A soil archaeal community responds to a decade of ecological restoration
Abstract
Large-scale restoration efforts are underway globally to mitigate the impact of decades of land degradation by returning functional and biodiverse ecosystems. Revegetation is a heavily relied upon restoration intervention, and one that is expected to result in associated biodiversity returns. However, the outcome of such restoration interventions rarely considers recovery to the soil microbiome, a mega-diverse and functionally important ecosystem component. Here we examine the archaeal component of the soil microbiome and track community change after a decade of eucalypt woodland restoration in southern Australia. We employed DNA metabarcoding to show that archaeal community composition, richness, and diversity shifted significantly, and towards a restored state 10 years after the restoration intervention. Changes in soil pH and nitrate associated with changes to the archaeal community, potentially relating to the pH responsive properties and close relationship with the nitrogen cycle of some archaea. Our study helps shed light on archaeal community dynamics, as no other study has used DNA metabarcoding to study archaeal responses across a restoration chronosequence. Our results provide great promise for the development of molecular monitoring of the soil microbiome as a future restoration monitoring tool.
Implications for Practice
- Replanting native vegetation can bring about a dramatic change in the soil archaeal community.
- Monitoring and assessing soil archaea changes with high-throughput amplicon sequencing has great promise.
- DNA could be developed into a more generalized tool for monitoring and assessing microbial community responses of restoration projects.
Introduction
Above and belowground biological components of terrestrial ecosystems interact to shape ecological communities (Wardle et al. 2004). Belowground microbiota process organic matter, influence soil fertility and structure, and are fundamental components of ecosystem health and biodiversity (Schmidt et al. 2011; Cotrufo et al. 2013). For example, the soil microorganisms that play vital roles in nutrient cycling can be detrimentally affected by degradation caused by land use changes (Bardgett & van der Putten 2014; Moon et al. 2016). Understanding the responses of these microbiota to restoration will help guide the repair of degraded land (Harris 2003; Harris 2009).
Ecological restoration prioritizes the recovery of biological assemblages, including microbial communities, and the processes needed to sustain these biota (Suding et al. 2015; Singh et al. 2019). Recovery of bacterial communities has been shown after revegetation programs (Banning et al. 2011; Gellie et al. 2017; Guo et al. 2019). Similarly, previous work has shown the fungal community can be shaped by revegetation, such as in our previous study in a eucalypt woodland catchment reserve (Yan et al. 2018), plus the work of others in post-mining contexts (Sun et al. 2017) and grassland restoration (Guo et al. 2019). Although research into environmental microbiota changes during ecosystem restoration is increasing, many knowledge gaps still remain, such as understanding the functional roles that these returning microbiota have in reference, degraded, and restored ecosystems (Breed et al. 2019).
Archaea are a domain of prokaryote microbes that are often associated with habitats that have unusual physicochemical properties (e.g. extreme temperatures, salinity, or pH; Walsh et al. 2005), and which can change with vegetation cover, land-use, and restoration practices (Zechmeister-Boltenstern et al. 2015; Guo et al. 2016; Belmok et al. 2019; Yuan et al. 2019; Zhang et al. 2019). However, molecular studies have shown that archaea are widespread and not limited to these extreme habitats (Flemming & Wuertz 2019). They represent approximately 2% (relative abundance) of soil microbiota across all biomes (Bates et al. 2011), and have pivotal roles in the cycling of nitrogen (e.g. oxidation and reduction of nitrogen; Nicol et al. 2008), soil carbon, and phosphorus (Hu et al. 2013; Yadav et al. 2015). They have been found to be the main driver of ammonium oxidation in soil environments (Yin et al. 2018), and knowledge from ecological restoration studies of archaea have helped unravel factors that regulate their distribution and function in soil environments (Bengtson et al. 2012; Singh et al. 2019). To date, even though archaea have important soil regulatory functions, their community structure, and the factors that determine their diversity, abundance, and ecology remain poorly understood (Flemming & Wuertz 2019; Korzhenkov et al. 2019).
Community profiling methods using environmental genomic DNA are rapidly developing and have led to improved ability to describe complex microbiomes (Ji et al. 2013; Valentini et al. 2016). High-throughput amplicon sequencing is one such profiling approach that has been used to characterize organismal occurrences, for instance, aquatic (Valentini et al. 2016) and invertebrate biodiversity (Fernandes et al. 2019). There is an obvious incentive to utilize amplicon sequencing for quantifying microbiota where culture-dependent methods have little success (Cavicchioli 2010), or where morphological identification is problematic (Taberlet et al. 2012; Valentini et al. 2016). Indeed, amplicon sequencing has been used to great effect to investigate soil bacteria and fungi (Fierer et al. 2007; Ji et al. 2013; Tedersoo et al. 2014; Bissett et al. 2016), providing a cost-effective, high-throughput, and easy-to-standardize approach to broadly quantify biodiversity (Deiner et al. 2017). Despite the appeal, using DNA metabarcoding to explore biological (e.g. microbiota, fauna) changes in a restoration context lags behind its other ecological applications, such as biosecurity and biosurveillance, and is still an emerging field of research (Breed et al. 2019).
In this study, we tested the hypothesis that replanting the native plant community into an ex-pasture will lead to significant compositional shifts in the soil archaeal community towards that found in the reference ecosystem. To test this hypothesis, we used amplicon sequencing of the 16S ribosomal RNA (rRNA) gene to explore how the archaeal community changed across a 10-year restoration chronosequence. We used samples from 0–10 and 20–30 cm soil depths across the chronosequence, which included sites that were 6, 7, 8, and 10 years post-revegetation, and included remnant sites (i.e. best-on-offer reference sites) and cleared sites (i.e. pre-revegetation as pastures). We analyzed these environmental samples to address the following questions: (1) Does a change in the plant community with revegetation alter the soil archaeal community? (2) If change does occur, which archaeal taxa are characteristic of the different ages of ecological restoration? (3) Does restoration return the archaeal community to one resembling the reference state? (4) How do archaeal community changes relate to soil physicochemical characteristics?
Methods
Site Description and Sampling
We studied a restoration site at Mt Bold, a water catchment reserve of the Mt Lofty Ranges in South Australia (35.07°S, 138.42°E), described in detail in Gellie et al. (2017). This catchment was dominated by open eucalypt woodland, but was cleared and grazed early in the 20th century. Grazing ceased in 2003, and restoration began in 2005. The goal of restoration was to recreate the local Eucalyptus leucoxylon grassy woodland community (i.e. reference site, known hereafter as remnant). Our restoration chronosequence included sites that were restored via native plant species revegetation between 6 and 10 years ago. Restoration approaches were consistent throughout this time period and were administered through active revegetation of the same local, native plant species. The species mix included an overstory of South Australian blue gum (Eucalyptus leucoxylon) and manna gum (E. viminalis), a shrub layer that included golden wattle (Acacia pycnantha), sticky hop bush (Dodonaea viscosa), and sweet bursaria (Bursaria spinosa ssp. spinosa). We sampled three remnant sites (Remnant A, B, C), and each was located less than 10 km away from the revegetation sites. Prior to 2005, when restoration began, Remnant A was minimally cleared and had low-density grazing, Remnants B and C were protected from clearing and had minimal human impact. The cleared sites are a legacy of grazing, are adjacent to current revegetation areas, are managed annually for exotic weeds, and have no history of active restoration.
Sequences and edaphic data used for this work were generated by the Biomes of Australian Soil Environments (BASE) project and are available in the BASE database (https://data.bioplatforms.com/organization/about/australian-microbiome, samples 102.100.100/19281–19322) (Bissett et al. 2016). Below, we briefly describe the BASE methods, which are described in detail in Bissett et al. (2016). In January 2015, we sampled soil from three randomly selected 25 x 25 m quadrats at each of eight sites, including sites restored 6, 7, 8, and 10 years before sampling, a cleared site, and three remnant sites (Gellie et al. 2017), giving a total of 24 quadrats. Some sites did display spatial dependency of treatment replicates; however, treatments were spatially independent of the chronosequence (see Fig. 1 in Gellie et al. 2017). All Remnant C samples and one Remnant B sample were omitted as they had high rates of unclassified archaea Operational Taxonomic Units (OTUs) at the phylum level (all >30% of total reads; all other samples <5%). Consequently, all Remnant A and B samples are analyzed together (known as Remnant hereafter), and across our study, we present and analyze data from 40 samples across the two soil depths.
Nine soil samples per quadrat were pooled into a sterile plastic bag, transported to the laboratory immediately after collection in sterile 50 mL falcon tubes on dry ice, stored at −20°C for DNA analysis. Three hundred grams of homogenized soil were also sampled for soil physicochemical analysis, quantifying soil moisture, ammonium, nitrate, available phosphorus, sulfur, organic carbon, and soil pH (H2O).
Genomic Analyses
DNA was extracted and then pooled from 3 × 0.25 g soil samples per replicate at the Australian Genome Research Facility (Adelaide, Australia) using MoBio Powersoil DNA extraction kits following manufacturer's instructions. DNA extracts were pooled per sample, and the 16S ribosomal RNA (rRNA) gene was PCR amplified for each replicate with negative controls (lab-grade water), as described in Bissett et al. (2016) (details on reagents, volumes, and final concentrations found at www.bioplatforms.com/wp-content/uploads/BASE_Illumina_A16S_SOP_v2.pdf).
PCR products were screened for negative control contamination with gel electrophoresis, purified using the Agencourt AMPure XP bead PCR product purification kit as per manufacturer's instructions, concentration normalized to 10 nM, and sized on an Agilent Bioanalyze. Equal volumes of products were pooled, diluted to 4 nM, and sequenced on the Illumina MiSEQ platform with MiSeq Reagent Kit v3 600 cycle chemistry, to produce 300 bp paired end reads.
Read analysis was also done as per Bissett et al. (2016), which included quality checking, merging, and read screening prior to 97% OTU picking. We discarded OTUs not identified as belonging to archaea and those that were unidentified at phylum level, as in Gellie et al. (2017). OTUs were classified using the ribosomal database project Bayesian Classifier against SILVA132 at 60% Bayesian probability. The OTU data are available in Dongfeng et al. (2019), and the Fastq sequence data used in this study is available from NCBI sequence read archive under bioproject PRJNA317932 and biosample accessions SAMN07488642–SAMN07488681.
Statistical Analyses
R v 3.4.4 (R Core Team 2018) was used to run all statistics. OTU abundance was rarefied to the replicate with the lowest number of reads (32,315 and 26,874 reads, in 0–10 and 20–30 cm soil samples, respectively) with the rarefy function in vegan v 2.5-1 (Oksanen et al. 2018), subsampling without replacement. OTU richness was estimated using the Chao 1 nonparametric richness estimator. Diversity was estimated as the effective number of species (Jost 2006) using the Shannon-Wiener index (H) and the Gini-Simpson index (D), where the Shannon-Wiener index and Gini-Simpson index were transformed by using the formula exp(H) and 1/(1-D), respectively, to estimate the true diversity of the archaeal community.
Differences in rarefied abundance, diversity indices, phyla, classes, and soil characteristics across the restoration chronosequence, soil depths, and the interaction between restoration site and soil depth were analyzed using permuted analysis of variance (PERMANOVA) with the aovp function implemented in lmPerm 2.1.0 package (Wheeler & Torchiano 2016) with 5,000 permutations. The Tukey honest significant difference test was used to determine whether the relationships between two restoration sites were statistically significant or not.
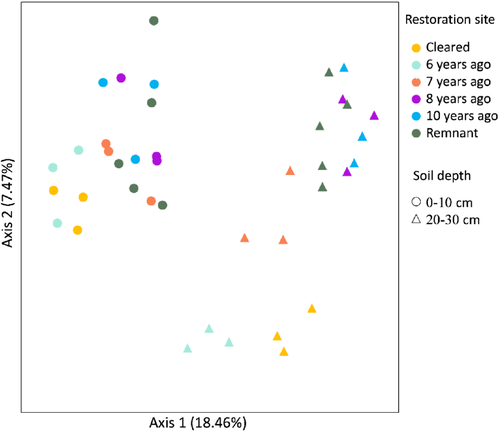
Principal coordinates analysis (PCoA) was used with Jaccard (presence–absence) dissimilarity matrices to visualize the effect of the restoration chronosequence on archaeal community composition, which were created with the functions vegdist, metaMDS, stressplot, and ordiplot in vegan. Analysis of similarities (ANOSIM) (9,999 permutations) with the anosim function in vegan was used to assess the differences in archaeal community compositions across the restoration chronosequence at the 0–10 and 20–30 cm soil depths, estimating r values, where r close to 1 indicates high separation between groups (e.g. between restoration ages) and r close to 0 indicates little separation between groups.
Distance-based redundancy analyses (db-RDA) were used to visualize the relationships between soil physical and chemical variables and archaeal community composition based on Jaccard distances. Prior to the ordination, the soil physicochemical variables were normalized using the decostand function in vegan. Permutation test (9,999 permutations) with the permutest function in vegan was introduced after db-RDA to measure the significant association between archaeal community composition and soil physical and chemical variables.
Results
Soil Physicochemistry
Notable changes in soil physical and chemical characteristics were observed across the restoration chronosequence (Tables S1, S2). Soil nitrate and phosphorus significantly decreased with time since restoration, and organic carbon and ammonium significantly increased. Phosphorus, organic carbon, ammonium, and sulfur significantly decreased, and pH increased with soil depth.
Archaeal Community Composition
We generated a total of 2,483,000 raw 16S reads across the 40 samples (62,075 ± 23,355 SD per sample). A total of 2,456,998 archaeal sequences (61,425 ± 23,563 SD per sample) and 2,740 archaeal 97% OTUs (69 ± 27 SD per sample) remained for further analysis after quality filtering.
The filtered data set comprised four archaeal phyla: Thaumarchaeota, Euryarchaeota, Nanoarchaeaeota, and Crenarchaeota, and was dominated by phylum Thaumarchaeota, representing 99.749 and 99.467% of the total rarefied archaeal sequences, at the 0–10 and 20–30 cm soil depths, respectively (Tables 1 & 2). The sequence abundance of Euryarchaeota significantly increased at the two soil depths with time since revegetation, while the sequence abundance of other taxa did not show any trends with time since revegetation (Table 2).
| Level | Taxon | Site | Direction of Effect | Depth | Direction |
|---|---|---|---|---|---|
| Phylum | Thaumarchaeota | 0.009 | Variable | <0.001 | Decreasing |
| Class | Nitrososphaeria | 0.007 | Variable | <0.001 | Decreasing |
| Group_1.1c | 0.036 | Variable | <0.001 | Increasing | |
| Genus | Candidatus Nitrosocosmicus | 0.041 | Variable | <0.001 | Decreasing |
| Nitrososphaera | 0.767 | 0.005 | Increasing | ||
| Unclassified Nitrososphaeraceae | <0.001 | Variable | <0.001 | Increasing | |
| Unclassified Nitrosotaleaceae | 0.155 | 0.002 | Increasing |
| Level | Taxon | Depth (cm) | Cleared | 6 years ago | 7 years ago | 8 years ago | 10 years ago | Remnant |
|---|---|---|---|---|---|---|---|---|
| Phylum | Thaumarchaeota (99.749%) | 0–10 | 32,248 ± 6 | 32,256 ± 7 | 32,214 ± 26 | 32,175 ± 51 | 32,231 ± 12 | 32,130 ± 49 |
| Class | Nitrososphaeria (99.676%) | 0–10 | 32,235 ± 28 | 32,255 ± 6 | 32,182 ± 56 | 32,067 ± 140 | 32,229 ± 10 | 32,129 ± 49 |
| Family | Nitrososphaeraceae (99.637%) | 0–10 | 32,235 ± 28 | 32,255 ± 6 | 32,182 ± 56 | 32,066 ± 140 | 32,218 ± 14 | 32,129 ± 49 |
| Genus | Candidatus Nitrosocosmicus (85.968%) | 0–10 | 28,744 ± 676 | 29,877 ± 966 | 24,809 ± 115 | 26,514 ± 3,044 | 30,792 ± 882 | 26,567 ± 2,071 |
| Genus | Nitrososphaera (0.003%) | 0–10 | 2 ± 2 | 1 ± 2 | 0 | 2 ± 3 | 1 ± 1 | 0 ± 1 |
| Genus | Unclassified Nitrososphaeraceae (13.666%) | 0–10 | 3,444 ± 644 | 2,371 ± 965 | 7,367 ± 91 | 5,547 ± 3,134 | 1,421 ± 898 | 5,556 ± 2,068 |
| Family | Nitrosotaleaceae (0.006%) | 0–10 | 0 | 0 | 1 | 4 | 11 ± 12 | 1 |
| Genus | Candidatus Nitrosotalea (<0.001%) | 0–10 | 0 | 0 | 0 | 1 | 0 | 1 |
| Genus | Unclassified Nitrosotaleaceae (0.006%) | 0–10 | 0 | 0 | 1 | 3 | 11 ± 12 | 0 |
| Class | Group_1.1c (0.073%) | 0–10 | 40 | 2 | 32 ± 30 | 324 | 8 | 2 |
| Phylum | Euryarchaeota (0.187%) | 0–10 | 34 ± 7 | 26 ± 7 | 65 ± 26 | 105 ± 49 | 48 ± 13 | 75 ± 38 |
| Phylum | Nanoarchaeaeota (0.041%) | 0–10 | 1 | 0 | 1 | 4 | 3 | 51 ± 71 |
| Phylum | Crenarchaeota (0.023%) | 0–10 | 0 | 0 | 3 ± 2 | 0 | 2 ± 1 | 26 ± 29 |
| Unclassified Classes (0.196%) | 0–10 | 34 ± 6 | 26 ± 7 | 65 ± 26 | 105 ± 53 | 48 ± 14 | 86 ± 27 | |
| Unclassified Genus (0.253%) | 0–10 | 79 ± 39 | 32 ± 1 | 74 ± 30 | 110 ± 50 | 53 ± 17 | 118 ± 37 | |
| Phylum | Thaumarchaeota (99.467%) | 20–30 | 26,713 ± 9 | 26,706 ± 19 | 26,659 ± 33 | 26,595 ± 113 | 26,619 ± 66 | 26,343 ± 352 |
| Class | Nitrososphaeria (94.227%) | 20–30 | 26,363 ± 467 | 26,557 ± 138 | 25,935 ± 377 | 23,803 ± 1,699 | 25,011 ± 906 | 24,117 ± 1,587 |
| Family | Nitrososphaeraceae (85.087%) | 20–30 | 26,250 ± 569 | 26,472 ± 269 | 25,729 ± 499 | 18,187 ± 1,667 | 23,282 ± 1,932 | 19,352 ± 6,531 |
| Genus | Candidatus Nitrocosmicus (41.850%) | 20–30 | 12,627 ± 5,455 | 13,844 ± 4,263 | 13,236 ± 2,357 | 6,326 ± 2,305 | 11,697 ± 4,002 | 10,094 ± 4,914 |
| Genus | Nitrososphaera (0.058%) | 20–30 | 25 ± 14 | 13 ± 21 | 2 ± 2 | 16 ± 21 | 16 ± 26 | 19 ± 27 |
| Genus | Unclassified Nitrososphaeraceae (43.179%) | 20–30 | 13,513 ± 4,855 | 12,140 ± 4,040 | 12,489 ± 2,543 | 11,835 ± 3,303 | 11,554 ± 3,817 | 9,235 ± 4,217 |
| Family | Nitrosotaleaceae (8.592%) | 20–30 | 113 ± 104 | 85 ± 132 | 206 ± 129 | 5,615 ± 2,185 | 1,729 ± 1,123 | 4,765 ± 5,779 |
| Genus | Candidatus Nitrosotalea (3.474%) | 20–30 | 78 ± 64 | 52 ± 90 | 99 ± 72 | 2,877 ± 2,992 | 96 ± 30 | 1,792 ± 2,339 |
| Genus | Unclassified Nitrosotaleaceae (5.118%) | 20–30 | 34 ± 40 | 33 ± 42 | 107 ± 101 | 2,633 ± 752 | 1,617 ± 1,090 | 2,817 ± 3,651 |
| Class | Group_1.1c (5.239%) | 20–30 | 350 ± 458 | 149 ± 150 | 724 ± 385 | 2,792 ± 1,721 | 1,608 ± 930 | 2,226 ± 1,580 |
| Phylum | Euryarchaeota (0.374%) | 20–30 | 8 ± 9 | 12 ± 13 | 39 ± 32 | 118 ± 108 | 67 ± 29 | 254 ± 306 |
| Phylum | Nanoarchaeaeota (0.027%) | 20–30 | 0 | 0 | 3 | 6 | 7 | 26 ± 22 |
| Phylum | Crenarchaeota (0.132%) | 20–30 | 2 | 12 | 23 ± 6 | 7 ± 2 | 34 ± 50 | 99 ± 189 |
| Unclassified Classes (0.129%) | 20–30 | 8 ± 9 | 12 ± 13 | 40 ± 33 | 117 ± 106 | 68 ± 28 | 282 ± 319 | |
| Unclassified Genus (1.081%) | 20–30 | 95 ± 56 | 490 ± 164 | 65 ± 35 | 244 ± 48 | 134 ± 52 | 539 ± 313 | |
At the class level, the community mainly consisted of Nitrososphaeria and Group_1.1c (both Thaumarchaeota), representing 99.676%, 0.073% and 94.277%, 5.239% of the sequence abundances, at the 0–10 and 20–30 cm soil depths, respectively (Table 2). The relative abundance of Group_1.1c significantly increased at the 20–30 cm soil depth with time since revegetation, and was significantly higher than that at the 0–10 cm soil depth (Table 2). Other classes Thermoprotei (Crenarchaeota), Archaeoglobi, Halobacteria, Methanomicrobia (all Euryarchaeota), and Nanohaloarchaeia and Woesearchaeia (both Nanoarchaeaeota) were also present, but each had very few reads (<0.01% total sequence abundance across two soil depths).
Archaeal community richness at the genus level showed an increasing trend with time since revegetation at 0–10 cm soils (Fig. S2). Among all classified genera, Candidatus Nitrosocosmicus (family Nitrososphaeraceae, class Nitrososphaeria, phylum Thaumarchaeota) dominated the archaeal community, representing 85.959 and 41.850% of the sequence abundances, at the 0–10 and 20–30 cm soil depths, respectively (Table 2). Among all key classified genera (>0.01% total sequence abundance across two soil depths), Nitrososphaera and Candidatus Nitrosotalea were higher in relative sequence abundances at 20–30 cm than 0–10 cm soil depths, while Candidatus Nitrosocosmicus showed the opposite trend (Table 2).
Archaea Community Responses to Restoration and Soil
We identified clear directional changes in the archaeal community across the restoration chronosequence (Figs. 1 & 2). Recently revegetated sites had a similar archaeal community to cleared sites, and older revegetated sites were similar to remnant sites at both soil depths. These results were supported by ANOSIM, which showed that the archaeal community was significantly dissimilar across the revegetation sites based on Jaccard dissimilarity (absence or presence) at both 0–10 (r = 0.411, p < 0.001) and 20–30 cm (r = 0.532, p < 0.001) soil depths.

Archaeal richness (observed and Chao 1) and diversity (Shannon-Wiener index, Gini-Simpson index) varied significantly across the restoration chronosequence, with greater richness and diversity at lower depths and at older revegetation sites. Archaeal richness (observed) increased with time since revegetation at the 20–30 cm soil depth (Table 3). Archaeal diversity increased with an increase in pH at the 0–10 cm soil depth; however, no obvious associations were present with the other edaphic soil characteristics (Fig. S1).
| OTUs (±SD) | Diversity (±SD) | ||||
|---|---|---|---|---|---|
| Site | Depth (cm) | Observed | Chao 1 | Shannon | Simpson |
| Cleared | 0–10 | 37 ± 4B | 46 ± 10B | 1.52 ± 0.03abB | 1.25 ± 0.05abA |
| 6 years ago | 0–10 | 34 ± 3B | 42 ± 6B | 1.33 ± 0.11bB | 1.16 ± 0.07bB |
| 7 years ago | 0–10 | 49 ± 10A | 57 ± 13A | 2.19 ± 0.14aB | 1.63 ± 0.02aB |
| 8 years ago | 0–10 | 46 ± 12B | 56 ± 16B | 1.99 ± 0.67abB | 1.48 ± 0.33abB |
| 10 years ago | 0–10 | 47 ± 8B | 56 ± 10B | 1.27 ± 0.09bB | 1.10 ± 0.06bB |
| Remnant | 0–10 | 48 ± 1B | 64 ± 15B | 1.93 ± 0.31abB | 1.45 ± 0.20abB |
| Cleared | 20–30 | 71 ± 3abcA | 79 ± 4bcA | 4.81 ± 1.64cA | 3.33 ± 1.33bA |
| 6 years ago | 20–30 | 52 ± 5cA | 69 ± 4cA | 3.34 ± 0.46bcA | 2.55 ± 0.57bA |
| 7 years ago | 20–30 | 65 ± 11bcA | 69 ± 11cA | 5.07 ± 0.36bcA | 3.23 ± 0.61bA |
| 8 years ago | 20–30 | 106 ± 15aA | 128 ± 18aA | 11.12 ± 1.97aA | 6.88 ± 1.22aA |
| 10 years ago | 20–30 | 94 ± 11abA | 116 ± 5abA | 7.39 ± 2.36abcA | 4.14 ± 1.68abA |
| Remnant | 20–30 | 94 ± 17abA | 120 ± 10abA | 7.65 ± 2.42abA | 4.60 ± 1.63abA |
| PERMANOVA p values | Site | <0.001 | <0.001 | <0.001 | 0.010 |
| Depth | <0.001 | <0.001 | <0.001 | <0.001 | |
The archaeal community strongly associated with soil physical and chemical variables based on Jaccard dissimilarity and permutation tests at 0–10 (F7,12 = 4.185, p < 0.001) and 20–30 cm (F7,12 = 4.016, p < 0.001) (Fig. 2). In total, the proportion of variation in the archaeal community explained by the first two axes was 45.65 and 25.29%, 48.59 and 21.50%, at 0–10 cm and 20–30 cm soil depths, respectively, and a distinct bacterial community structure across the restoration chronosequence was observed (Fig. 2).
The permutation test between archaeal community composition and the soil variables showed that soil moisture, nitrate, and organic carbon at 0–10 cm, and pH, organic carbon, sulfur, and phosphorus at 20–30 cm soil depth, were the abiotic factors that had significant effects on the archaea community across the restoration chronosequence (Fig. 2).
Soil depth significantly influenced the archaeal community, with clear separation between 0–10 and 20–30 cm communities based on Jaccard dissimilarity (Fig. 1) and ANOSIM test (r = 0.482, p < 0.001), and the richness (observed and Chao 1) and diversity (Shannon and Simpson) of archaea was significantly higher at the 20–30 cm than the 0–10 cm soil depth (Table 3).
The relative sequence abundance of the genus Candidatus Nitrosocosmicus (85.968% of all sequence abundance) significantly decreased, while Nitrososphaera and Candidatus Nitrosotalea significantly increased, from 0–10 to 20–30 cm soil depths (Tables 1 & 2).
Discussion
Our study explored archaeal community composition changes across soil samples from a restoration project that has experienced a decade of eucalypt woodland revegetation in the Mt. Bold Reservoir catchment, South Australia. We used metabarcoding of 16S rRNA gene fragments to explore changes in the archaeal community across a 10-year revegetation chronosequence. To describe relative changes in the archaeal community, we compared the archaeal communities from the revegetation chronosequence to reference systems, including both remnant vegetation and un-restored (pasture) sites. We found that changes in the archaeal community were associated with changes in the physical and chemical properties of the soil across this restoration chronosequence. Further, sites that had been more recently revegetated (less than 6 years) had similar archaeal communities to un-restored sites, and sites that had been revegetated for 8–10 years were more similar to sites supporting remnant vegetation.
These findings are important in a restoration context because even though it is clear that strong associations exist between above- and belowground biota (Wardle et al. 2004), and excess soil nitrogen can inhibit restoration (e.g. it can widen the environmental filter for invasive species; Funk 2008), the contribution of archaea to mediate the recovery of degraded lands is not well understood. In our study, nitrate levels in the surface soil horizon of cleared land were an order of magnitude higher than soils of remnant sites (16.0 vs. 1.70 mg/kg). The trend of decreasing nitrate at older restored sites is largely consistent with a previous study of abandoned agricultural land (Cunningham et al. 2015). This trend is likely to reflect conversion of nitrogen-enriched pastures by a combination of plant assimilation and a suite of denitrifying organisms. However, nitrate reduction is only one of several components to the nitrogen cycle that affects nitrogen availability for plants. Further work on metagenomic (i.e. functional genes) and metatranscriptomic (total content of RNA; i.e. transcribed copies of genes) would help to qualify what role the archaea community plays in the nitrogen cycle, and the mechanism of change following restoration.
Soil pH plays a substantial role in regulating microbial diversity and distribution, where it can be viewed as an integrated index of soil condition that is related to other soil properties, such as nutrient availability (Lauber et al. 2009). We observed soil pH to associate with archaeal diversity, with higher archaeal diversity in samples with higher pH, which is consistent with a study in paddy soils (Yuan et al. 2019). We observed pH varied significantly with archaeal community change at the 20–30 cm soil depth, which is consistent with our previous work on the same study system for bacteria (Gellie et al. 2017) and fungi (Yan et al. 2018). The study site soils were not acidic sensu stricto (acidic soil is defined to be pH < 5.5 in the surface layers; Von Uexküll & Mutert 1995), but with a pH range 5.3–6.1, the study site soil was generally acidic. In semi-arid regions, the composition of archaeal communities has been shown to be distributed according to an aridity gradient, where archaeal richness linearly decreased with increasing aridity (Huang et al. 2019; Zhang et al. 2019). Such variations in species richness were thought to be largely driven by changes in Thaumarchaeota, a deep-branching archaeal division, which is among the most abundant organisms on Earth (Weber et al. 2015).
Thaumarchaeota is well known to contain important ammonia-oxidizing archaea (AOA) (Kimble et al. 2018; Fan et al. 2019). This taxon is recognized to exert primary control over ammonia oxidation in terrestrial, marine, and geothermal habitats (Stahl & Torre 2012). The relative abundance of Thaumarchaeota varied significantly across our study, but did not appear to coincide with time since restoration. Within Thaumarchaeota, Group_1.1c is known to be abundant in highly organic and acidic soils and is closely related to ammonia oxidation (Timonen & Bomberg 2009). Our study found that the relative abundance of phylum Euryarchaeota and class Group_1.1c increased with organic carbon and pH increases at the 20–30 cm soil depth with time since revegetation. Soil pH has been shown as the main environmental factor to shape the biogeographic patterns of Euryarchaeota (mostly methanogens) (Hu et al. 2015; Yuan et al. 2019), which may affect the availability of substrates to archaeal methanogens and ammonia oxidizers (Anderson et al. 2009).
We observed an increase in diversity (observed and Chao 1) and richness (Shannon-Wiener and Gini-Simpson indexes) in the 20–30 cm compared to the 0–10 cm horizon. These depth trends were not consistent with the current literature. In a study in mixed Norway spruce-beech forest, a significant decrease in diversity of Euryarchaeota was observed with increasing soil depth (Pesaro & Widmer 2002). Soil depth has also been shown to have no effect on archaeal community structure (Poplawski et al. 2007), or conversely, depth has been shown to strongly structure and stratify the archaeal community (Bundt et al. 2001; Hansel et al. 2008). Furthermore, Eilers et al. (2012) examined archaeal community abundance using deep soil pits (up to 180 cm) and found higher abundance and a higher ratio of archaea to bacteria at depth, leading to the inference that such heterogeneity of effects of soil depth on archaea was likely a function of soil chemistry and opportunistic autotrophic nitrification by AOA. It is likely that soil chemistry and soil depth act as significant filters for archaea diversity, but additional work on the ecology of archaea is required to clarify their responses to changes in soil type, land use, soil depth, nutrient cycling, weathering, and revegetation.
Ecological restoration often focuses on restoring the aboveground plant community, with an expectation of broader biodiversity recovery, including the belowground microbiota (e.g. a field of dreams approach as discussed in Strickland et al. 2017). A key barrier to validating this assumption is the shortage of empirical studies that link restoration interventions with desirable restoration outcomes. Our results provide evidence that DNA metabarcoding is a suitable method for assessing difficult-to-observe soil community components, such as archaea. Furthermore, by demonstrating the significant correlations between the age of restoration, change in soil physicochemistry, and shifts in the soil archaeal community, our findings highlight the potential importance of this taxonomic group for tracking functional restoration outcomes and the overall utility of this method for monitoring restoration outcomes (Fernandes et al. 2018; Breed et al. 2019). However, the spatial layout and number of sample sites in our study was not ideal to infer the effect of revegetation on the archaeal community. As a result, and due to a general absence of such studies, we recommend that well-designed and well-replicated studies are established to confirm the associations in this and related studies.
Land use legacies (e.g. nutrients, weeds) are known to limit restoration success (Yates et al. 2000), but understanding the direct and indirect ecological pathways of the impact of these legacies on archaeal communities will be necessary to help to improve restoration practices. Our study suggests that replanting native plants can shift the soil archaeal community in a predictable trajectory towards a desired reference state, a finding which was consistent with the bacterial (Gellie et al. 2017) and fungal communities (Yan et al. 2018) at the same study site. Soil depth appears to filter archaea diversity and composition (potentially due to changes in pH and available nitrogen); however, with limited taxonomic resolution and scant functional attribution, basic ecological questions remain about the functional role of archaea. In conclusion, our study helps to describe aspects of archaeal community structure during restoration, but importantly, it highlights that greater investigation of this cryptic, but potentially functionally important, domain of life is urgently required.
Acknowledgments
This work was supported by the China Scholarship Council [201408410176 to D.Y.] and Bioplatforms Australia through the Australian Government National Collaborative Research Infrastructure Strategy. We thank S. Kennedy, Z. Baruch, S. Caddy-Retalic, L. Clarke, I. Fox, M. Laws, K. McCallum, and J. McDonald for technical and field assistance. We are grateful for support from the Australian Genome Research Facility, Bioplatforms Australia, and SA Water. We would like to acknowledge the contribution of the Biomes of Australian Soil Environments (BASE) consortium (https://data.bioplatforms.com/organization/pages/bpa-base/acknowledgements) in the generation of data used in this publication. The authors declare no conflicts of interest.




