Evaluation of different hormonal treatments on oestral and ovarian responses in Moroccan Beni Arouss goats during anoestrus and breeding season
Abstract
The efficacy of eight combinations of fluorogestone acetate (FGA, 20 or 40 mg as intravaginal device during 11 days), equine chorionic gonadotropin (eCG, 300 or 500 UI injected 48 hr before FGA removal) and prostaglandin F2α (cloprostenol, 0 or 50 μg injected 48 hr before FGA removal) aiming at induction and synchronization of oestrus and ovulation was evaluated during the anoestrus season in spring and during the breeding season in autumn in adult Beni Arouss goats. Oestrous behaviour was recorded between 12 and 60 hr after FGA removal. Blood samplings allowing to assess onset of the pre-ovulatory LH surge and increase of progesterone as sign of an active corpus luteum were performed, respectively, between 20 and 60 hr and 3, 5, 8 and 15 days after FGA removal. No season-related differences (spring vs. autumn) were observed for oestrous response (95% vs. 93%), pre-ovulatory LH surge (94% vs. 84%) and luteal response after 3–8 and 11–15 days post-treatment (respectively 92% vs. 66% and 92% vs. 98%). The onset of oestrus (21 [13–53] vs. 32 [12–54] hr) and LH surge (26 [20–60] vs. 38 [22–60] hr) occurred significantly later in autumn. FGA (40 vs. 20 mg) in autumn significantly delayed the onset of oestrus (36 [16–54] vs. 23 [12–47] hr) and LH surge (44 [26–58] vs. 33 [22–60] hr). Significant treatment-related differences were recorded for onset of LH surge (earliest for 20 mg FGA, 300 IU eCG, 50 μg PGF2α) and onset of luteal phase (latest for 40 mg FGA, 300 IU eCG, 50 μg PGF2α). In conclusion, the hormone combinations tested appeared equally effective in terms of oestrous and ovulation rates. Season has influenced significantly the onset of oestrus and LH surge, and the high dose regimen of FGA delayed the ovarian response in autumn.
1 INTRODUCTION
In the Northern of Morocco, goat farming despite its low productivity contributes at 70% to rural population incomes (Chentouf, Bister, & Boulanouar, 2011) and therefore plays an important socio-economic role. To improve livestock farms productivity and hence goat producers' income, a genetic improvement programme of local goats is highly needed.
Beni Arouss is an indigenous North Moroccan goat breed, recently recognized by Moroccan Ministry of Agriculture (official Journal of Kingdom of Morocco; No. 6,430; 01/2016) whose name was derived from the geographical location. This breed is characterized by a good milk production performance (El Otmani, Hilal, & Chentouf, 2014) and an excellent adaptation to local conditions and resistance to pests and diseases. Presently, this breed is under a breeding programme aiming to preserve and improve its production potential. Artificial insemination is critical to support this programme by accelerating the identification of superior bucks at younger age and dissemination of the genetic progress.
The seasonality of reproduction in Beni Arouss goats impacts negatively on productivity and consequently on the management of animal products availability (Chentouf et al., 2011). The breeding season begins approximately in July and peaks from September to December. A seasonal anoestrus was recorded from April to June (Chentouf et al., 2011). In order to control reproduction and to accompany the goat breeding programme in this region, the development of hormonal protocols to induce and synchronize oestrus during anoestrus season but also during the breeding season is necessary. Indeed, no protocol adapted to the local goat breeds is available.
The routine protocol used for induction and/or synchronization of oestrus in goats is 11 days of treatment with intravaginal progesterone sponges impregnated with fluorogestone acetate (FGA) and intramuscular injection of equine chorionic gonadotropin (eCG) and prostaglandin (PGF2α) or its analogues 48 hr prior to sponge removal (Baril, Remy, Vallet, & Beckers, 1992; Freitas, Baril, Bosc, & Saumande, 1996a; Freitas Baril, & Saumande, 1996b, 1997; Leboeuf et al., 2003; Leboeuf, Restall, & Salamon, 2000). This protocol is effective during both seasons. Two doses of FGA that are commonly used for synchronization and/or induction of oestrus in goats are 20 and 40 mg of FGA. In their study, Leboeuf et al. (2003) found that both dose regimens are equally effective for the induction of oestrus, ovulation and fertility, leading to the use of the lowest dose. Beni Arouss goat has a little size with an average adult body weight of 37.5 kg (Hilal, Otmani, Chentouf, & Boujenane, 2013) and an average milk production of 0.5 kg per day (El Otmani, Hilal, & Chentouf, 2013). Depending on milk production and season, Leboeuf et al. (2000) recommended for French breeds a dose of eCG varying from 400 to 600 IU in combination with 50 µg of cloprostenol. Up to now, the most appropriate treatment combination for Beni Arouss goats is still unknown.
The objective of this study was to compare eight treatment combinations of FGA, eCG and PGF2α used in spring and autumn in Beni Arouss goats. Prevalence and time point of onset of oestrous behaviour, pre-ovulatory LH (luteinising hormone) surge and luteal phase after treatment were used as parameters to compare treatments efficiency.
2 MATERIALS AND METHODS
2.1 Animals and management
The study was carried out at the experimental station of INRA, Regional Center of Tangier, located at the North of Morocco (latitude 35°44′N, longitude 5°54′O), during the period of anoestrus (spring) and during the breeding season (autumn).
In both studies, the goats were maintained in a semi-intensive system under natural photoperiod. Oat hay and concentrate feed mixture were distributed once a day according to the maintenance requirements (Jarrige, 1988) with water and mineral salt available ad libitum. All animal procedures were approved by the Animal Ethics Committee at INRA.
2.2 Study design and treatments
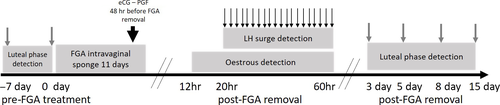
This first part of the study was conducted during the month of April, characterized as anoestrus season in Beni Arouss goat (Chentouf et al., 2011). The second part of the study was performed during November of the same year. The study design as depicted in Figure 1 was used at both time points, and the number of goats enrolled for each hormonal treatment combination is shown in Table 1. A total of 64 (in spring) and 58 (in autumn) non-lactating adult Beni Arouss goats were randomly assigned to eight treatments according to their age (5.0 ± 1.0 years) and body weight (30.0 ± 4.1 kg). Treatment combinations were based on dose of FGA (40 and 20 mg applied as vaginal sponge during 11 days; Chronogest LC®, Intervet, France), eCG dose (300 or 500 IU administered intramuscularly 48 hr before FGA sponge removal; Synchro-part, Ceva) and use or non-use of PGF2α (0 or 50 μg administered intramuscularly 48 hr before FGA sponge removal; Estrumate®, MSD animal Health, Morocco).
| Season | FGA | eCG | PGF2α | Animals planned per group |
|---|---|---|---|---|
| Spring (n = 64) | 40 mg | 300 IU | 0 µg | 8 |
| 50 µg | 8 | |||
| 500 IU | 0 µg | 8 | ||
| 50 µg | 8 | |||
| 20 mg | 300 IU | 0 µg | 8 | |
| 50 µg | 8 | |||
| 500 IU | 0 µg | 8 | ||
| 50 µg | 8 | |||
| Autumn (n = 58) | 40 mg | 300 IU | 0 µg | 7 |
| 50 µg | 7 | |||
| 500 IU | 0 µg | 7 | ||
| 50 µg | 8 | |||
| 20 mg | 300 IU | 0 µg | 7 | |
| 50 µg | 7 | |||
| 500 IU | 0 µg | 7 | ||
| 50 µg | 8 |
- Abbreviations: eCG, equine chorionic gonadotropin; FGA, fluorogestone acetate; PGF2α, prostaglandin F2α.
2.3 Blood samples and progesterone assays
Blood samples were collected from all goats via the jugular vein using 9-ml heparinized vacutainer tubes. To establish the reproductive status of goats, blood samples were collected 7 days before sponge insertion, the day of sponge insertion and 3, 8, 11 and 15 days after sponge removal. Plasma was separated by centrifugation at 800 g for 20 min, transferred into 1.5 ml microcentrifuge tubes and stored at −20°C until analysis. Progesterone (P4) concentrations were measured in duplicate using a commercial ELISA kit (Human DS-EIA-Steroid-Progesterone, DSI). The intra- and inter-assay coefficients of variation were 3% and 9.4%, respectively. Goats were in luteal phase when their plasma progesterone concentration was higher than 2 ng/ml at two successive blood samplings. They were in anoestrus if the samples performed at −7 and 0 days before FGA treatment revealed progesterone concentrations below 2 ng/ml.
2.4 Oestrous detection
In both seasons and in each group, the occurrence of behavioural oestrous signs was monitored between 12 and 60 hr after FGA sponge removal by using one buck per treatment group equipped with a plastic apron and a colour harness. Animals were observed by two same investigators at 2-hr intervals. The goats were in oestrus only if they stood while be mounted by the bucks and displayed colour marks left by the bucks' harness.
2.5 Pre-ovulatory LH surge
Blood samples were collected by jugular venipuncture every 2 hr from 20–60 hr after sponge removal. Plasma was separated by centrifugation (800 g for 20 min) and stored at −20°C until use. LH concentrations were determined in duplicate samples using a commercial ELISA kit (LH Detect, Repropharm, INRA). The intra- and inter-assay coefficients of variation were 4.3% and 4.4%, respectively. Onset of pre-ovulatory LH surge was determined as the time of the maximum LH concentration with at least a fivefold amount of the basal LH concentration.
2.6 Statistical analysis
Based on plasma progesterone analysis performed on samples collected prior to hormonal treatment (−7 and 0 days before FGA treatment), goats displaying ovarian activity in spring (n = 1) and goats displaying the absence of ovarian activity in autumn (n = 14) were excluded from the data set. The number and proportion of animals within each season and each treatment group displaying signs of oestrus, a pre-ovulatory LH surge and onset of a luteal phase after 3–8 and after 11–15 days after FGA sponge removal were calculated. The interval between FGA sponge removal and onset of oestrus and LH surge was recorded for each animal and expressed as medians with their minimum and maximum in function of season and dose regimens.
Descriptive statistics for all the data were calculated. The Shapiro–Wilk test was used to verify the data normality. Non-normal quantitative data were analysed by means of the Kruskal–Wallis variance analysis with season or treatment as main effects. Medians are compared by Wilcoxon sum rank test. Frequency data were assessed by Fisher's exact test.
For all tests, the R statistical software (version 3.5.1) was used. The level of significance was set at p < 0.05.
3 RESULTS
Results of season- and treatment-related differences for oestrous response, pre-ovulatory LH surge and onset of luteal phase are shown in Table 2. No season-related differences (spring vs. autumn) were recorded for oestrous response (95% vs. 93%, p > 0.05), pre-ovulatory LH surge (94% vs. 84%, p > 0.05) and luteal response after 3–8 and after 11–15 days after treatment (respectively 92% vs. 66% and 92% vs. 98%; p > 0.05). Treatment-related differences were observed in autumn during the breeding season where the percentage of goats displaying a luteal phase within 3–8 days after sponge removal was significantly reduced after the 40 mg FGA, 300 IU eCG and 50 µg PGF2α treatment as compared with 20 mg FGA and 300 or 500 IU eCG treatment (p < 0.05). All treatments including the 40 mg FGA dose regimen used in autumn tended to a delayed onset of the luteal phase. Although non-significant, the absence of PGF2α treatment in autumn further tended to decrease the rate of LH surges occurring within 60 hr after FGA sponge removal (Table 2).
| Season | FGA | eCG | PGF2α | Animals per group | Oestrus detected | LH surge | Luteal phase after 3–8 days | Luteal phase after 11–15 days |
|---|---|---|---|---|---|---|---|---|
| Spring (n = 64) | 40 mg | 300 IU | 0 µg | 8 | 100% | 100% | 100% | 100% |
| 50 µg | 8 | 100% | 100% | 100% | 100% | |||
| 500 IU | 0 µg | 8 | 88% | 88% | 100% | 100% | ||
| 50 µg | 7 | 86% | 71% | 71% | 71% | |||
| 20 mg | 300 IU | 0 µg | 8 | 100% | 100% | 88% | 88% | |
| 50 µg | 8 | 88% | 88% | 88% | 88% | |||
| 500 IU | 0 µg | 8 | 100% | 100% | 100% | 100% | ||
| 50 µg | 8 | 100% | 100% | 88% | 88% | |||
| Autumn (n = 58) | 40 mg | 300 IU | 0 µg | 5 | 80% | 60% | 40% ab | 80% |
| 50 µg | 5 | 100% | 100% | 20% b | 100% | |||
| 500 IU | 0 µg | 6 | 83% | 67% | 50% ab | 100% | ||
| 50 µg | 5 | 100% | 100% | 40% ab | 100% | |||
| 20 mg | 300 IU | 0 µg | 6 | 100% | 83% | 100% a | 100% | |
| 50 µg | 5 | 100% | 100% | 80% ab | 100% | |||
| 500 IU | 0 µg | 6 | 83% | 67% | 100% a | 100% | ||
| 50 µg | 6 | 100% | 100% | 83% ab | 100% |
- Note: Data are shown as absolute numbers and proportions of responding goats. Data of a same column with different letters significantly differ (p < 0.05).
- Abbreviations: eCG, equine chorionic gonadotropin; FGA, fluorogestone acetate; PGF2α, prostaglandin F2α.
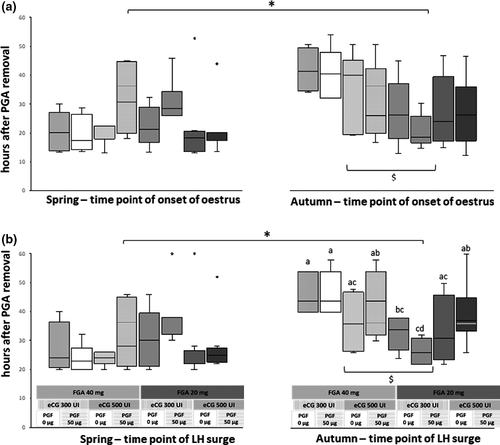
Figure 2a and 2b shows the onset of oestrus and LH surge in spring and autumn and in function of each treatment regimen. Oestrus occurred significantly later in autumn than in spring (32 [12–54] vs. 21 hr [13–53]; p < 0.05), as well as LH surge (38 [22–60] vs. 26 [20–60] hr; p < 0.05). FGA doses of 40 mg (vs. 20 mg) used in autumn significantly delayed the onset of oestrus (36 [16–54] vs. 23 [12–47] hr; p < 0.05) and LH surge (44 [26–58] vs. 33 [22–60] hr; p < 0.05). A significant treatment-related difference was recorded for onset of LH surge (earliest for 20 mg FGA, 300 IU eCG, 50 μg PGF2α) and onset of luteal phase (latest for 40 mg FGA, 300 IU eCG, 50 μg PGF2α).
4 DISCUSSION
In small ruminants, oestrous induction and/or synchronization protocols are used for genetic improvement and to manage reproductive activity over the year. Beside the efficiency in terms of ovulation rate, the variability of the time points of onset of oestrus, LH surge and development of corpus luteum are important parameters for AI protocols. Indeed, the ideal time point for single or repeated IA is predetermined without a need for oestrous detection (Leboeuf et al., 2000; Menchaca & Rubianes, 2004).
The present study comes up with original results allowing to characterize (a) the induction rates of oestrus, LH surge and corpus luteum and (b) the variability of onset of oestrus, LH surge and corpus luteum in function of the season (spring vs. autumn) after eight FGA-eCG-PGF2α treatment combinations in Beni Arouss goats. Before discussing the major findings of this study, some drawbacks need to be addressed: as several goats did not respond to the inclusion criteria of seasonal anoestrus in spring (i.e. the absence of luteal phase prior FGA treatment) or cyclicity in autumn (i.e. the presence of luteal phase prior FGA treatment), the group sizes decreased, thereby reducing somewhat the statistical power. Another point concerns the animals used during spring and autumn: around 60% of the goats were used at both occasions, whereas others had been replaced by new animals. Consequently, a pairwise evaluation of treatment efficiency could not be performed.
The eight treatments used in this study were found equally effective in inducing oestrus in Beni Arouss goats during the anoestrus and the breeding season. Within 60 hr after sponge removal, there were no significant differences between treatment groups in terms of oestrous response (Table 2). During anoestrus and the breeding season, a high oestrous rate was observed following sponge removal (95% and 93%, respectively). This was within the range of 82%–100% reported in studies using treatments with FGA or MAP intravaginal sponges maintained for 9, 11 or 14 days plus an intramuscular injection of eCG and PGF 48, 24 or 0 hr prior to sponge withdrawal (Freitas, Baril, & Saumande, 1996b; Leboeuf et al., 2003; Amarantidis, Karagiannidis, Saratsis, & Brikas, 2004; Dogan, Nur, Gunay, Soylu, & Sonmez, 2004; Dogan et al., 2005; Fonseca & Torres, 2005; Modu-Bukar, Yusoff, & Haron, 2012; Omontese et al., 2013; Pietroski, Brandao, Souza, & Fonseca, 2013; Dogan, Nur, & Dogan, 2016), or only using intravaginal sponges with eCG (Amarantidis et al., 2004; Blaszczyk, Udala, & Gaczarzewicz, 2004; Lohloenya, Greyling, & Schwalbach, 2005; Montlomelo, Greyling, & Schwalbach, 2002). During anoestrus, the overall oestrous response reported in our study was higher than what is reported when this protocol was applied to synchronize oestrus in the North African Maure goat in Tunisia (75%) (Rekik, Ben Othmane, Lassoued, & Sakly, 2014).
During anoestrus, there are no significant differences in onset of oestrus between groups. The oestrous response occurred between 13 and 53 hr after sponge removal (median value: 21 hr), with the highest percentage of females in oestrus observed between 13 and 44 hr (97%). The results of our trial showed a poor agreement with previous findings of Zarazaga, Gatica, Gallego-Calvo, Celi, and Guzman (2014), where the onset of oestrus occurred after 24 ± 0.4 hr. Leboeuf et al. (2003) reported that the oestrus occurred between 18 and 30 hr after using sponge impregnated with 20 or 40 mg FGA combined with 500 IU of eCG and 50 µg of cloprostenol. However, a similar range of oestrous onset (20–44 hr) was reported by Freitas, Baril, and Saumande (1996b) using sponges impregnated with 40 mg FGA combined with 500 IU of eCG and 50 µg of cloprostenol. Variations in oestrous response as observed in this study compared with previous reports could be due to differences in breed, location, nutrition, age, parity and management (Simoes et al., 2008; Whitley & Jackson, 2004; Wildeus, 2000). It can be hypothesized that seasonal anoestrus is less pronounced in does kept under optimal feeding and housing conditions than under field conditions.
The results of onset of oestrus recorded during the breeding season showed no significant differences between treatment groups and are in line with previous studies reporting onset of oestrus between 18 and 60 hr (Dogan et al., 2016), 18 and 66 hr (Dogan et al., 2005), 18–30 hr (Leboeuf et al., 2003) and around 29.4 (±1.4) hr (Zarazaga et al., 2014). However, a FGA dose effect was found: onset of oestrus was significantly delayed when treatment combinations including 40 mg of FGA were used (Figure 2a). Similar results were reported by other authors (Crosby, Boland, & Gordon, 1991; Greyling, Erasmus, Taylor, & Merwe, 1997). Moreover, the onset of oestrus after sponge removal in goats investigated in autumn during their breeding season was significantly delayed when compared to oestrous onset recorded in spring during the period of anoestrus. The most likely explanation for this difference is an increased progesterone-mediated negative feedback of the hypothalamic–pituitary–ovarian axis leading to a delayed onset of oestrus after FGA withdrawal in autumn (Freitas, Baril, Bosc, et al., 1996a). This negative feedback seems further enhanced by 40 mg FGA sponges.
During both seasons, a high pre-ovulatory LH surge response was recorded (94% and 84%, respectively, in anoestrus and breeding season) regardless of the regimen treatment. During the anoestrus season, the overall percentage of females displaying LH surge reported was similar to that recorded in dairy goats treated, during both seasons, with the same protocol (Leboeuf et al., 2003). By the use of 20 mg FGA, 450 IU of eCG and 6 mg of luprostiol, Zarazaga et al. (2014) found that all the goats treated during anoestrus and in the breeding season displayed a pre-ovulatory LH surge. Although non-significant, a reduced rate of LH surges observed within 60 hr after FGA sponge removal was recorded in goats that did not received PGF2α during their reproduction period in autumn (Table 2). As almost all goats developed later a corpus luteum, it is likely that LH surges in goats without PGF2α treatment occurred after the 60-hr sampling period. Previous studies reported that prostaglandin is effective during the breeding season as a synchronizing agent responsible for luteolysis after priming with FGA (Amarantidis et al., 2004; Greyling & Van, 1991; Romano, 1996).
In the present study, the interval from sponge removal to the pre-ovulatory LH surge was ranged from 20–60 hr in most animals. During the period of anoestrus, LH surge occurred significantly earlier than during autumn (Figure 2b). Our results are in line with those reported by Zarazaga et al. (2014) in Blanca Andaluza goats where the interval between 20 mg FGA sponge removal and pre-ovulatory LH surge was of 29.3 hr (±0.6) during anoestrus versus 35.9 hr (±1.8) during the breeding season. No effect of regimen treatment was evidenced in spring (Figure 2b), but as for onset of oestrus, high dose FGA sponges (40 mg) led to a delayed LH surge in autumn (Figure 2b). These observations are in agreement with earlier studies (Leboeuf et al., 2003) and in line with the above-mentioned progesterone-mediated negative feedback. Further significant differences between treatments performed in autumn were found. Even these results warrant confirmation by enrolling a larger number of goats, it is worth to mention that the 20 mg FGA, 300 IU eCG and 50 µg PGF2α treatment induced the most rapid and best synchronized LH surge (occurring between 22 and 32 hr after sponge removal) (Figure 2b).
Onset of a functional corpus luteum seemed delayed during the breeding period, and an FGA dose effect was detected as for onset of oestrus and LH surge (Table 2). Although non-significant, the percentage of goats that did not develop corpus luteum was slightly lower (92% vs. 98%) in spring than in autumn. This absence of ovulation (despite a LH surge) may be related to insufficient luteinizing hormone released by the pituitary, a poor ovarian response to eCG or individual variation in responsiveness to eCG by goats (Rubianes & Menchaca, 2003).
5 CONCLUSION
In conclusion, the hormone combinations used in the present study were equally efficient in inducing oestrus and ovulation in Beni Arouss goats during the anoestrus and in the breeding season. The 20 mg FGA, 300 IU eCG and 50 μg PGF2α treatment induced the best synchronized ovulation in both seasons. Season-related effect and FGA dose-related effect lead to a delayed ovarian response in autumn. No eCG dose effect was evidenced, whereas PGF2α should be recommended when oestrous induction is performed during the reproduction period.
ACKNOWLEDGEMENTS
This research was supported by the Belgian Academy for Research and Higher Education- Development Cooperation Committee (ARES-CCD). It was conducted at INRA, Regional Center of Tangier. The authors thank staff of this centre for their assistance with animal handling and care during experimentations.
CONFLICT OF INTEREST
The authors confirm that there are no conflicts of interests.
AUTHOR CONTRIBUTIONS
The designed study was performed by Sara El Kadili, Mouad Chentouf, Nathalie Kirschvink, Marianne Raes and Jean-Loup Bister. In the experimental period, the samples were collected by Sara El Kadili. The results were analysed by Sara El Kadili, Nathalie Kirschvink, Mouad Chentouf, Marianne Raes, Jean-Loup Bister, Jean-François Beckers, Gaston Amzati and Bouchaib Archa. The manuscript was drafted by Sara El Kadili. All authors are contributed to revising and reviewing the manuscript.




