) to promote nutrient and water uptake by host plants, facilitate host plant growth and resistance to stresses and diseases, enhance soil organic matter decay via enzymatic and non-enzymatic degradation and improve availability of soil phosphorus (Liu et al. 2020). Establishment of many pioneer plants in wastelands is facilitated by ectomycorrhizal fungi (Liu et al. 2020), which may play an important role in land reclamation.
Molecular methods, such as high throughput metabarcoding, are powerful tools to study fungal communities. We used the Illumina sequencing (MiSeq) method, which is known to achieve deeper sequencing than 454 metabarcoding approaches, due to utilization of shorter reads for sequencing (Schmidt et al. 2013). Several comparative studies have shown the taxonomic resolution of internal transcribed spacer region 1 (ITS1) and ITS2 can significantly differ for Basiodiomycota and Ascomycota and in relation to taxonomic level (Wang et al. 2015). Since fungal groups can be over- or under-represented (Blaalid et al. 2013), we used a sequencing approach targeting ITS1 and ITS2 regions to identify root-associated Ascomycota and Basiodiomycota of Populus balsamifera L. (balsam poplar) on a phosphogypsum (PG) reclamation site in Alberta, Canada.
PG (CaSO4·2H2O) is a gypsum based by-product of phosphate fertilizer production (Macías et al. 2017; Bituh et al. 2021) and composed mainly of solid gypsum, phosphoric acid, trace elements, and small amounts of radionuclides (Plyatsuk et al. 2019; Haneklaus et al. 2022; Turner et al. 2022). For every tonne of phosphate (P2O5) produced as phosphoric acid, 4–6 tonnes dry mass of PG are generated, with an expected global total of 258 × 106 tonnes in 2018 (Saadaoui et al. 2017). Over half of this PG is deposited in large stockpiles (Rutherford et al. 1994), often spanning hundreds of hectares in area which can potentially pose environmental hazards, including ground water contamination with fluoride, trace elements, acidity, or radionuclides, radon gas, gamma radiation, and atmospheric contamination by fluoride (Saadaoui et al. 2017; Chernysh et al. 2021; Haneklaus et al. 2022). PG stack reclamation focuses on limiting exposure of sulfur dioxide (SO2), sulfur trioxide (SO3), trace elements, and radionuclides to the surrounding environment by capping with soil (hereafter topsoil) and usually vegetating with grass. A recent study (Robinson et al. 2022) on whether rapidly growing woody vegetation could establish on PG stacks was conducted using P. balsamifera, a tree species occurring naturally in northern North America. There are many studies on Populus tremuloides (trembling aspen), Populus deltoides (eastern poplar), Populus trichocarpa (black poplar), and other species and hybrids involving fungal root microbiome (Karlinski et al. 2010; Bonito et al. 2016; Velez et al. 2017). Yet to our knowledge, there is no study on the root-associated fungal microbiome of P. balsamifera.
Populus balsamifera is a pioneer tree species in Alberta, Canada that establishes well after disturbance at sites with favorable water and nutrients. Growing short rotational forestry systems (bioenergy plantations) on PG reclamation sites could provide environmental and economic benefits relative to traditional grass cover (Robinson et al. 2022). PG stack reclamation requires large volumes of topsoil, which are often unavailable. A minimum 8–15 cm topsoil cover is necessary for successful revegetation and hydrology on PG stacks (Jackson et al. 2011; Turner et al. 2022). Soil building on PG using a minimum of topsoil with amendments of different origins may be an important alternative when topsoil availability is limited. Many industries produce waste materials that are high in organic matter and can contain plant nutrients, such as animal manure, municipal waste compost, humic acids, humates, and humate-based products such as biochar. Application of mycorrhizal inoculants is an alternative to organic amendments that is gaining attraction in sustainable improvement of disturbed soils (Bitterlich et al. 2020).
When new reclamation strategies are tested, organisms such as fungi that directly interact at the soil–plant interface are of interest due to their potential impact on plant growth and health, and overall revegetation success. Root-associated fungal diversity, quantity and spatial distribution can be highly influenced by soil characteristics such as organic and mineral soil layers, and some types of organic matter can significantly reduce pathogenic fungi in soil (Sun et al. 2016). We hypothesize that the root-associated fungal community of P. balsamifera is affected by soil treatments, which ultimately influence tree growth. We predicted that application of amendments that are rich in organic material (manure, compost, biochar) would significantly decrease potential fungal pathogens of balsam poplar roots and this reduction would improve tree growth on the PG reclamation site. Our objectives were to determine whether (1) soil amendments influenced the P. balsamifera root-associated fungal community, (2) roots harbored a core group of fungi, and (3) roots in PG and topsoil had different fungal compositions. Results of this study will contribute to a better understanding of how soil amendments impact root-associated fungal communities of balsam poplar in a reclamation soil, which will help to improve PG reclamation practices to promote beneficial fungal communities and/or to reduce pathogenic fungal communities to promote overall plant health.
Methods
Site Description
The research site was at Nutrien Nitrogen Operations (53.73′N, 113.19′W) in the Parkland Natural Region of Alberta, Canada. Mean annual precipitation is 459 mm, with 353 mm as rain and 104 mm as snow (Government of Canada 2022). Mean annual temperature is 2.4°C; highest 17.1°C in July and lowest −10.4°C in December.
This research was conducted on a PG stack produced in the 1970s and PG was derived from Florida phosphate rock. The PG was gray colored, of silt loam texture (silt 55.4%, sand 36.5%, clay 8.1%). During decommissioning, 15–20 cm of sandy loam topsoil (sand 80%, silt 9.6%, clay 10.4%) from an old pasture site 3 km away was used for capping PG in 2015. The PG stack was seeded in fall 2015 with a reclamation grass seed mix to prevent soil erosion in spring. Detailed chemical properties of PG and topsoil with treatments are presented in Table S1.
Experimental Design and Treatments
The experimental design was a randomized complete block with four soil amendment treatments (compost, manure pellets, biochar [minimum organic matter 80%] and mycorrhizal fungi) and a control (unamended topsoil). Mycorrhizal fungi (2015 Canadian version) contained only arbuscular mycorrhizal fungal spores of Rhizophagus irregularis in a perlite-peat mix. MYKE Tree and Shrub was used for mycorrhizal fungi and acquired from MYKE Natural Powerful, Canada (MYKE 2016). Amendments were selected for their nutrient amending properties, availability and relatively low cost. They needed to be easy to apply in small or large amounts in various scale scenarios.
Replicates were arranged in a complete randomized block design as 10 blocks, each with 5 plots (2.5 × 6.25 m) assigned to 5 soil amendment treatments, with 2.5 m unplanted buffers between them. This resulted in 10 plots for each treatment, and 50 plots in total; each plot had 5 P. balsamifera trees (clone Okanese). Soil amendments of compost (20 tonnes/ha), manure (20 tonnes/ha) and biochar (0.67 tonnes/ha) were hand spread and raked to ensure homogeneous distribution over the plot, then incorporated into the top 15 cm of soil using a rear tine rototiller. Mycorrhiza (125 mL/plant) were placed in each planting hole during tree planting. Populus balsamifera cuttings (25 cm long, with approximately 1.0 cm diameter with 5–8 buds per cutting) were soaked in water 12 hours prior to planting, then planted in rows, spaced 1.25 m apart in a grid. When planted, all cuttings appeared to be of the same health status with no obvious visible differences.
Soil Analyses
In June 2016, soil was sampled from nine plots per treatment at the center of the plots at 0–10 cm depth. Three samples were randomly composited into one so that 3 samples per treatment were analyzed (3 samples × 5 treatments = 15 samples). Samples were analyzed at a commercial laboratory for inorganic and organic carbon and total nitrogen by Leco combustion (Nelson & Sommers 1996); ammonium and nitrate by extractable 2 N KCl (McKeague 1981); available potassium and phosphorus by modified Kelowna (Ashworth & Mrazek 1995); pH and electrical conductivity by saturated paste (Carter & Gregorich 2008).
Three randomly selected samples from the control topsoil and from underlying PG were analyzed at Brandenburg University of Technology, Cottbus, Germany. Total sulfur was determined via infrared detection (DIN ISO 10694, 1994). For total phosphorus, potassium, calcium, magnesium, and sodium 200 mg of PG or soil powder were treated with 1 mL nitric acid (HNO3, 65%; Loftfield) prior to digesting in a pressure vessel. Samples were oven dried at 160°C for 7 to 9 h at 12 bars pressure for complete digestion, then filtered through 0.2-μm filters (Schleicher and Schuell, Dassel, Germany, filter 512, phosphorus free) into a flask and filled to 25 mL with ultra-pure water. In this extract, element concentrations were determined by inductively coupled plasma spectrometry (iCAP 6000 series, Thermo Scientific, Karlsruhe, Germany).
Tree Parameters Assessment
Height, stump diameter, and total aboveground biomass were measured in September 2017 for each planted P. balsamifera trees. End of growing season height of each tree was measured from the base of the trunk to the end of the tallest branch, and stem diameter was measured using a tree caliper at the ground surface. Aboveground biomass was clipped at the soil surface and bagged, then dried at 80°C for 48 hours to constant weight. Health of each individual tree was visually assessed during tree parameters measurement.
Root Harvesting and Preparation
Tree roots were sampled for microbiome analyses in September 2017 with a shovel. Out of 10 blocks, only 6 were randomly selected for root sampling; uneven numbers of samples (4 = control topsoil, 3 = control PG, 3 = mycorrhiza topsoil, 3 = mycorrhiza PG, 3 = manure topsoil, 1 = manure PG, 3 = compost topsoil, 3 = compost PG, 3 = biochar topsoil, 3 = biochar PG) were taken from the treatments as there was only sufficient funding to analyze 30 samples (6 block × 5 plot). We planned for the same number of samples in topsoil and PG for each treatment; however, in some sample locations there were no roots in the PG, thus we took more samples on some treatments. For topsoil and PG, we sampled the cuttings that grew deeper roots in PG and depending on the depth we separated topsoil and PG root samples. From each sampled tree, roots from topsoil (0–15 cm) and subsoil PG (>15 cm; clearly separable colors and textures) were cut and stored separately in plastic bags in a cooler. Roots (diameter 0.5–1.5 mm) were washed under running tap water, then in sterile water; then surface dried on paper towel, and ground in liquid nitrogen in a mortar. Samples were frozen at −20°C before further processing.
DNA Extraction and Sequencing
Hundred milligrams of root powder were suspended in 400 μL SFG1 buffer of the E.Z.N.A. SP Fungal DNA Mini Kit (Omega Bio-Tek, Norcross, GA, U.S.A.). Genomic DNA was extracted according to the manufacturer's instruction and eluted in 100 μL elution buffer. Samples were adjusted to a final DNA concentration of 50 ng/μL before amplification. High-throughput sequencing of the ITS region was performed by Eurofins Genomics (Ebersberg, Germany) on an Illumina MiSeq platform. Amplicons were generated using a two-step polymerase chain reaction (PCR) protocol for ITS1 and ITS2 regions using template specific primers (ITS1 Fwd GGAAGTAAAAGTCGTAACAAGG, Rev. GCTGCGTTCTTCATCGATGC [ITS5/ITS2]; ITS2 Fwd GCATCGATGAAGAACGCAGC, Rev. TCCTCCGCTTATTGATATGC [ITS3/ITS4]; White et al. 1990), containing a universal overhang. Amplicons were cleaned and set up for the index PCR with specific primers directed to universal overhangs. Final amplicon libraries were cleaned, quantified and pooled equimolarly. The resulting library pool was quantified and sequenced using v3 chemistry (2 × 300 bp paired end reads).
Sequencing reads were processed according to primer sequences with in house scripts. Only read pairs with expected forward and reverse primers were kept for further analysis. No mismatches were allowed for identification of primer sequences. Paired end reads were merged using FLASH (2.2.00, Magoč & Salzberg, 2011), which considers all possible overlaps at or above a minimum length between reads in a pair and chooses the overlap resulting in lowest proportion of mismatched bases in the overlapped region. FLASH computes a consensus sequence in the overlapped region by selecting the base with higher quality value at each overlapped position. If both bases had an identical quality value, one was selected randomly. Pairs were merged with a minimum overlap size of 10 bp to reduce false positive merges. The %Q30 (% bases with Phred quality score 30 or higher) represents an inferred base call accuracy of 99.9% and was on average 73% for ITS1 and 77% for ITS2. Most Illumina runs should generate 70–80% of Q30 data or higher to be indicative of a successful sequencing run.
Bioinformatics and Statistical Analyses
Eurofins Genomics performed the microbiome analysis. All reads with ambiguous bases (“N”) were removed; chimeric reads were identified and removed based on the de-novo algorithm UCHIME (Edgar et al. 2011) as implemented in the VSEARCH package (Rognes et al. 2016). The remaining set of high quality reads was processed using minimum entropy decomposition (MED; Eren et al. 2015), which provides a computationally efficient means to partition marker gene datasets into operational taxonomic units (OTUs), each representing a distinct cluster with significant sequence divergence to any other cluster.
By employing Shannon entropy, MED uses only information rich nucleotide positions across reads and iteratively partitions large datasets while omitting stochastic variation. MED outperforms classical, identity-based clustering algorithms. Sequences can be partitioned based on relevant single nucleotide differences without being susceptible to random sequencing errors. This allows decomposition of sequence data sets with a single nucleotide resolution. MED identifies and filters random noise in the dataset, sequences with a very low abundance (≤0.02% of average sample size).
Assignment of taxonomic information to each OTU, DC-MEGABLAST was done through alignments of cluster representative sequences to the sequence database. A specific taxonomic assignment for each OTU was then transferred from the set of best matching reference sequences (lowest common taxonomic unit of all best hits); sequence identity of 70% across at least 80% of the representative sequence was a minimal requirement for considering reference sequences. Further processing of OTUs (abundance rarefication, richness) and taxonomic assignments was performed using QIIME software package (version 1.9.1, http://qiime.org/; Caporaso et al. 2010). Abundances of fungal taxonomic units were normalized to 100% using lineage specific copy numbers of relevant marker genes to improve estimates (Angly et al. 2014). NCBI_nt (release 7 July 2018) was the reference database. With a total of 4,295,313 (ITS1) and 5,577,113 (ITS2) raw reads, and after lineage specific copy number correction 3,986,357 (ITS1) and 4,860,221 (ITS2), reads were obtained at a depth of 0–15 cm. Reads per sample were generally similar (Figs. S1 & S2). Median sequence length after processing was 280 ± 15 bp (ITS1) and 350 ± 18 bp (ITS2). Calculations for rarefaction curves were performed in Excel.
Soil data, number of fungal orders and genera, relative abundances of the fungal genus Fusarium (most abundant species), and tree growth parameters were tested for normality of distribution (Shapiro–Wilk). With normality and equal variance, one-way analysis of variance (ANOVA) followed by pairwise multiple comparison procedures (Bonferroni t-test) were performed. Without normality, a Kruskal–Wallis one-way ANOVA on ranks and all pairwise multiple comparison procedures (Student–Newman–Keuls method) was performed. Analyses were carried out with Sigma Plot 12.2 (Jandel Scientific, San Raphael, CA, U.S.A.). Multivariate Statistical Package (MVSP 3.1, Kovach Computing Services, Pentraeth, Isle of Anglesey, United Kingdom) was used for indirect detrended correspondence analyses (DCA) of root-associated Ascomycota and Basidiomycota species. DCA incorporates an ecologically realistic measure of distance between samples and is typically better at extracting the primary gradient (Clapham 2011), and was performed with relative abundances (means of replicates per treatment). For DCA ordinations data were log(e) transformed and rare species were downweighted. Joint plots with variables (fungal genera of Ascomycota and Basidiomycota) and cases (treatments) were created. Further nonparametric permutational ANOVA was performed with permutational multivariate analysis of variance (PerMANOVA) where amendments were used as fixed factors and individual sample plot used as a random factor. Pairwise multiple comparison (if significant) and PerMANOVA analyses were conducted using the package vegan 2.5–2 (Oksanen et al. 2017). Affinity of species to particular treatment units was evaluated using indicator species analysis with the function multipatt of the package indicspecies version 1.7.9 (De Cáceres 2013). The R statistics system version 4.0.3 was used for PerMANOVA and indicator species analyses (R Core Team 2020).
Results
Root-Associated Ascomycota Classes with ITS1 and ITS2 Sequencing
Root-associated Ascomycota were found in topsoil and associated PG in all treatments (ITS1, ITS2). Every treatment, except PG control and PG manure (ITS1), contained Ascomycota that could not be resolved to class level (Fig. 1). Eight root-associated fungal classes were identified, with highest number in the control (ITS1, ITS2). Sordariomycetes, Leotiomycetes, and Dothiodeomycetes were dominant in most samples, with mainly higher relative abundances of Leotimycetes in PG and of Sordariomyctes in topsoil.
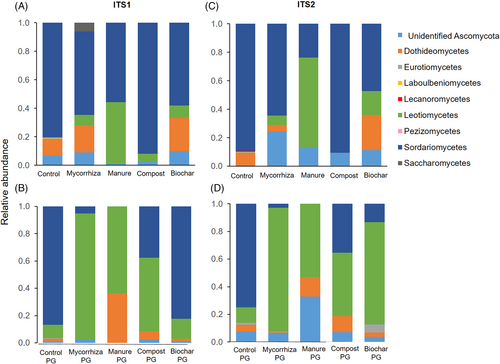
Laboulbeniomycetes and Lecanoromycetes were only found in low abundance in roots in the control (Fig. 1). Pezizomycetes were not found in topsoil but in control PG (ITS1, ITS2) and biochar PG (ITS2). With ITS2 sequencing a higher number of sequences could be assigned to fungal orders and species than with ITS1 sequencing. Thus, for Ascomycota in the following steps we focus on ITS2 sequencing results.
Effect of Amendments on Root-Associated Ascomycota
ITS2 sequencing resulted in 16 fungal orders and a large proportion of fungi assigned only to a higher taxonomical level (Fig. 2). Treatment and whether roots were in topsoil or PG influenced composition and abundance of root-associated fungal orders. In topsoil, there were more fungal orders in the control (6.3 ± 3.8, mean ± standard deviation) than in other treatments: mycorrhiza (3.5 ± 2.9), biochar (3.0 ± 1.0), manure (0.7 ± 0.6), compost (0.7 ± 0.6). There was a trend toward higher diversity of fungal orders in PG than topsoil with 8.3 ± 3.5 orders for control PG topsoil, 4.0 ± 1.7 for mycorrhiza PG, 6.0 ± 2.6 for biochar PG, 3.5 ± 1.0 for compost PG and 1 for manure PG.
Hypocreales was the core fungal order in all topsoil treatments and in PG, except in manure PG (might be related to lack of replicates; Fig. 2). Relative abundance of Hypocreales was significantly higher in control topsoil than in all other treatments except mycorrhiza topsoil (Fig. S3). When considering number of reads as an estimation of Hypocreales significance, the pattern was similar except control PG and biochar PG did not significantly differ from control topsoil (Fig. S3). There were no statistically significant differences in abundance of Capnodiales, Helotiales, Pleosporales and Sordariales.
Some fungal orders were limited to specific treatments. Glomerellales, Eurotiales, and Pyxidiophorales that were only in control topsoil. Others were only found in PG of different treatments such as Chaetothyriales, Magnaporthales, Onygenales, Thelebolales, and Pezizales (Fig. 2). Control PG had higher relative abundance of Sordariales than all other treatments. Control, mycorrhiza and especially compost topsoil increased abundance of Microascales, found in PG only and rarely in control PG. In control topsoil and PG relative abundance of unidentified Ascomycota and those that could not be resolved to order level were low relative to other treatments. Relative abundance of unidentified Ascomycota, especially that of unidentified Sordariales, was significantly higher (p < 0.016) in control PG than in topsoil.
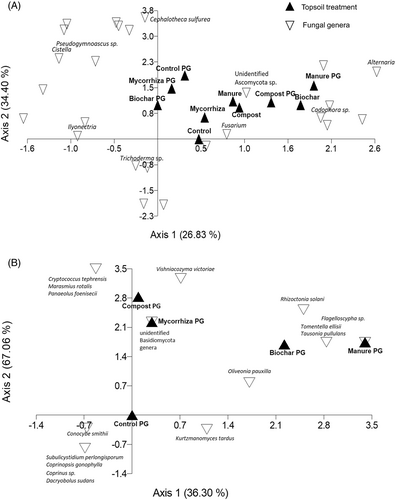
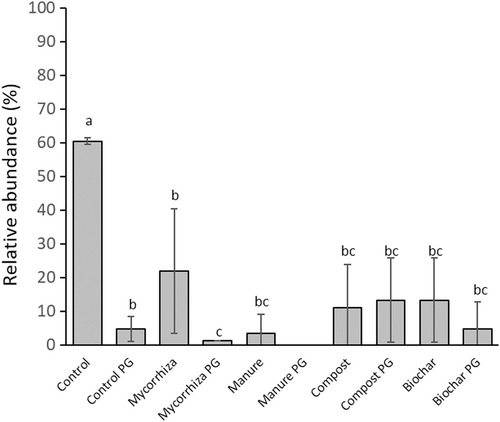
PerMANOVA analysis showed a significant soil amendment treatment (p < 0.001) effect on species composition and further pairwise comparison revealed a significant difference between control versus mycorrhiza (p = 0.032), and close to significant between control versus compost PG (p = 0.061), control PG versus mycorrhiza (p = 0.064) and mycorrhiza versus mycorrhiza PG (p = 0.053). Indicator species analysis showed species Fusariella sinensis and genera Pleurophragmium were significantly associated with control and control PG soil amendment treatments. Further DCA analyses (Fig. 3A) portrayed distribution of root-associated Ascomycota genera between topsoil and associated PG, where control PG, biochar PG, and mycorrhiza PG formed a cluster separated from other treatments, suggesting similar fungal communities. Many genera were strongly associated with PG. DCA illustration indicates that within topsoil there was an effect of treatments on root-associated fungal communities relative to the control along Axes 1 and 2. Four of 10 treatments (control topsoil, control PG, compost PG, biochar PG) harbored treatment specific root-associated Ascomycota genera or species (Table S2); however, most genera were found in more than two treatments. Overall, the most abundant detected genus was Fusarium, found in 9 of 10 treatments (Table S2; Fig. 4). The main trend was that number of reads assigned to Fusarium sp. was higher in control topsoil and PG than in all other treatments, with significantly higher numbers of reads in control topsoil than in compost topsoil and manure topsoil, and in control PG versus biochar PG (Fig. 4).
Since many fungi could not be assigned to species level, we used genera richness to compare treatments (Table 1). Genera richness was significantly higher between control topsoil and control PG; and between manure topsoil and control PG and compost topsoil; and was higher in PG than associated topsoil (excluding manure PG) in all other treatments.
| Treatments | ITS1 | ITS2 |
|---|---|---|
| Control topsoil | 9 (3) ab | 9 (4) ab |
| Control PG | 15 (6) a | 18 (9) a |
| Mycorrhiza topsoil | 4 (4) ab | 5 (4) ab |
| Mycorrhiza PG | 4 (3) ab | 6 (3) ab |
| Manure topsoil | 1 (1) b | 1 (1) b |
| Manure PG | 1 | 2 |
| Compost topsoil | 1 (1) b | 1 (1) b |
| Compost PG | 5 (3) ab | 5 (2) ab |
| Biochar topsoil | 1 (2) ab | 4 (4) ab |
| Biochar PG | 8 (8) ab | 13 (7) ab |
Root-Associated Basidiomycota Classes with ITS1 and ITS2 Sequencing
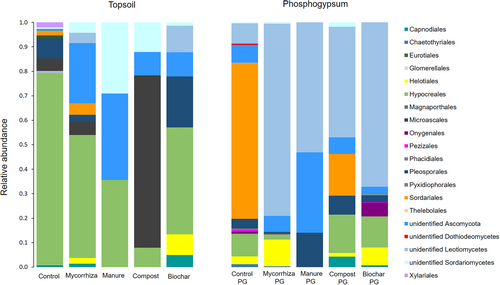
Root-associated Basidiomycota were predominantly found in roots in PG (ITS1, ITS2). In topsoil they were only in 2 of 17 samples (mycorrhiza topsoil). Thus, data analysis for Basidiomycota focused on PG. Three classes of Basidiomycota, Agaricomycetes, Agaricostilbomycetes, and Tremellomycetes were identified with ITS1 and ITS2 sequencing (Fig. 5A & 5B), with better resolution of fungal classes (more detected) in control and biochar PG with ITS1 sequencing. Thus, further analysis for Basidiomycota focused on ITS1 sequencing results. Agaricomycetes, Agaricostilbomycetes, and Tremellomycetes were detected in compost and biochar PG, while in control PG there were no Tremellomycetes. In manure PG only Agariomycetes was detected, while in mycorrhiza PG fungal OTUs could not be resolved to class level (Fig. 5A & 5B).
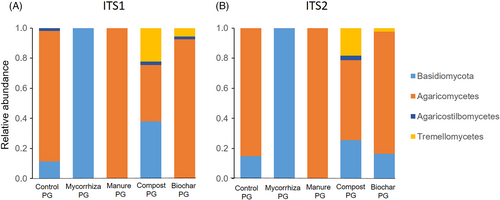
Effect of Amendments on Root-Associated Basidiomycota
Eleven fungal orders of Basidiomycota were detected with ITS1 (Fig. 6) and 8 with ITS2 sequencing (data not shown). In mycorrhiza PG, fungi could not be classified to order. Topsoil treatments had an influence on relative abundance and number of root-associated fungal orders in PG (Fig. 6). The highest number of Basidiomycota orders was in compost and biochar PG (4.3 ± 3.5, 4.0 ± 3.5), followed by the control (2.7 ± 2.5), and mycorrhiza and compost PG (0.7 ± 1.2) and one fungal order. No fungal order was found in all treatments; Agaricales were most abundant, in 4 of 5 treatments (Fig. 6).
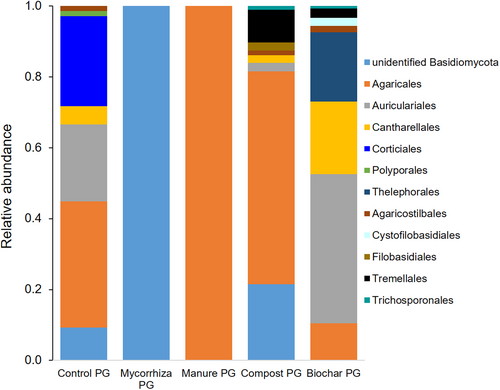
PerMANOVA analysis did not show significant soil amendment effects on root-associated Basidiomycota species composition; however, indicator species analysis showed only Flagelloscypha sp. was significantly associated with manure PG amendment treatment. DCA analyses shows the pattern of root-associated fungi in relation to soil treatments based on relative abundance. Many detected fungi were found only in one sample per treatment and thus no statistical validation was possible. No fungal genus and/or species occurred in all treatments. Most Basidiomycota species occurred in biochar PG and control PG (Table S3), with 5 species specific to control PG (Table S3; Fig. 3B). Tomentella ellisii was specific for biochar PG and Marasmius rotalis for compost PG (Table S3). Oliveonia pauxialla was found in roots in control, compost and biochar PG treatments but not in the other two treatments. All treatments except manure PG harbored unknown Basidiomycota species (Table S3). Four root-associated Basidiomycete yeasts (or yeast like) species were identified. Cryptococcus tephrensis, Filobasidium oeirense, and Vishniacozyma victoriae were detected in compost PG, Tausonia pullans was only found in biochar PG together with Filobasidium oeirense and Vishniacozyma victoriae.
Samples of control topsoil and PG were more diverse, with number of fungal OTUs increasing more than in other samples with increasing number of sequences (Fig. S4). Samples of other treatments were less diverse, as number of fungal OTUs did not increase much regardless of number of sequences. For some samples the curves did not reach their asymptote, which means complete fungal diversity might not be fully assessed, likely since not all OTUs were covered with the sampling. This is the case for some samples of biochar, mycorrhiza PG and topsoil and manure PG.
Effect of Amendments on Tree Growth
All soil treatments tended to positively influence tree height, stump diameter and total above ground biomass relative to the untreated control. No visible health issues were identified. Application of mycorrhiza and biochar had the most positive effect, but due to low number of replicates, there is no statistical significance (Table S4).
Discussion
Populus balsamifera roots in our study were associated with diverse Ascomycota, consistent with results of Bonito et al. (2016) who found a high diversity of endophytic fungi in roots of Populus deltoides and Populus trichocarpa. In our study, equal presence of Ascomycota in roots in topsoil and PG trending to higher diversity and abundance in PG suggest favorable soil conditions for certain fungi. Soil amendments influenced root-associated fungi in topsoil and associated PG, with a reduction of fungal richness and relative abundance after treatment with manure, compost, and mycorrhiza. The higher number of treatment specific Ascomycota species in control and control PG, most likely resulted from lack of soil organic amendments or input of exogenous species via commercial inocula, (Sun et al. 2016; Martignoni et al. 2020). Topsoils with manure and compost had higher potassium and phosphorus concentrations than other treatments, which is beneficial for tree growth and may decrease potential pathogenic root fungi. Shakya et al. (2013) who investigated fungal and bacterial community structure of Populus deltoides found that soil properties had a large impact on fungal community structure. Sun et al. (2016) found that the addition of organic matter (straw, manure) decreased relative abundance of potential pathogenic fungi in soil. Zaccardelli et al. (2013) found that the use of compost inhibits growth of Rhizoctonia solani, Fusarium sp., and other plant pathogens due to groups of microorganisms in compost that produce metabolites with specific suppressive activity against soil-borne pathogens.
Most root-associated Ascomycota in our study are considered omnipresent in root-and soil-associated fungal communities, and under certain abiotic and biotic conditions are potential plant pathogens, such as Fusarium, Ilyonectria, and Alternaria. Although no visible health issues were found in P. balsamifera, some Fusarium species can lead to dieback of poplar (Boyer 2011; Bao et al. 2020; Su et al. 2021), Cryptosphaeria multicontinentalis, an exemplary fungus detected only in control topsoil, is a known pathogen for canker and dieback on poplar (Moyo et al. 2017). Although Fusarium was found in all samples, its greatest relative abundance in control topsoil and control PG suggests soil amendments (manure, compost, biochar, mycorrhiza) greatly reduced its abundance, which can positively influence tree establishment on PG reclamation sites. Ascomycota Cephalotheca sulfurea, a fungus found only in control and compost PG, is known to have growth promoting effects on soybean (Hamayun et al. 2012), and its presence only in PG may indicate it positively contributed to P. balsamifera growth. The Cadophora fungi genera was predominantly detected in PG (control, compost, mycorrhiza, biochar), indicating PG may provide favorable conditions for its establishment. Exophiala, a genus with some species, is able to produce yeasts under specific circumstances, was also identified and limited to PG control, mycorrhiza and biochar treatments. Although Exophiala is a common genus in soils and associated with plant roots worldwide, it seems to be host specific to poplar roots (Bonito et al. 2016). This genus showed ability to grow in extreme habitats and can positively change soil properties for plant growth over time (Ferrari et al. 2011). With few exceptions Basidiomycota were only detected in PG. Soil type (topsoil vs. PG) and its chemical and physical variations may be responsible for spatial segregation of root-associated Basidiomycota, as many species are ectomycorrhizal. Bruns (1995) found ectomycorrhizal fungi in forests were influenced by soil depth associated with a vertical environmental gradient (pH, water content, nutrient availability, organic and mineral materials). Dickie et al. (2002) found that half of ectomycorrhizal species were restricted to soil mineral horizons and saprophytic fungi showed niche partitioning. In our study, PG on the reclamation site likely provided favorable conditions (high concentrations available and total phosphorus and ammonium) for Basidiomycota, leading to their preferential growth in PG in all treatments. Topsoil amendments had a large impact on the PG root fungal community. Chemical alteration and introduction of microorganisms due to amendments in topsoil alter characteristics of underlying PG over time and thus lead to changed root-associated fungal communities.
Consistent with the literature (Hibbett et al. 2007), Agaricomycetes was the dominant fungal class within three identified Basidiomycota classes in our study. One exception was the mycorrhiza treatment, where only unidentified Basidiomycota were detected with ITS1 and ITS2 sequencing. Thus, mycorrhiza differs from the other treatments, with the difference potentially indicating suppression of indigenous root-associated Basidiomycota (and other fungi) of P. balsamifera by introduced AMF fungi. In control and biochar PG Basidiomycota species richness was similar, although each amendment led to a highly treatment specific root fungal community in PG, suggesting high treatment related soil changes on composition of root-associated Basidiomycota. Three molecularly identified Basidiomycota known as ectomycorrhizal, including Ceratobasidium sp. (Tedersoo et al. 2010; Kinoshita et al. 2016), Oliveonia pauxilla (Taylor & Bruns 1999; Smith & Smith 2001), and Tomentella ellisii (Danielsen et al. 2012) were found in control, compost, and biochar PG, suggesting a positive influence of these amendments on ectomycorrhizal fungi. Basidiomycete ectomycorrhizal fungi diversity in our study was low relative to 20–30 ectomycorrhizal species typically found in forests (even monoculture forest) (Bruns 1995; Dickie et al. 2002). That may be due to the young age of trees, since with increasing age poplar roots typically increase ectomycorrhizal fungal richness (Smith & Read 2008), and/or due to soil properties of the reclamation site. Our study found that compost and biochar application on topsoil provided favorable conditions for Basidiomycete yeasts (root endophytic fungi; Turchetti et al. 2011; Joubert & Doty 2018). Cryptococcus tephrensis, Kondoa sorbi, Vishniacozyma (Cryptococcus) victoriae, and Filobasidium oeirense were found in compost and biochar treatments indicating these amendments can provide favorable conditions for these endophytic fungi. Although root-associated (endophytic) Basidiomycete and Ascomycete yeasts and their biological and ecological role are poorly understood (Joubert & Doty 2018), recent studies showed they may play an important role in transformation of nitrogen, phosphorus, and carbon and probably contribute to binding of soil particles by formation of long hyphae beneath the soil, and to adaptation to contaminated soils (Deng et al. 2012; Buzzini et al. 2017; Li et al. 2020). All soil amendments influenced tree growth positively, increasing tree height, stump diameter, and total aboveground biomass. This is supported by Robinson et al. 2022 who found P. balsamifera growth parameters were positively influenced by soil amendments irrespective of types. Since amendments changed soil nutrients and the root-associated fungal community, both aspects are interconnected in their influence on poplar growth and vitality.
Our results advance the knowledge of fungal diversity associated with P. balsamifera on a PG reclamation site with amendments. Sequencing data from root samples in topsoil and in PG showed all amendments significantly influenced the root-associated fungal community in topsoil and PG. The strong layering of reclamation soil may increase fungal niche space and enhance fungal diversity that could be favorable for P. balsamifera. PG specifically provided favorable conditions for Basidiomycota, including important ectomycorrhizal fungi and plant growth promoting yeasts. All treatments shaped root-associated fungal communities in topsoil and PG, and led to treatment specific fungal species, especially Basidiomycota. Most Ascomycota found are known as potential plant pathogens, such as Fusarium sp.; however, amendments greatly reduced abundance of Fusarium sp., which may be important in suppressing plant diseases. Compost and biochar were associated with reduced potential fungal pathogens and a high number of potential plant growth promoting Basidiomycota in biochar. This study did not investigate effects of amendments on soil physicochemical properties and used only one plant species. Further studies on multi species with extensive sampling in different seasons and including amendment effects on soil physicochemical properties could elucidate underlying mechanisms to better understand the effects of topsoil amendments on plant–microorganism interactions and ascertain their implications in reclamation.
Acknowledgments
The assistance of Udo Bröring (Brandenburg University of Technology Cottbus-Senftenberg) for help with multivariate statistics is gratefully acknowledged. The authors thank Stefanie Schulz (Helmholtz Zentrum München) for assistance with the analysis of sequencing data. This portion of the research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.




