Evaluation of DNA fragmentation in dog sperm using the sperm chromatin dispersion test
Abstract
The study's objective was to adapt the Sperm Chromatin Dispersion (SCD) protocol to evaluate sperm DNA fragmentation and implement a fragmentation control in dogs. Correlation between DNA status and routine sperm parameters was also analysed. To adapt the SCD, two different mercaptoethanol (ME) concentrations were assayed (2.5% and 5%) in fourteen ejaculates from seven dogs and semen incubation with 0.3 M NaOH for 15 min at room temperature was assayed as a control for sperm DNA fragmentation. Data were analysed using a Mann–Whitney test and either Pearson's or Spearman's correlation. The selected ME concentration to use in the SCD test was 5%, as it produced the largest DNA dispersion halo while preserving the core nucleus structure. Four DNA halo patterns were identified as follows: large dispersion halos, medium halos, small halos and nuclei without halos. Semen incubated with NaOH showed 100% sperm without halos (damaged DNA). A significant positive correlation was observed between sperm with fragmented DNA and sperm with coiled tails. Thus, it was possible to adapt the SCD protocol to evaluate dog sperm DNA fragmentation in raw semen without using a commercial kit and establish incubation with NaOH as a DNA fragmentation control. Only coiled tails showed correlation with DNA fragmentation.
1 INTRODUCTION
For many years, routine semen analysis, including evaluation of sperm motility, morphology, membrane function, viability and sperm concentration, has been used to study male infertility. However, in many cases, fertility may be altered even when these semen parameters are within normal values (Prinosilova et al., 2012; Sakkas et al., 2003). Therefore, increasing attention has been paid to evaluation of the sperm nucleus, and one of the parameters proposed to determine fertility is chromatin structure (López-Fernández et al., 2007; Oleszczuk et al., 2016). Moreover, compared with conventional semen parameters, sperm DNA integrity analysis is now considered a better marker of male fertility potential, both in humans and in animals (Evenson, 2016; Love, 2005; Zeqiraj et al., 2018; Zini et al., 2001).
Assessment of sperm DNA fragmentation can be performed by two different kinds of techniques: those that evidence double and simple strand breaks, such as terminal dUTP nick-end labelling (TUNEL) (Lopes et al., 1998); and those that assess chromatin susceptibility to denaturalize after being treated, for example, the sperm chromatin dispersion (SCD) test and the sperm chromatin structure assay (SCSA) (Evenson, 2013; Fernández et al., 2003). Several of these techniques are laborious, require expensive equipment or depend on the use of enzymes that often show irregular activity and accessibility to DNA breaks (Enciso et al., 2006; Fernández et al., 2005). Furthermore, the need for expensive flow cytometers for some of the aforementioned techniques restricts their use in clinical situations in companion animals (Prinosilova et al., 2012).
The SCD test is a simple technique, which does not require expensive equipment and has allowed effective evaluation of sperm DNA fragmentation in different species (human: Fernández et al., 2003, 2005; horse and llama: Carretero et al., 2010; Carretero, Arraztoa, et al., 2012; Carretero, Lombardo, et al., 2012). In this test, sperm that have a high level of DNA fragmentation show small or no halos while those with a low level of DNA fragmentation show large halos (Carretero et al., 2010; Carretero, Lombardo, et al., 2012; Fernández et al., 2003, 2005). Visualization and analysis of halo size is very clear using either fluorescent or bright-field microscopy. This method, originally developed by Fernández et al. (2003), was adapted to become a commercial kit (Halosperm®) to evaluate human sperm DNA (Fernández et al., 2005). After some modifications, this kit started to be commercialized for use in different species (boar: Enciso et al., 2006; bull: García-Macías et al., 2007; horse: López-Fernández et al., 2007; ram: López-Fernández et al., 2008; donkey: Cortés-Gutiérrez et al., 2009; cat: Vernocchi et al., 2014; dog: Hidalgo et al., 2010, 2015; Urbano et al., 2017). However, these kits are expensive, they are not available in some countries and, what is more important, do not include the use of a DNA fragmentation control.
As the sperm head consists almost entirely of DNA, it has been hypothesized that sperm head morphometric parameters may reflect chromatin organization (Lange-Consiglio et al., 2010). Studies have been carried out in dogs that relate DNA damage to sperm head morphometry (Núñez-Martinez et al., 2005; Lange-Consiglio et al., 2010; Urbano et al., 2017); but few studies evaluate the association between DNA damage and other routine sperm parameters (Pereira et al., 2017; Prinosilova et al., 2012).
In this context, the objectives of this study were to adapt and evaluate the effectiveness of the SCD technique to assess DNA fragmentation in dog sperm and evaluate the efficiency of a sperm DNA fragmentation control. In addition, correlation between DNA status and routine sperm parameters was analysed.
2 MATERIALS AND METHODS
2.1 Reagents
Sperm chromatin dispersion and HOS test reagents were provided by Global Lab. S.A. and Sigma Chemicals, Argentina.
2.2 Animals
This study was approved by the ethics committee of the Faculty of Veterinary Sciences of Buenos Aires University (CICUAL Nº 2014/24). Seven clinically and reproductively healthy male dogs, 2 mongrels and 5 Beagle, between 2 and 5 years old, were included in the study. They were housed in dog pens at the Faculty of Veterinary Sciences (University of Buenos Aires) in Argentina and were fed with commercial concentrates and had free access to fresh water.
2.3 Semen collection
A total of 14 ejaculates were obtained from seven dogs (n = 7, replicates =2) and for each male dog, the period between collections was 15 days. Semen collection was performed by applying manual massage of the penis collecting both the first and second portions of the ejaculate (Linde-Forsberg, 2006). Ejaculates were individually assessed.
2.4 Routine seminal characteristics evaluation
The routine seminal characteristics evaluated in raw semen were as follows: volume and pH, sperm motility, vigour, concentration, membrane function and sperm morphology. Sperm progressive motility was evaluated using a computer analysis system (AndroVision™; Minitüb GmbH, Tiefenbach) using the manufacturer's set-up for canines with images being captured at twenty-five frames/second. According to the system configuration, sperm cells were considered non-motile when the amplitude of lateral head displacement (ALH) and beat cross frequency (BCF) were lower than 4 μm and 4 Hz, respectively. Sperm were considered to be moving in their place (non-progressive) when velocity curved line (VCL) and velocity straight line (VSL) were lower than 40,000 μm/s and 20,000 μm/s, respectively. Sperm vigour was estimated placing a drop of sample between a slide and coverslip using a warm stage (37°C) and phase contrast microscopy (100×), where 5 =maximum vigour and 0 =no vigour. Sperm concentration was calculated using a Neubauer hemocytometer and a dilution of 1:200 in buffered formol saline (BFS). Membrane function was evaluated using the hypoosmotic swelling test (HOST) according to England and Plummer (1993). Briefly, 5 µl of semen was incubated in 50 µl of hypoosmotic fructose-sodium citrate solution (150 mOsmol/L) at 37°C for 30 min. A minimum of 200 sperm were evaluated using a phase contrast microscope (400×). Sperm morphology was evaluated using the Casarett stain (Casarett, 1953) and light microscopy (1000×). Briefly, a smear was made with raw semen and left to air-dry. Once dry, it was placed on a warm plate and covered in Casarett stain for 5–7 min, then rinsed with distilled water and again left to air-dry. A minimum of 200 sperm were evaluated in each slide and classified into one of the following categories: normal sperm, abnormal heads, detached heads, proximal cytoplasmic droplets, distal cytoplasmic droplets, distal midpiece reflex, broken tails and coiled tails. Thus, percentages of spermatozoa with normal or altered morphology were determined.
2.5 SCD test and selection of mercaptoethanol (ME) concentration for lysing solution 1
The SCD test was carried out according to Fernández et al. (2003) modified by Carretero, Lombardo, et al. (2012). Ejaculates from each male dog were diluted in PBS to obtain final concentrations of 5–10 × 106 sperm/ml. Then, samples were divided in two aliquots, one to evaluate DNA fragmentation (sample) and the other to use as a positive control. The control was achieved by inducing DNA fragmentation prior to the SCD test by means of incubation with 0.3 M NaOH for 15 min at room temperature. For the SCD test, 40 µl of each sperm suspension (sample and control) was mixed at 37°C with 100 µl of 1% low-melting-point aqueous agarose (0.7% final agarose concentration). Aliquots of 50 μl of each agarose-sperm suspension (3 per slide: two replicates of the sample and one control; see Figure 1: Experimental design) were pipetted onto a glass slide that had previously been coated with 0.5% normal-melting-point aqueous agarose. Immediately, a coverslip was placed over each drop, and the glass slides were placed at 4°C for 10 min to solidify. After that, coverslips were gently removed and slides were immersed horizontally in a tray with freshly prepared acid solution (0.08 N HCl) for 7 min at room temperature. The slides were rinsed with distilled water and transferred to another tray with a neutralizing and lysing solution (solution 1: 50 mM EDTA, 0.4 M Tris, 1% Lauryl sarcosine and 2.5% or 5% ME; pH 7.5) for 10 min at room temperature, to remove proteins. Two different ME concentrations were tested for the solution 1: 2.5% and 5% ME, to replace the dithiothreitol of the original protocol. Next, the slides were rinsed and incubated for 5 min at room temperature in lysing solution 2 (2 M NaCl, 0.4 M Tris and 1% Lauryl sarcosine; pH 7.5). Subsequently, slides were rinsed, dehydrated in sequential ethanol baths (70°, 85° and 96°) for 2 min each and then air-dried. Finally, slides were stained with Giemsa for 15 min, rinsed with distilled water and again air-dried. Four slides were prepared for each ejaculate (two with 2.5% ME in solution 1 and two with 5% ME in solution 1). Sperm head images were captured using a Leica DC180 camera (Leica Microsystems Co.), acquiring 400 images per ejaculate (200 for each solution 1 tested), employing a sensitivity of 16 bits per channel. To select the most adequate ME concentration, two binary images were obtained for each sperm: one of the core (residual nucleus) and one of the halo (Figure 2). Finally, both areas were automatically detected and measured in μm2 using the QWin Plus V3 program (Leica Microsystems Co.). The program is calibrated using a micrometric slide that allows it to create a factor relating the quantity of pixels that correspond to 100 microns. Based on the data acquired, the relative halo size was calculated as follows: surface of the halo/total surface (halo +core) × 100 (μm2). In addition, sperm were classified into different patterns according to the size and presence of the halo: large, medium, small and without halo. To minimize errors, the same person carried out all evaluations.
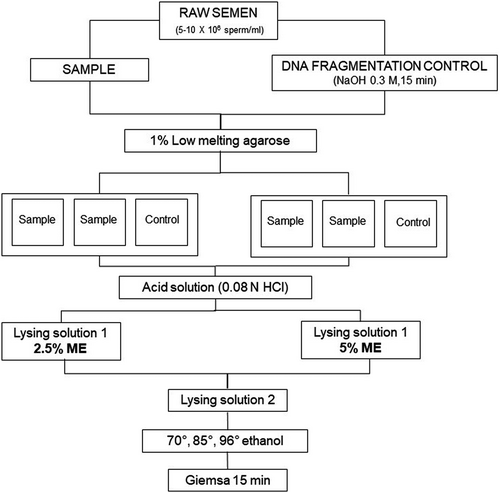
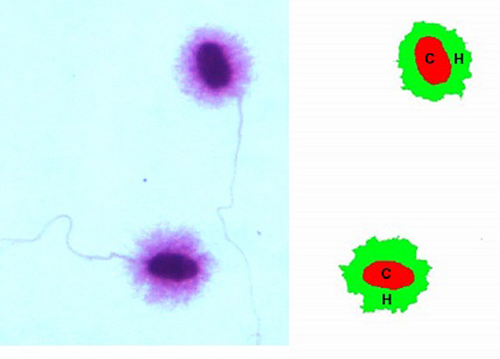
2.6 Statistical analysis
Statistical analyses were conducted using the InfoStat program. Values are expressed as mean ± SD. Differences were considered significant when p values were < .05. Normal distribution of the data was verified using the Shapiro–Wilk test. Consequently, the effect of the ME concentrations (2.5% and 5%) in Solution 1 on the core and halo areas, relative halo size and the different SCD patterns, was analysed using the non-parametric Mann–Whitney test. To assess the correlation between the different SCD halo patterns and each of the routine semen parameters evaluated (volume, concentration, total sperm count, sperm motility, sperm membrane function and sperm morphology), Pearson's test was used for those showing a normal distribution and Spearman's test was used for those that did not. This was carried out separately for each ME concentration. Regarding sperm morphology, correlation was analysed between SCD patterns and the percentage of sperm with normal morphology, primary, secondary and total abnormalities. In addition, SCD patterns were correlated with the percentage of abnormal heads (abnormal and detached heads), tail abnormalities (coiled, broken and distal midpiece reflexes) and the presence of proximal and distal droplets.
3 RESULTS
3.1 Routine seminal characteristics
Routine seminal characteristics were all within the normal range for the species and most are shown in Table 1. Regarding abnormal sperm morphology, samples presented 0.1% ± 0.2% abnormal heads, 5.2% ± 3.8% detached heads, 2.2% ± 3.3% proximal cytoplasmic droplets, 1.8% ± 1.5% distal cytoplasmic droplets, 6.7% ± 2.4% distal midpiece reflexes, 0.3% ± 0.8% broken tails and 5.8% ± 2.6% coiled tails.
| Volume (ml) | pH | PM (%) | Vigour | Concentration (x106 sperm/ml) | Total sperm (x106) | HOS (%) | Normal morphology (%) |
|---|---|---|---|---|---|---|---|
| 2.0 ± 0.8 (1.0–3.5) | 6.2 ± 0.1 (6.0–6.3) | 74.7 ± 4.8 (60.0–90.0) | 3.1 ± 0.6 (2.0–4.0) | 512.1 ± 290.4 (120–1000) | 907.0 ± 445.7 (340–1500) | 92.7 ± 3.8 (82.5–97.0) | 77.9 ± 4.9 (70.0–85.0) |
Note
- Ranges are included between brackets.
- Abbreviations: HOS, sperm with intact membrane function; PM, progressive motility.
3.2 SCD test
3.2.1 Selection of ME concentration for lysing solution 1
Regarding sperm core area, no significant differences were observed between the different ME concentrations evaluated in lysing solution 1 (p > .05). However, the absolute and relative halo areas were significantly greater in sperm incubated in the lysing solution 1 containing 5% ME (p ˂ .05; Table 2). Thus, the ME concentration selected to use in the lysing solution 1 of the SCD test was 5%, since it preserved the core structure and produced the largest DNA dispersion halo.
| ME concentrations (%) | Core area (µm2) | Halo area (µm2) | Relative halo size (µm2) |
|---|---|---|---|
| 2.5 | 22.5 ± 6.7 a (8.2–67.3) | 48.8 ± 20.0 a (1.0–223) | 67.0 ± 11.0 a (3.2–100) |
| 5 | 22.4 ± 6.7 a (8.7–81.1) | 58.7 ± 33.7 b (1.3–801) | 70.5 ± 9.8 b (6.8–100) |
Note
- Different mercaptoethanol (ME) concentrations (2.5 and 5%) were tested. Values are expressed as mean ± SD (n = 7; replications =2). Ranges are included in brackets. a,bDifferent letters between rows indicate significant differences (p ˂ .05).
3.2.2 SCD patterns
Four sperm DNA dispersion patterns were identified as follows: (I) nuclei with large DNA dispersion halos; (II) nuclei with medium halos; (III) nuclei with small halos and (IV) nuclei without halo (Figure 3). According to Fernández et al. (2003, 2005) patterns I and II should be considered sperm without DNA fragmentation and patterns III and IV, sperm with fragmented DNA and this classification was respected.
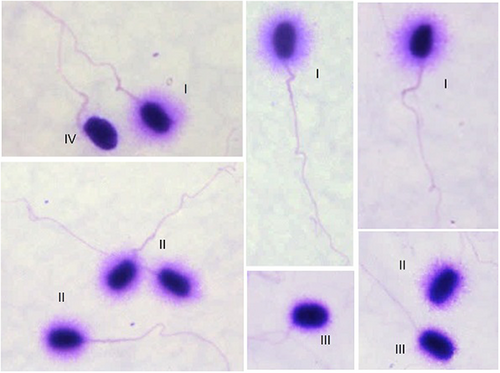
No significant differences were observed in the mean percentages of sperm with small halos and without halo (p > .05) between the different ME concentrations. However, significant differences were observed in the mean percentages of sperm with large and medium halos (p ˂ .05), with the highest percentage of large halos observed with the 5% ME concentration (Table 3). These results support the selection of 5% ME for use in lysing solution 1.
| ME concentrations (%) | Large halos (%) | Medium halos (%) | Small halos (%) | Without halos (%) |
|---|---|---|---|---|
| 2.5 | 43.8 ± 35.2 a (22.0–94.8) | 52.0 ± 32.5 a (25.5–94.5) | 3.7 ± 3.2 a (0–13.5) | 0.5 ± 0.4 a (0–2.2) |
| 5 | 71.4 ± 26.3 b (30.7–97.0) | 26.0 ± 25.0 b (15.0–63.9) | 2.0 ± 2.0 a (0–7.3) | 0.6 ± 2.5 a (0–1.9) |
Note
- Different ME concentrations (2.5 and 5%) were tested in the lysing solution 1, (n = 7, replications =2). Large and medium halos are sperm with undamaged DNA while small and without halos correspond to sperm with fragmented DNA. Values are expressed as mean ± SD. Ranges are included in brackets. a,bDifferent letters between rows indicate significant differences (p ˂ .05).
Finally, no significant differences between the 2.5% and 5% ME concentrations of the lysing solution 1 were observed in the percentage of sperm with intact DNA (95.8% ± 5.5% versus. 97.4% ± 2.7%, respectively) or in the percentage of fragmented DNA (4.2% ± 3.5%; versus. 2.6% ± 2.5%, respectively).
Semen samples incubated with NaOH showed 100% sperm without halo (sperm with damaged DNA).
3.2.3 Correlation between SCD patterns and routine seminal parameters
No correlation was detected between routine seminal parameters (volume, concentration, total sperm count, sperm motility, sperm membrane function and sperm morphology) and the different SCD patterns when using 2.5% ME in the lysing solution. However, when using 5% ME, the percentage of sperm with coiled tails showed a positive correlation with fragmented DNA (r = 0.79; p = .01) and a negative correlation with intact DNA (r = −0.78; p = .002).
4 DISCUSSION
Artificial insemination is the most accurate method to determine the fertility of a male; however, in dogs, it is costly and slow, as female dogs only cycle 1–3 times per year (Concannon, 2011). Consequently, using sperm DNA fragmentation analysis to evaluate an individual's fertilizing potential has gained increasing interest in recent years. In this study, a simple, inexpensive, non-commercial SCD test to evaluate sperm DNA fragmentation was adapted for use in dogs, together with a reliable DNA fragmentation control for the technique. The method was first developed for use in human semen (Fernández et al., 2003) and later modified for use in equines, South American camelids and felines (Allera et al., 2017; Carretero et al., 2010; Carretero, Arraztoa, et al., 2012; Carretero, Lombardo, et al., 2012).
According to results in this study, the most appropriate concentration of ME to use in the lysing solution of the SCD test was 5%. Three factors were considered in the selection: (1) Sperm incubated with 5% ME preserved the core structure and formed large halos of intact DNA around the core, (2) 5% ME produced a greater number of large halos, which simplified sample evaluation and (3) sperm incubated with 5% ME showed a larger relative halo size. This third factor is essential for interpreting the test's results, because sperm cells may have different nuclear sizes, so this relative parameter prevents the distortion that would result if only absolute size were to be considered.
Regarding SCD patterns, four categories were observed in dog spermatozoa in this study. These patterns coincided with those reported in equine, South American camelids and feline sperm (Allera et al., 2017; Carretero et al., 2010; Carretero, Arraztoa, et al., 2012; Carretero, Lombardo, et al., 2012), using a similar SCD technique. Whereas in human sperm, together with the same four patterns as those observed in the present study, a fifth pattern identified as ‘degraded’ sperm (cells without halos but weakly or irregularly stained) was also reported (Fernández et al., 2003, 2005). These authors stated that this fifth pattern is observed more frequently when using the Halosperm® kit compared with the original SCD protocol. This fifth pattern was not observed with either of the ME concentrations tested in the present study, possibly because the protocol is similar to the original SCD method or because ME was used in the lysing solution as an alternative to dithiothreitol, the reagent used by Fernández et al. (2003).
In porcine, a new kit was developed in 2006 to evaluate sperm DNA fragmentation, introducing an adaptation to the original Halosperm® involving omitting the step of incubation with the acid solution (Sperm-Sus-Halomax®; Enciso et al., 2006). Using this modified SCD, the interpretation of the halo patterns is reversed, with the nuclei showing extensive dispersion halos corresponding to spermatozoa with fragmented DNA. Galaz-Leiva et al. (2012) proposed that in nuclei with intact DNA, the use of acidic solutions, NaCl 2 M, polyanions or detergents to extract proteins will cause the formation of ‘saline’ double-strand DNA halos due to the dispersion of supercoiled DNA loops. However, in cells with fragmented DNA, free rotation of polynucleotide ends would avoid supercoiling of the DNA without forming a halo, when proteins are extracted with acid solutions. This would explain the halo patterns observed in the original protocol developed for human sperm and in the current adaptation we used in dog sperm, where the protocol involves a step in an acid solution. In contrast, Galaz-Leiva et al. (2012) propose that DNA alkaline denaturation prevents the formation of halos in normal cell nuclei, because there are no fragments to diffuse from the nucleus and proteins are insoluble due to their high alkaline isoelectric point. Thus, under the alkaline denaturation used in the Halomax® kits developed for various species in which the acid solution step was omitted, the nucleus with DNA fragmentation exhibits extensive denaturation and the release of these fragments, forming a halo of ‘alkaline’ single-strand DNA. According to Fernández et al. (2003), in the original SCD technique, single-strand DNA interacts within the sperm head in such a way as to avoid the removal of nuclear proteins, thus preventing the dispersion of the DNA fragments, leading to the absence of a halo in sperm with fragmented DNA. Carretero et al. (2010), Carretero, Lombardo, et al. (2012) successfully used the original SCD protocol, developed for use in humans, to evaluate horse and llama semen and proposed that DNA fragments interact through complementary bases (guanine-cytosine; adenine-thymine) in the sperm head to form cohesive ends, which then fail to produce a halo, and this is the hypothesis proposed for dog sperm too.
It is important to consider that most studies that use the SCD technique do not include DNA fragmentation controls. Exceptions to these are two studies performed in llamas and horses that used semen incubation with NaOH (0.3 M) for 30 min, UV radiation exposure for 2 hr and incubation at 100°C for 30 min to damage sperm DNA, and all these treatments proved to be successful controls (Carretero et al., 2010; Carretero, Lombardo, et al., 2012). More recently, Allera et al. (2017) reported the effectiveness of incubating cat sperm with 0.3 M NaOH to induce DNA fragmentation. Considering these reports, in this study, raw dog semen was incubated with 0.3 M NaOH for 15 min, leading to 100% sperm with damaged DNA (sperm without halo) thus proving to be an effective control for this species.
Regarding sperm DNA fragmentation in canine raw semen, Hidalgo et al. (2010) reported a total fragmentation of 2.0% ± 0.1% in dog semen evaluated by light microscopy and 2.3% ± 0.5% in samples evaluated by fluorescence microscopy, while Urbano et al. (2013) reported a total fragmentation of 1.4% ± 0.1%. These values, obtained using commercial SCD kits, are similar to the values observed in this study (2.6 to 4.2%) and to reports by other authors using different techniques such as SCSA and TUNEL (averaging 0.4%–2.9%; Núñez-Martinez et al., 2005; Koderle et al., 2009; Kim et al., 2010; Prinosilova et al., 2012).
The DNA fragmentation index resulting from the various methods used to evaluate sperm DNA has been used to estimate the fertility potential of an ejaculate. In human medicine, an index higher than 30% has been associated with infertility (Fernández et al., 2003; Larson et al., 2000). In bulls and boars, fertility decreases when DNA damage is around 15% and 20%, respectively (García-Macías et al., 2007; Rybar et al., 2004). In this study, the percentage of DNA fragmentation ranged from 0% to 8.3%, using the lysing solution with 5% ME. Although in canine sperm, a DNA fragmentation threshold indicating a reduced fertilization potential has not been established, Prinosilova et al. (2012), using SCSA, reported that all dogs with recurring fertility problems and showing poor semen quality had a DNA fragmentation index higher than that of dogs considered to be fertile (0.8% – 9.0% versus 0.3% – 4.2%, respectively). Regardless of the method (SCSA or SCD) used to assess DNA fragmentation in dog semen, it seems that this species shows a lower fragmentation than the above-mentioned species. This could be due to a greater stability of dog sperm nuclei which is reflected, for instance, in the long incubation times with dithiothreitol that semen samples require to produce chromatin denaturation in this species compared to equines and camelids (Monachesi et al., 2019).
Regarding the relationship between the different SCD patterns and routine sperm characteristics, no correlation was observed in this study. Prinosilova et al. (2012) reported that the SCSA DNA fragmentation index in raw dog semen negatively correlated with the total sperm count (r = −0.564, p < .01), the percentage of total and progressive sperm motility (r = −0.285 and −0.299, respectively, p < .05), sperm viability (r = −0.297, p < .05) and the percentage of morphologically normal spermatozoa (r = −0.284, p < .05). Moreover, Urbano et al. (2013), similar to the results observed in this study, reported no correlation between the SCD DNA fragmentation index and total and progressive sperm motility in frozen-thawed dog semen (r = −0.13, p = .63 and r = −0.12, p = .65; respectively). However, these same authors reported that the percentage of sperm with normal morphology (evaluated on slides stained with Diff-Quick®) negatively correlated with the percentage of sperm with fragmented DNA (r = −0.74; p = .001) and this was contrary to our results. In this particular case, the difference in results could perhaps be attributed to the type of sample evaluated in each study (raw semen in the current study and frozen-thawed samples in Urbano´s study) as freeze-thawing exposes sperm to further stress and DNA damage. In this study, we did not observe a correlation between the degree of DNA fragmentation and sperm head abnormalities either, while other studies in canine semen report a correlation between altered DNA and different parameters of head shape using a computerized morphometric analysis (Núñez-Martinez et al., 2005; Lange-Consiglio et al., 2010; Urbano et al., 2017). The lack of association between DNA damage and morphologically abnormal sperm heads observed in this study could be explained by the fact that sperm head morphology was not evaluated using morphometry, as in other reports, the Casarett stain was only used to evaluate general morphology. Regarding the rest of sperm morphological abnormalities, only the percentage of sperm with coiled tails, an anomaly related to abnormal spermatogenesis, showed a positive correlation with DNA fragmentation. Coiled tails are considered a primary morphological alteration; hence, their presence could be indicating a defect of some sort during spermatogenesis that, although not evidenced by an alteration of the morphology of the sperm heads, is still affecting the formation of sperm and which could include chromatin damage. It would be interesting to increase the number of animals studied, to ascertain if the general lack of correlation with routine semen parameters is maintained. It would also be worthwhile to include animals with known problems of fertility, as it would possibly increase the probability of finding an association between DNA fragmentation and other semen parameters in these animals.
5 CONCLUSIONS
It was possible to adapt the SCD protocol to evaluate DNA fragmentation in raw dog semen without the use of a commercial kit and to determine the different DNA dispersion patterns. In addition, incubation of canine semen with NaOH (0.3 M) for 15 min at room temperature proved to be useful as a DNA fragmentation control for this technique. An association was observed between the percentages of sperm with fragmented DNA and those of sperm with coiled tails. The SCD test is simple, inexpensive and can provide evidence of sperm DNA alterations that cannot be observed in the routine sperm tests, suggesting it would be a beneficial adjunct to routine seminal assessment in this species.
AUTHOR CONTRIBUTIONS
NEM: performed the study and wrote the manuscript. MFG: collaborated in data analysis and in writing the manuscript. DMN: critically revised the manuscript and discussed the results. MIC: designed the study, collaborated in data analysis and critically revised the manuscript.
ACKNOWLEDGEMENT
This research was endorsed by grants from Universidad de Buenos Aires (UBACyT 20720190200004BA and 20020190200084BA).
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
Open Research
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.




