Diversity stabilizes but does not increase sapling survival in a tree diversity experiment
Author contributions: JDP, SCC-P designed and established the experiment; RAK, JDP conceived the paper idea; SCC-P, JP collected data; RAK conducted statistical analyses; JP, RAK made figures and tables; RAK wrote the manuscript; RAK, JP, SCC-P, JDP edited the manuscript.
Abstract
Tree plantings have the potential to increase species diversity and sequester carbon, yet planting failure and early mortality pose significant barriers to their success. Biodiversity-ecosystem function theory suggests that diverse tree plantings could improve survival outcomes through either the portfolio or facilitation effect, yet there remain few tests of this hypothesis. Here, we use a large-scale tree-diversity experiment (BiodiversiTREE), with monitoring of nearly 8,000 individual trees to test whether (1) tree species diversity increases survival rates, (2) tree diversity stabilizes the risk of planting failure, and/or (3) diversity effects are important relative to other common drivers of seedling mortality (e.g. herbivory and soil moisture). We found that only species identity significantly impacted the likelihood of survival, not plant functional diversity nor plot species richness nor phylogenetic diversity. There were minor effects of elevation and soil moisture on survival, but both explained a very small amount of variation in the data (r2marg ≤ 0.011). Higher tree diversity did, however, strongly reduce variation in survival across plots, with nearly 2-fold higher coefficients of variation in monocultures (30.4%, 28.4–32.6% 95% bootstrapped confidence interval) compared to 4- (16.3%, 13.8–18.7%) and 12-species plots (12.8%, 10.8–14.7%). Ultimately, our results suggest that employing diverse species can lower the risk of planting failure (i.e. the portfolio effect), but that species selection still plays a large role in early establishment.
Implications for Practice
- Saplings survive equally well in diverse mixtures and monocultures, indicating that planting diverse species mixtures does not come with added mortality risk.
- Small increases in tree diversity can substantially reduce the risk of planting failure.
- The benefits of including more species could increase over time as growing uncertainty about future climate conditions makes it challenging to identify suitable species for planting efforts.
Introduction
Tree plantings are popular options for restoring tree cover given the potential to safeguard species diversity and sequester carbon (C). Recent estimates highlight the large potential C sink from tree cover restoration, with the capacity to sequester 1.60 Pg C/year across 349 Mha (Cook-Patton et al. 2020). Emerging research also suggests that more diverse stands store more C than monoculture plantations, at least in aboveground biomass (Ma et al. 2020; Feng et al. 2022). Restoration of tree cover can also help maintain or increase biodiversity (Derhé 2017), leading to numerous additional ecosystem services (Alexander et al. 2011; Lewis et al. 2019). Such increases in diversity and ecosystem functioning are often key goals of tree planting projects (Brancalion & Holl 2020). Therefore, planting efforts that include higher levels of tree diversity may offer a greater range of benefits than single-species plantations.
However, current planting efforts focus primarily on monoculture plantations (Lewis et al. 2019). Planted forest area increased by 0.11 billion hectares between 1990 and 2015 (Zhang et al. 2021), yet this increase predominantly came from planting monocultures: e.g. monocultures made up >80% of the planted stands in a recent survey of China's Grain-for-Green Program (Hua et al. 2018; Holl & Brancalion 2020). Monoculture plantations are unlikely to have the capacity to safeguard biodiversity or meet other key goals of efforts to restore forest cover (Di Sacco et al. 2021). A further disadvantage of monocultures in tree planting could be an increased risk of planting failure. Low survival rates for tree plantings are a key barrier to success and plantings can require substantial input of time and money to maintain. Seedlings face mortality risk from numerous sources, including vertebrate herbivory (e.g. deer; Côté et al. 2004), environmental stressors (e.g. drought/low soil moisture; Allen et al. 2010), site conditions (e.g. topography, elevation; Yang et al. 2013), and disease (Ostry et al. 2012). Monocultures may exacerbate the risk of planting failure if the selected species are particularly vulnerable to those mortality risks, or if they are not well suited to site conditions (Truax et al. 2018). As multi-species plantings could mitigate mortality risks by reducing the odds that all species would succumb to the common drivers of seedling mortality, increasing diversity could be an effective strategy to not only increase ecosystem functions but also to reduce the odds of planting failure.
Biodiversity-ecosystem function (BEF) theory suggests a few mechanisms through which increasing diversity could improve the success of tree planting efforts. First, more diverse plantings could have higher survival through selection effects—whereby through planting more tree species, there is a greater likelihood of including species with higher survival rates (Potvin & Gotelli 2008; Grossman et al. 2018). Similar to the selection effect, a more diverse planting could also have greater survival through the insurance or portfolio effect, whereby higher diversity has a “buffering” effect by increasing the likelihood of including species that are suited to microclimatic conditions (Van de Peer et al. 2016; Loreau et al. 2021). Finally, more diverse plantings could exhibit complementarity in survival through niche partitioning or facilitation. In niche partitioning, planting mixtures rather than monocultures could reduce competition for resources as species differ in their traits, resource demand, and acquisition strategies (Johnson et al. 2017; Williams et al. 2017), potentially leading to greater survival. Facilitation could improve survival if certain species alter the environment or resource conditions in a way that makes it more favorable for neighboring species, e.g. a tall shade-intolerant species planted concurrently with a shade-tolerant species can reduce light stress (Kothari et al. 2021). With multiple pathways by which diversity could increase survival, planting mixtures offer a promising way to improve tree planting efforts.
Though selection, portfolio, and complementarity effects are well documented in many BEF experiments, there remain fewer tests of these theories in forest ecosystems, especially for survival (Grossman et al. 2018). When tested, studies have found evidence for the “insurance” (i.e. portfolio) effect with survival (Tuck et al. 2016; Van de Peer et al. 2016) but mainly observed no effect or even a negative effect of tree diversity on survival (Potvin & Gotelli 2008; Yang et al. 2013). One potential reason for the lack of a diversity effect in previous studies is their primary emphasis on species richness as a test of diversity. Other diversity indices, including functional and phylogenetic diversity, are also relevant to explain variation in survival because they quantify differences among species in additional dimensions. For example, both variation in plant functional traits (i.e. functional diversity) and phylogenetic diversity can explain tree performance as well as or better than species richness (Hao et al. 2018). Similarly, the range of diversity levels tested is also frequently low, with the highest level of species richness in most of these experiments is only six species, though it is common for a natural forest stand to have ≥15 species/ha in temperate forests and more than 200 species/ha in tropical forests (Chisholm et al. 2013). Additional studies testing BEF predictions for diversity and survival are therefore needed to accurately understand diversity effects in tree plantings and help promote the most effective options for restoring tree cover.
Here, we use a large-scale tree-diversity experiment (BiodiversiTREE) to test key predictions of BEF theory on tree survival. Specifically, we ask whether (1) increasing tree diversity increases the likelihood of survival, (2) increasing tree diversity stabilizes the risk of planting failure, and (3) the strength of the diversity is important relative to other common drivers of seedling mortality that can vary within and across sites (e.g. herbivory, soil moisture, distance to forest edge; Smith & Beese 2021), and elevation (Yang et al. 2013). We tested these metrics using species richness, phylogenetic diversity, and functional diversity and, to examine scale-dependence of BEF relationships, at both the plot and neighborhood levels.
Methods
Study Site and Experimental Design
We studied the BiodiversiTREE project at the Smithsonian Environmental Research Center in Edgewater, Maryland, U.S.A. (38°52′7″N, 76°33′6″W), part of a global network of tree diversity experiments (TreeDivNet: https://treedivnet.ugent.be/). Prior to establishment in 2013 the site had been in continuous Zea mays L. agriculture for over 35 years. The primary purposes of BiodiversiTREE are to restore tree cover within the imperiled Chesapeake Bay watershed, test BEF theory with trees rather than more commonly manipulated herbaceous species, and test factors that can accelerate progress toward forest restoration. The restoration goals are thus a combination of rehabilitation and partial recovery of native forest cover (Gann et al. 2019).
The site is warm-temperate with mean annual precipitation of 1,068 mm and mean annual temperature of 13.2°C (Fick & Hijmans 2017). Soils are loamy, fluviomarine deposits and are classified as fine-loamy, mixed, active, mesic Typic Hapludults or fine-loamy siliceous, semiactive, mesic Typic Hapludults (Soil Survey Staff 1999).
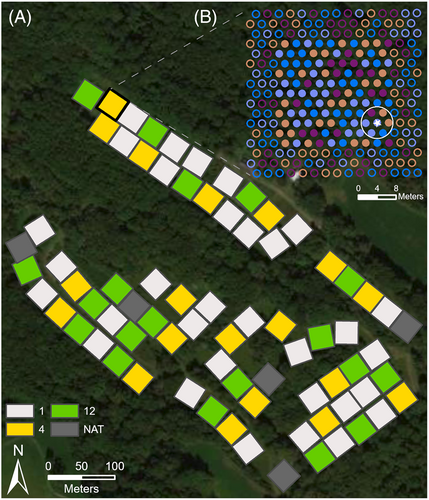
There are 70 plots planted with three levels of species richness (Fig. 1): 1-species (n = 32, 2 per species), 4-species (n = 19), or 12-species (n = 19). Each 35 × 35–m (1,225 m2) plot has 255 trees planted in a hexagonal grid with 2.4-m spacing between trees. Over one hundred local community members volunteered to plant the trees as 1-year-old bare root seedlings in winter 2013. The 16 planted species are some of the most common native species found in intact local forests (Table 1), and richness treatments span typical species richness levels found in nearby forests (Parker & McMahon 2011). Seedlings were allowed to develop fungal associations in situ and were not inoculated with fungi prior to planting. Species mixtures for polyculture plots were generated semi-randomly with the composition of each plot adjusted so that each species mixture occurred only once and each species occurred with equal frequency across the experiment. Plot diversity treatments are maintained with annual mowing and selective pruning to remove volunteer trees and reduce understory vegetation (Fig. S1). This maintenance precludes natural tree regeneration typical of some restoration projects. There are also five natural regeneration plots for comparison, but these are not monitored for survival so we do not include them here. See Griffin et al. (2019) and Devaney et al. (2020) for additional planting details.
| Latin name | Common name | MF Type | Shade Tolerance | Number in Study |
|---|---|---|---|---|
| Acer rubrum (L.) | Red maple | AM | Intermediate | 498 |
| Carpinus caroliniana (Walt.) | Ironwood | ECM | Tolerant | 489 |
| Carya spp. (Nutt.) | Hickory | ECM | Intermediate | 957 |
| Cornus florida (L.) | Dogwood | AM | Tolerant | 501 |
| Fagus grandifolia (Ehrh.) | Beech | ECM | Tolerant | 507 |
| Fraxinus pennsylvanica (Marshall) | Green ash | AM | Intermediate | 478 |
| Liquidambar styraciflua (L.) | Sweetgum | AM | Intolerant | 515 |
| Liriodendron tulipifera (L.) | Tulip poplar | AM | Intolerant | 457 |
| Nyssa sylvatica (Marshall) | Black gum | AM | Tolerant | 426 |
| Platanus occidentalis (L.) | Sycamore | AM | Intolerant | 457 |
| Quercus alba (L.) | White oak | ECM | Intermediate | 514 |
| Quercus pagoda (Raf.) | Cherrybark oak | ECM | Intermediate | 494 |
| Quercus rubra (L.) | Northern red oak | ECM | Intermediate | 511 |
| Quercus velutina (Lam.) | Black oak | ECM | Intermediate | 468 |
| Ulmus americana (L.) | American elm | AM | Intermediate | 418 |
Data Collection and Calculations
Survival of individual trees was monitored yearly for the first 3 years (2013, 2014, and 2015) and then mostly biannually (2017, 2019, and 2022). Figure S2 illustrates tree growth for one species after 6 years. During each census, every tree was visited and marked as live or dead. In cases where a tree appeared dead but resprouted the following year, prior entries were corrected to indicate that the individual was alive. Nursery stock included some nontarget species that remain in the experiment as they are primarily closely related species and comprise <1% of all individuals in the experiment for most species. Excluding nontarget species did not substantially alter the proportion of surviving trees by diversity treatment (Table S1), so we retained all individuals in our dataset (coded as their intended species). Herbivory from deer was assessed in a previous study on a subset of focal trees (n = 50 per plot, n = 3,500 total). Briefly, browse damage from white-tailed deer (Odocoileus virginiana) was assessed visually in fall 2016 by estimating the number of branches showing evidence of browsing (0%, 1–25%, 26–50%, 51–75%, or 76–100%). See Devaney et al. (2020) for additional details.
The coordinates of each individual tree were used to calculate the elevation and distance to nearest forest edge. Elevation data were obtained from the NEON digital terrain model (DTM) (NEON 2022) by overlaying the coordinates of each tree and extracting the underlying elevation data from the DTM using ArcGIS Pro (ESRI, version 2.5.0). Distance to nearest forest edge was calculated as the Euclidean distance from each tree to the nearest forest boundary in ArcGIS Pro. We also collected soil moisture data (volumetric water content) via plot-level sensors starting in 2018, using Sentek Drill & Drop Triscan Probes SD1-12 (Sentek Sensor Technologies, Stepney, South Australia, Australia), with sensors every 10 cm starting at 2 cm depth down to 1 m. Readings were taken every 15 minutes, and we calculated plot-level averages through the final census date for each depth.
Finally, we calculated phylogenetic and functional diversity metrics for each plot and tree neighborhood. “Plot” indicates the entire 35 × 35–m plot and “neighborhood” includes only the six closest individual trees within the 2.4-m radius of the target tree (Fig. 1B). We use both metrics since early in the experiment neighborhood effects may trump plot-level effects, especially given the initial spacing and size of the trees planted (Li et al. 2014). Phylogenetic diversity was calculated using the pd function from the picante R package (version 1.8.2) to calculate Faith's phylogenetic diversity based on the tree generated using Phylomatic by Phylocom (Webb et al. 2008) and the stored tree from Zanne et al. (2014). We tested functional variation in two ways: using functional dispersion as an index of functional diversity, which estimates the dispersion of species in trait space (Laliberté & Legendre 2010), and with mycorrhizal type (arbuscular mycorrhizal [AM] or ectomycorrhizal [ECM]), which captures information on species' nutrient acquisition strategies and their associated traits (Phillips et al. 2013). To calculate functional dispersion, we used leaf traits related to species acquisitiveness (specific leaf area, chlorophyll concentration index, turgor loss point; Reich 2014), as well as their shade tolerance and mycorrhizal type. These traits were all measured on individuals within the experiment in a prior study. For mycorrhizal type, we calculated the proportion of AM-associated trees relative to ECM-associated trees planted at the plot and neighborhood levels.
Data Analysis
Survival data were analyzed with logistic regression (2022 survival status) and discrete time survival analysis (2013–2022). All analyses were conducted using R version 4.1.2 (R Core Team 2021). We excluded buffer trees (trees in the outer three rows of each plot; see Fig. 1B) to account for any edge effects caused by human disturbance around the plot perimeters, leaving a total of 7,770 trees (111 per plot) in the dataset.
We first analyzed the influence of deer browsing, elevation, distance to forest edge, and soil moisture on survival using generalized linear mixed-effects models (GLMMs) via the glmmTMB package version 1.1.2.3 (Brooks et al. 2017). We compared model performance using Akaike information criterion (AIC) values and evaluated the strength of individual models using Nakagawa's r2, both generated using the compare_performance function in the performance package version 0.10.1 (Lüdecke et al. 2021). We used these to determine if any of these factors had significant effects on survival and explained a large amount of variation in the data and thus should be included in the models with diversity. None of these variables met those criteria, and we did not include them in future analyses. We then tested whether survival varied with any of our diversity metrics (species richness, functional dispersion/proportion AM, and phylogenetic diversity) at the plot or neighborhood level. For the logistic regression, we analyzed survival data using GLMMs (glmmTMB) with species and plot as random effects, testing for significant diversity effects using the Anova function (car package) with Wald's chi-square tests. Discrete time survival analyses were conducted using GLMMs followed by Wald tests after converting data to person-period format using the discSurv package version 1.4.1 (Welchowski et al. 2022, dataLong function). We checked residuals for all models using the DHARMa package (version 0.4.5, Hartig 2022).
We tested for a portfolio effect of diversity on survival by calculating the coefficient of variation (CV) in survival at each diversity level followed by bootstrapping to generate 95% confidence intervals. For each year, we calculated the CV for each level of species richness by dividing the standard deviation in the proportion survival by the mean proportion survival. This was repeated 1,000 times via the boot function (boot package version 1.3-28, Canty & Ripley 2021) and then used to derive the bootstrap mean and 95% confidence intervals (percentile) for CV in each year. We used the 95% confidence intervals to determine significant differences in CV, interpreting nonoverlapping intervals to be significantly different. Finally, we examined the effect of diversity on survival for individual species by calculating the effect of polyculture on survival for each species relative to monocultures (polyculture survival − monoculture survival) for each year. We plotted this effect size versus monoculture survival and used major axis (MA) and ordinary least squares (OLS) regression with the lmodel2 package (version 1.7-3; Legendre 2008) to test whether diversity disproportionately altered survival of different species relative to monocultures. We report the OLS results with a permutation test given minor deviation from normality as recommended by Legendre (2008).
Results
Effects of Abiotic Factors and Deer Browse on Survival
To account for the potential influence of nontarget factors on survival, we tested the effects of distance to forest edge, elevation, soil moisture, and deer browsing on individual survival. Most models comparing different combinations of the abiotic factors had similar AIC values (ΔAIC < 3; Table S2), and the best models included elevation and/or soil moisture. However, the explanatory power for all models was low, with r2marg ≤ 0.011 for all models. Trees at higher elevations and in plots with higher soil moisture had a slight increase in the odds of survival (odds ratio [OR] = 1.23, p = 0.022; OR = 1.04, p = 0.023, respectively), while survival did not vary strongly or consistently with distance to forest edge (Table S3; Fig. S3). Due to the low explanatory power of the models, we did not include any of these variables in models with diversity.
The effect of deer browsing on sapling survival was similarly small. Only the highest level of deer browse (76–100% tips browsed) was associated with a decreased odds of survival (OR = 0.85; Fig. S4), the model explained a very small amount of variation in the data (r2marg = 0.004), and the effect was not significant (χ2 = 4.3, df = 4, p = 0.36). Additionally, only 116 deaths were observed out of all individuals monitored for deer damage (n = 2,774), and of those individuals, only approximately 60% of deaths occurred in individuals with deer browse damage. Thus, survival was high across the monitored individuals and deer browse had little influence on survival likelihood. Overall, the small and/or nonsignificant impacts of elevation, distance to forest edge, soil moisture, and deer browsing suggests that they are not the primary predictors of survival early in this experiment.
Tree Diversity Effects on Sapling Survival
Diversity metrics explained little variation in survival at both the plot and neighborhood levels. All models had r2marg < 0.004 (Table S4), which is small and similar or slightly less than the variation explained by models with elevation, soil moisture, and deer browse. Accordingly, all models had similar AIC values (ΔAIC < 3) and neither species richness, functional variation (functional dispersion and proportion AM), nor phylogenetic diversity were significant predictors of survival in either the logistic regression (p > 0.1 for all; Tables S5–S9) or survival analysis (p > 0.1 for all; Table S10). Of all models, species richness and phylogenetic diversity at the neighborhood scale had the highest r2marg (0.004 and 0.002, respectively) and the lowest p-values (p = 0.16 and p = 0.12, respectively), though these values still do not suggest strong effects of either diversity metric. Thus, the overwhelming majority of evidence suggests that diversity has a negligible influence on sapling survival in this experiment.
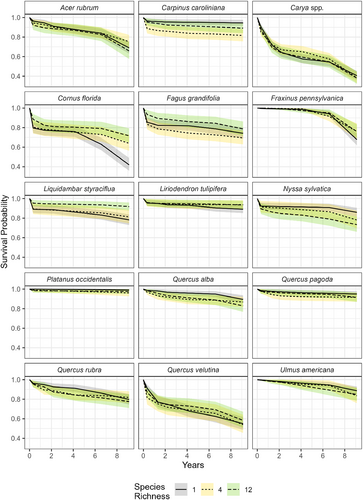
In contrast to diversity, tree species identity accounted for most variation explained in all models. The random effects explained circa 100 times more variation than fixed effects (Intraclass correlation coefficient [ICC] values = 0.279–0.281; Table S4), and the species random effect accounted for more variance (τ) than the plot random effect (Tables S6–S9). Mean survival rates therefore varied substantially across species, from close to 100% in Platanus occidentalis (99.1 ± 0.0%) to near 50% in the Carya spp. (38.6 ± 4.4%) (Table S1; Fig. 2). In contrast, mean survival rates only varied between 74.3 and 76.3% across diversity levels, much less than the variation encompassed by species. Thus, the traits or characteristics of individual species explained more variation in early sapling survival than any diversity metric.
Tree Species Richness and Variation in Survival Rates
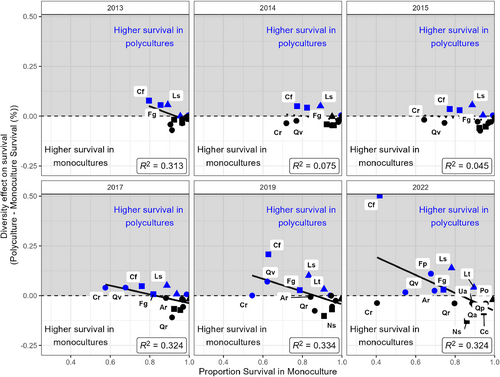
Though we did not see a direct effect of diversity on the probability of survival, higher species richness reduced plot-level variation in survival. Monocultures had a CV for survival rate 2–2.5 times higher than either the 4- or 12-species polycultures, and this effect increased through time (Fig. 3). The CV for survival in monocultures increased from 9% to circa 30% in 2022, while it only reached 12.8% in 12-species polycultures. The variation in survival is also evident in the broader range of survival rates in monoculture plots in 2022 (20.9–99.1%) compared to 12-species polycultures (49.4–93.7%). The 95% confidence intervals around the CVs for each polyculture also stopped overlapping with the monocultures in 2014, two growing seasons after planting. Interestingly, species with the lowest survival in monocultures also showed an increase in survival in polycultures, while species with the highest survival in monoculture had lower survival in polycultures (Fig. 4). The species with the most positive effect of diversity were not consistently late-successional or shade-tolerant species, e.g. the shade-intolerant species Liquidambar styraciflua had higher survival in polycultures, while shade-tolerant species like Nyssa sylvatica and Carpinus caroliniana had lower survival in polycultures. The magnitude of the slope also increased from −0.22 (95% CI: −0.03, −0.41) in 2017 to −0.45 (95% CI: −0.06, −0.84) in 2022, suggesting a strengthening of the diversity effect over time. While small, this effect suggests that the stabilizing diversity effect is not simply due to fewer poor performing species in the mixture plots (i.e. selection effect), but also due to an actual change in their survival rates (i.e. complementarity). Thus, diversity produces more consistent survival rates and improves survival for species with higher mortality rates even if it does not alter the overall likelihood of survival across all species.

Discussion
In a large-scale, multi-year tree diversity experiment, we tested whether diversity could increase the success of efforts of tree planting efforts by either (1) improving overall survival or (2) reducing variability in survival rates. Nine years after planting, higher levels of tree diversity were associated with increased stability in survival rates but not greater likelihood of survival across all species. Instead, species identity explained most variation in survival likelihood compared to any of our diversity metrics, herbivory, or site factors. Reduced variability in survival in more diverse plots suggests that diverse tree plantings may be beneficial by reducing the likelihood of planting failure. Furthermore, even though the diversity effect was not significant across all species, species with the lowest survival rates in monocultures had similar or greater survival at higher diversity levels. Planting higher levels of tree diversity could therefore be a strategy to boost survival for species with greater mortality risk when planning tree planting projects.
Limited Impact of Abiotic Factors and Deer Browse on Survival
We did not include any covariates in our survival models because abiotic site factors had little to no effect on survival. Elevation and soil moisture had slight impacts, which may be due to small variations in soil texture and soil moisture with elevation at our site, and greater soil moisture improved survival outcomes. However, both factors explained only a tiny fraction of the variation in survival relative to the amount explained by species identity alone. This contrasts with other studies of tree survival that have found that site conditions like slope, elevation, distance to forest edge, and soil conditions impact survival (Shen et al. 2020; Wang et al. 2022). However, the variation in elevation (from 5 to 25 m) at our site is much smaller than in previous studies with a significant elevation effect. For example, elevation varied by about 200 m in a tree diversity experiment finding positive effects of elevation on tree survival (Yang et al. 2013). Second, though distance to forest edge varied more than elevation (0–200 m), our analysis already accounted for edge effects by only analyzing survival on the interior trees in our plots. Previous studies have found significant edge effects on survival primarily within 5 m of the forest edge (Smith & Beese 2021), and our interior trees were at least 6 m from the plot edge. Finally, soil moisture data were only continuously available starting in 2018, 5 years after the initial planting. Many tree species quickly develop extensive root systems (Tomlinson et al. 2012), which could help to reduce mortality risk from low water availability by the time our continuous measurements started (Thompson & Schultz 1995). Our study period (2013–2022) also did not include any significant droughts prior to 2019, 6 years after planting, and earlier one-time measurements of soil moisture did not show any significant differences by diversity (data not shown). Given the humid summers of our study site, soil moisture may not have been particularly limiting during our study when species were most vulnerable.
Surprisingly, deer browse damage was not associated with a decrease in the odds of survival in our study. Only at the highest level of browse damage (>75% branch tips browsed) was there any indication of decreased survival. Deer browse pressure can be a major driver of tree seedling survival and species composition in temperate forests (Granger et al. 2017; Russell et al. 2017), especially for preferred browse species (Truax et al. 2018; Reed et al. 2021), and at densities observed in our experimental area (20 deer/km2; Russell et al. 2017). However, many of these studies are from natural forests or naturally regenerating seedlings, while our trees were planted as 1-year-old bareroot seedlings. As mortality is typically highest during the first year of life (Tyler et al. 2008), we may have bypassed the window when survival of saplings would have been most vulnerable to deer browse. Unfortunately, we do not have data for browse damage during the first 3 years of the experiment, so we cannot test whether deer browse influenced survival prior to 2016 (67% of all deaths recorded). Despite this gap in data, we think it unlikely that it would entirely explain the lack of a browse effect on survival. For example, the survival of Acer rubrum and Carpinus caroliniana, the two most-browsed species, was near or above the median survival for all species in the experiment. In contrast, the two Carya species had both the lowest survival and among the lowest incidence of deer browse (Devaney et al. 2020). Therefore, it seems unlikely that we are missing an effect of deer browse on survival because of missing data.
However, high survival in heavily browsed species does not mean deer have no impact on the trees. Devaney et al. (2020) found a negative relationship between deer browse damage and tree height at BiodiversiTREE, which supports previous work showing that deer have significant impacts on tree growth (Côté et al. 2004; Cook-Patton et al. 2014; Granger et al. 2017). Furthermore, we observed significant growth impacts for both C. caroliniana and A. rubrum: C. caroliniana shows an intense herbivory-induced branching response (Churski et al. 2022), producing a “cage” of branches to protect the leader from browse damage, while A. rubrum saplings are among the smallest trees in the experiment. Taken together, protecting individual trees from browse may not be necessary for survival, but protection could help heavily browsed species avoid significant delays in growth leading to prolonged suppression or eventual death.
Species Identity Rather Than Diversity Metrics Explain Most Variation in Tree Survival
We initially hypothesized that increased diversity at either the plot or neighborhood level would result in greater survival, yet no diversity metric had a significant impact on survival odds. Instead, species identity was the variable that explained most variation in survival across all models, similar to other tree diversity experiments (Potvin & Gotelli 2008; Philipson et al. 2014; Shen et al. 2020). The substantial range in mortality rates among individual species in our study adds to the body of evidence that individual characteristics are more important determinants of sapling survival than neighborhood diversity (Philipson et al. 2014; Shen et al. 2020). Our study also went beyond some previous work to evaluate functional and phylogenetic diversity impacts on survival, but we still found no evidence that diversity impacts survival. As these metrics tested hypotheses that diversity would increase survival via reduction in competition and/or facilitation, our results suggest that these are not key mechanisms explaining tree sapling survival.
The minimal impact of diversity on survival likelihood could also be attributed to our experimental design and the early nature of the experiment. We planted trees relatively far apart, especially in comparison to the density of seedlings in our natural regeneration reference plots (712.4 trees/plot vs. 255 trees/plot or 0.581 trees/m2 vs. 0.208 trees/m2). Wide spacing may have limited interactions between individual trees, especially in early years, leaving individual traits more important than species richness, functional diversity, or phylogenetic diversity for survival. Moreover, the experiment was primarily designed to test variation in species richness rather than functional or phylogenetic diversity. Although there was sufficient variation to test these latter factors in our analysis, it is possible that a study designed to maximize functional or phylogenetic variation could have found these to be more prominent.
The nature of our experiment may also have limited the potential effect of mycorrhizal type on survival. Most studies that found an effect of ECM abundance on survival have been done in natural forests (Teste et al. 2009). In natural forests, nurse trees may be substantially larger and better able to provide carbohydrates through a common mycorrhizal network, and light may be more limiting in the understory than it was in our open plots. In our experiment, trees are all the same age and may not be able to provide the same level of local facilitation. Furthermore, mycorrhizal networks may not have been particularly well established at the time of our study. The site was formerly in corn rotation for >35 years, so the appropriate fungal partners may not have had time to establish extensive networks (Hawkins et al. 2015).
Though we did not see a diversity effect across all species, certain species saw a slight positive effect of diversity on survival. Species with lower survival rates in monocultures tended to have higher survival in polycultures (e.g. Cornus florida, Fraxinus pennsylvanica), resulting in a negative relationship between monoculture survival and the diversity effect on survival. Competition from grasses and other understory species can reduce survival (Vandenberghe et al. 2006), and monocultures with low survival had little canopy closure and substantial understory vegetation in our experiment even with mowing once or twice a year. Canopy cover tended to increase with diversity and is associated with lower percent cover of understory vegetation (unpublished data). Thus, more diverse plots may have reduced competition and contributed to the increased survival observed for these species. Additionally, initial data from our experiment show that polycultures have lower maximum daily temperatures, higher minimum temperatures, and higher relative humidity than monocultures (J. Parker, unpublished data). Both of these findings support a slight facilitation effect of tree diversity which improves survival of certain species and helps to reduce variation in survival rates.
While we did not deliberately manipulate shade-tolerance in our planting, other restoration projects have found that it is possible to facilitate forest recovery by planting shade-intolerant species initially and then allowing natural recruitment of shade-tolerant species in the understory (Osuri et al. 2022). This can increase planting survival and reduce the costs of planting by requiring the planting of fewer species (Brancalion et al. 2019). Comparing this facilitation of natural regeneration to direct, simultaneous planting of shade-intolerant and shade-tolerant species would be a useful follow-up to understand the most effective techniques for recovering species diversity.
Tree Diversity Increases Stability in Survival and the Effect Increases Over Time
Though we did not see an effect of diversity on survival overall, we did find that increasing tree species richness increased stability in survival rates. Increases in stability with diversity are frequently found in BEF experiments (Isbell et al. 2015), though are not as frequently tested in tree diversity experiments. Where tested, our results echo previous tree diversity experiments in finding that monocultures have the most variable survival rates (Tuck et al. 2016; Van de Peer et al. 2016). Finding a strong portfolio effect is not surprising given that we had a mix of shade tolerance levels and ECM-AM species, and the portfolio effect is a result of species responding differently to site conditions (Loreau et al. 2021). Some of our species are well suited to regenerating in open field conditions (e.g. Platanus occidentalis, Liriodendron tulipifera) while others primarily regenerate in the understory and did not survive as well (e.g. C. florida, Carya spp.), causing the observed variation in survival rates. However, we extend these findings by showing that the CV for survival rates increased over time for monocultures until it was approximately double the CV of both polycultures.
While previous BEF work shows that diversity effects often increase over time (Reich et al. 2012), we do not know of any that document the portfolio effect in tree diversity experiments. Our observed increase in CV for survival in monocultures, especially over time, could be explained by increased exposure to varying environmental conditions or other stressors over time. The more vulnerable species in our experiment continue to suffer mortality, increasing the range of survival rates observed for different species. We also observed that the greatest reduction in CV occurred between monocultures and 4-species plots; increasing diversity from 4- to 12 species did not further reduce variation in survival. Though we expected further reduction in CV with 12-species plots, it might be that these effects saturate more quickly than we anticipated, or that the CV for 4-species plots may be increasing slow enough that it is not yet observable. Continuing to monitor survival will help determine whether differences between polycultures emerge over time.
Overall, we show that increasing diversity can lead to greater stability in early survival and therefore increase the likelihood of successful tree planting efforts. Though we did not observe an increase in mean survival at higher levels of species richness, the greater stability in survival rates at higher levels of species richness, especially over time and in the face of increasing uncertainty, offer compelling reasons to increase diversity in tree plantings. We also add support to the portfolio effect hypothesis in the BEF literature. Our findings are also particularly relevant given that we tested higher levels of species richness than many previous studies of the portfolio effect in tree diversity experiments, and we found that even 4-species mixtures offer substantial benefits over monocultures. Future studies could test whether certain trait combinations produce higher survival and/or greater complementarity, measure additional ecosystem functions, and continue to observe the impacts of deer browse and pathogens on survival. With recent estimates of global forest loss ranging from circa 1.5 million km2 (Hansen et al. 2013) to circa 0.8 million km2 (Winkler et al. 2021), active efforts are essential to restore tree cover and maintain biodiversity on the landscape. Our results indicate that the simple act of increasing species richness in tree planting efforts is a key part of an effective strategy and we hope researchers and managers continue to explore these mechanisms to improve restoration outcomes into the future.
Acknowledgments
The authors thank all of the people who have helped work on the BiodiversiTREE experiment to establish trees, collect data, and maintain treatments, especially W. Hoot, J. Shue, L. Klimesova, A. Cawood, S. Alley, S. Bennett, K. Burghardt, E. Butz, D. Doublet, E. DuBois, C. Cecil, J. Devaney, T. Eaves, A. Galvin-Manico, E. Griffin, K. Hill, A. Lienesch, F. Kashon, M. Knaack, K. Komatsu, A. Lewis, M. McCormick, K. McGurrin, S. Newkirk, A. Nordseth, M. Palmer, C. Rhodes, R. Rich, A. Santana, G. Schilling, L. Schmitt, J. Taylor, D. Valet, and dozens of volunteers. Funding for this work was provided by the Smithsonian Grand Challenges Consortium, a generous gift from John and Ann Ryan, NSF DEB 2044406, NSF Macrosystems 2106014, and NSF REU 1156799, 1659668, 1950656. The Bezos Earth Fund covered a portion of SCC-P's time on this work.




