Effect of environmental factors and changes in the body condition score on the onset of the breeding season in mares
Data Availability Statement: The data supporting the findings of this study are available from the corresponding author on request.
Abstract
Several methods have been proposed to advance the onset of the breeding season in horses. Most of them are based on the exposure to an artificial lighting period combined with hormonal treatments. Mares exposed to an artificial photoperiod are most often housed indoors where the ambient temperature is often higher than the outside temperature. Mares held in barns are also exposed to different daylight intensities than horses kept outside, depending on the architecture. In the current study, we evaluated the impact of ambient temperature, daylight intensity and changes in body condition score (BCS) on the timing of first ovulation after winter anestrus in mares exposed to an artificial photoperiod. Mares (n = 211) were housed in barns with different ambient temperature and daylight exposure but with the same artificial photoperiod exposure (except for a natural photoperiod control group). Artificial photoperiod as well as an increase in BCS over the winter significantly advanced the first spring ovulation. The BCS at the start and end of the anestrus period did not have an effect on the interval to first ovulation and neither did the modest increase in ambient temperature in the barn. However, a higher light intensity during the daytime significantly advanced the first spring ovulation. The results of this study suggest that exposure to more sunlight advances the onset of the breeding season. This effect is likely mediated through the biological effect of short wavelength blue light and its impact on melatonin suppression and biological rhythms. We suggest that greater/direct exposure to the blue light component of daylight improves the response to the artificial photoperiod. The results of the present study can further assist to optimize the conditions that lead to an efficient spring transition of breeding mares.
1 INTRODUCTION
The horse (Equus caballus) is a seasonal polyestrous breeder. During the winter, approximately 85% of warmblood and thoroughbred mares and over 90% of pony mares will cease normal cyclic activity and enter an anestrus period of variable length (Ginther, 1974; McKinnon, Squires, Vaala, & Varner, 2011; Nagy, Guillaume, & Daels, 2000). In mares, the circannual rhythm of reproduction is mainly regulated by photoperiodic changes with the increase in day length preceding the onset of the breeding season (Driancourt, Prunier, Palmer, & Mariana, 1983; Hart, Squires, Imel, & Nett, 1984; Nagy et al., 2000). During the anestrous period, mares typically show minimal to moderate follicular growth and the absence of periodic ovulations as a consequence of the lower gonadotropin secretion (Ginther, 1979). Melatonin is one of the main mediators in the regulation of gonadotropin-releasing hormone (GnRH) secretion. Melatonin is synthesized and secreted by the pineal gland during darkness, and its secretion ceases immediately after exposure of the melanopsin receptors in the eye to low-intensity blue light (Arendt, 1994; Brainard et al., 2001; Lerner, Case, Takahashi, Lee, & Mori, 1958; Malpaux, Migaud, Tricoire, & Chemineau, 2001; Thapan, Arendt, & Skene, 2001). The action of melatonin involves a network of neurons that relay the melatonin signalling from its target cells in the pre-mammillary region of the hypothalamus to the GnRH neurons in the pre-optic area (Malpaux et al., 2001). During winter anestrus, the extended melatonin secretion during the long periods of darkness has an inhibitory effect on GnRH secretion which leads to reduction in gonadotropin secretion, luteinizing hormone (LH) and to a lesser extent follicle-stimulating hormone (FSH) (Murphy, 2019; Nagy et al., 2000). During the spring, the increasing day length results in a shortening of the duration of melatonin secretion, thereby removing the inhibitory action on the hypothalamus and increasing GnRH pulse frequency (Cleaver et al., 1991). Once the inhibitory influences are lifted, short day length being the most important, the resumption of gonadotropin secretion results in restoration of cyclic ovarian activity (Donadeu & Watson, 2007; Ginther, 1979). Generally, as the days lengthen in the spring, temperatures also increase, and horses are turned out to graze on lush spring grass. Ambient temperature and nutrition have also been suggested as factors that influence the return to reproductive activity, but researchers agree that photoperiod is the dominant external factor that influences the circannual endogenous reproductive rhythm in horses (Ball, 2005; Carnevale, Hermenet, & Ginther, 1997; Gentry et al., 2002; Kubiak, Crawford, Squires, Wrigley, & Ward, 1987; Nagy et al., 2000; Salazar-Ortiz et al., 2011).
It has been reported that mares in good body condition are more likely to cycle year around, and mares in good body condition that enter anestrus will start reproductive activity on average 1 month earlier than mares with a lower body condition score (BCS) (Waller, Thompson, Cartmill, Storer, & Huff, 2006). Similarly, an increasing plane of nutrition or grazing on lush green spring grass has been associated with an earlier return to reproductive activity (Carnevale et al., 1997; Fitzgerald, Reedy, Sessions, Powell, & McManus, 2002; Van Niekerk & Van Heerden, 1972). Similar to the body condition, the ambient temperature is also considered by several authors as a modulating factor during the spring transition, with warm weather during spring initiating earlier cyclicity (Allen, 1987; Guerin & Wang, 1994; Oberhaus & Paccamonti, 2013). In a study in Thoroughbred mares in the United Kingdom, it was reported that cold weather delays the spring transition (Allen, 1987). In a 10-year survey of breeding records on a Thoroughbred farm in Australia, the onset of reproductive activity was closely related to minimum and maximum environmental spring temperatures (Guerin & Wang, 1994). However, the environmental winter temperatures did not demonstrate any effect on the onset of breeding season (Cooper & Wert, 1976). Breeders and veterinarians often report that a sudden extreme drop in temperature during the spring transition will cause mares to return to anestrus (Oberhaus & Paccamonti, 2013). However, in all the aforementioned studies, there was no control group to measure the effect of ambient temperature under similar conditions of photoperiod, nutrition and/or management system. Therefore, it cannot be excluded that the presumed effect of temperature in these studies was biased by other climatic changes, such as the intensity of daylight (more sunny days during a warm spring) and quantity and quality of available food (increased growth of grass during warm spring) (Carnevale et al., 1997; Walsh, Prendergast, Sheridan, & Murphy, 2013).
Several treatment regimens have been implemented in an effort to advance the onset of the breeding season. Most of these treatments have focused on increasing the photoperiod (number of hours of light per day), and it has been repeatedly demonstrated that an artificial, long photoperiod effectively advances the time of the first ovulation in mares (Daels, 2006; Kooistra & Ginther, 1975; Malinowski, Johnson, & Scanes, 1985; Murphy et al., 2014; Oxender, Noden, & Hafs, 1977; Palmer, Driancourt, & Ortavant, 1982). To a lesser extent, various schemes of administering gonadotropins, dopamine antagonist and/or progesterone, have also been shown to stimulate an early onset of reproductive activity (return to cyclicity) during the deep anestrous season or the transitional period (Alexander & Irvine, 1991; Besognet, Hansen, & Daels, 1996; Donadeu & Thompson, 2002; Evans & Irvine, 1979; Harrison, Squires, Nett, & McKinnon, 1990; Hyland et al., 1987; McCue, Logan, & Magee, 2007; McKinnon, Vasey, Lescun, & Trigg, 1997; Panzani et al., 2011; Squires, Heesemann, Webel, Shideler, & Voss, 1983; Turner & Irvine, 1991). Manipulating environmental conditions such as nutrition and ambient temperature have also been recommended to shorten the anestrous period (Carnevale et al., 1997; Guerin & Wang, 1994). However, the effect of temperature under similar conditions of photoperiod has not yet been critically evaluated.
Climatic conditions differ from year to year and cannot be controlled. However, housing conditions can be adapted to satisfy specific requirements for temperature (increase/decrease insulation of barns), light and general comfort of the mares. Therefore, it is important to identify the optimal conditions for an efficient transition to cyclic reproductive activity. This will enable breeders to adapt their management and indoor facilities with regard to ambient temperature (insulation and ventilation), quantity/quality of light (intensity and colour of light, duration and artificial vs. natural light) and feeding (quantity, quality and stress-free access to food and water in group housing) to ensure an early onset of the breeding season. Especially in large breeding farms and embryo transfer centres where large numbers of non-pregnant recipient mares are housed over the winter in preparation for the next breeding season, it is of significant economic relevance to optimize the housing conditions in function of an earlier onset of the breeding season. Currently, there is no objective information available about the effect of barn types, insulation and ambient temperature duration/intensity/type of light, and feeding systems on the onset of breeding season in mares.
In the presented study, we have evaluated the effect of ambient temperature on the onset of ovarian activity in mares that were housed under artificial photoperiod. We hypothesized that under similar conditions of photoperiod, nutrition and management, an increase in ambient temperature might further stimulate the return to reproductive activity in mares under artificial photoperiod. We also evaluated the effect of the changes in weight and BCS on the interval to first ovulation. Last, we evaluated the effect of the intensity of light during the daytime in the barns on the interval to first ovulation.
2 MATERIALS AND METHODS
2.1 Animal care and ethics
This study was performed retrospectively based on data provided by Keros Embryo Transfer Center (Passendale, Belgium www.keros.be). Data were generated as part of routine monitoring of the recipient mare herd at the Keros Embryo Transfer Center in the context of optimization of nutrition and housing of the non-pregnant, recipient mares over the winter and clinical evaluations of recipient mares in anticipation of the breeding season. All mares were client-owned and were property of the Keros Embryo Transfer Center. No research animals were used in this study. All personnel who work with the mares to obtain the samples were veterinarians and were trained in ethical handling of the animals.
A total of 211 non-pregnant embryo recipient mares were used in this study. The median age of the mares was 10 years (range: 4–20). To minimize the effect of breed on the outcome of this experiment, only standardbred and warmblood mares were included in the study. All mares were regularly vaccinated and dewormed according to the farm protocol and had the same vaccination and deworming status at the start of the study. All mares had ad libitum access to corn silage, straw and water and received a daily ration of carrots and concentrates.
2.2 Housing and environmental factors
All mares were housed at the Keros Embryo Transfer Center. Mares were accustomed to living in groups ranging from 12 to 40 mares in closed or half-open barns. Mares were housed in six different barns (Barns 1–6) located at northern hemisphere (50°51′11.8″N 3°01′36.8″E). The groups were established at least 1 month before the start of this study and remained unchanged during the study. All barns, except Barn 5, were closed barns with half-shaded windows and ventilation mainly through the ceiling. The middle isle between pens consisted of head gates used for feeding in the central path. Barn 5 was a half-open construction consisting of one full-height wall on the longitudinal (back) side of each pen and three open sides with head gates on the outside (front) side of the barn. Feeding in Barn 5 was done outside under open sky at the front side of the pen and had a predominant south-west exposure.
Mares in Barns 1–5 were exposed to the same schedule of artificial photoperiod. The artificial photoperiod was started on December 15, continued until April 14 and consisted of overhead lighting (fluorescent tubes) during 2 hr from 3:00 a.m. to 5:00 a.m. (Palmer et al., 1982; Palmer & Guillaume, 1992). No additional light was provided during daytime. Mares in Barn 6 (closed barn) were not submitted to artificial photoperiod and were only exposed to natural photoperiod (control group for photoperiod treatment).
Since heating horse barns is not economically justifiable nor practically feasible, we were primarily interested in evaluating if insulating horse barns, with naturally increased ambient temperature (relative to the outside temperature), has an effect on the interval to first ovulation. To study the effect of the ambient temperature on the interval to first ovulation, the temperature was recorded hourly throughout the study using an automated recording system (Crijns Energy Controlling, Malden, The Netherlands). The ambient temperature was expressed as the difference between the temperature in the barns and the outside temperature (∆T° = average daily inside temperature in each barn − average daily outside temperature) and was calculated for every barn for each day of the study. The ∆T° was used to quantify the relative temperature, with higher ∆T° reflecting a higher level of insulation and better protection from the outside climatic conditions.
Light intensity was measured using a Lux Light Meter (iPhone application by Elena Polyanskaya) in all barns within an interval of less than 1 hr on the same day at midday using the same device. The light intensity was measured while standing in the middle of each pen holding the measuring device at the level of the mare's eye and aiming laterally (perpendicular to the floor) in four directions perpendicular to the walls or sides of the pen. At least eight measurements (two in each direction in each pen) were made. The average daytime light intensity was compared between the different barns.
2.3 Body weight and body score condition
Body weight and BCS were recorded on December 19 and again on February 21. The weight was estimated using the following formula: estimated weight (kg) = (heartgirth2 × body length)/(11,880 cm3) as previously described (Hall, 1971). The Henneke BCS scoring system, with a score from 1 to 9, was used with 1 having a poor BCS and 9 being extremely fat (Henneke, Potter, Kreider, & Yeates, 1983).
2.4 Monitoring ovarian activity
Blood samples were collected at 10-day intervals throughout the study. Serum progesterone concentrations were measured with an in-house ELISA assay using 3-CMO progesterone-HRP antibody (65-IP27; Fitzgerald Ind.) as the progesterone antibody (Wynn et al., 2018). The inter-assay and intra-assay coefficients of variation were 7% and 8%, respectively, and the minimal detectable concentration was 0.2 ng/ml. Mares with three consecutive serum progesterone concentrations <1 ng/ml at the beginning of the study were considered in seasonal anestrus and were included in the study. The approximate date of the first ovulation was determined based on elevated serum progesterone (>1 ng/ml) and confirmed by the presence of a corpus luteum on ultrasonography (Terblanche & Maree, 1981). For analysis, the interval to first ovulation was calculated as the number of days after January 1.
2.5 Recording and analysis of data
Data on age, parity, foaling history, body weight, BCS, barn and interval to first ovulation were imported to an Excel spreadsheet (Microsoft Corporation, Seattle, WA, USA). Statistical analyses were performed using the jmp software (JMP 12.1.0, SAS Institute, Buckinghamshire, UK). To assure the uniformity of mares in each barn, the distribution of age, parity, foaling in the previous season (yes/no), weight loss/gain and BCS loss/gain was compared between the barns using an analysis of variance test (ANOVA) and a Mann–Whitney–Wilcoxon test for parametric and non-parametric values, respectively.
Daily ∆T° was compared between the barns using ANOVA to confirm differences in ambient temperatures between locations. The effect of temperature (∆T°), body weight changes, BCS changes and barn and their interactions on the interval to first ovulation (from January 1st to the first ovulation) was evaluated using mixed model. Spearman's correlation was used to analyse the correlation between interval to first ovulation and changes in BCS. The effect of the intensity of daylight on the interval to first ovulation was evaluated by ANOVA. Significance level was set at p < 0.05.
3 RESULTS
Even distribution between groups was confirmed for age, parity, foaling in the previous year, starting weight and starting and ending BCSs (p > 0.05) (Table 1). Out of 211 mares, 177 mares (84%) were confirmed to be in anestrus based on three consecutive progesterone concentrations <1 ng/ml and were included in the study.
| Median | Min | Max | |
|---|---|---|---|
| Age | 10 | 4 | 20 |
| Parity | 2.5 | 0 | 6 |
| Weight at the start of the study | 560 | 440 | 730 |
| Weight at the end of the study | 553 | 418 | 713 |
| BCS at the start of the study | 4 | 2 | 7 |
| BCS at the end of the study | 4 | 1 | 8 |
During the entire study period, the ∆T° was significantly higher in Barn 4 (mean + 4.6°C) than in Barns 1 (+1.9°C), 2 (+1.9°C), 3 (+2.0°C), 5 (−0.5°C) and Barn 6 (+2.6) (p < 0.001) (Table 2). The ∆T° in Barns 1, 2 and 3 were similar and significantly higher than in Barn 5 (half-open barn) (p < 0.0001) (Figure 1, Table 2). The temperature in Barn 6 (under natural photoperiod) was significantly higher than in Barns 1, 2, 3 and 5 (p < 0.001). No significant effect of ∆T° on the interval to first ovulation was observed.
| Barn | Artificial photoperiod | ∆T° | Light intensity midday (Lux) | Interval to first ovulation (days) |
|---|---|---|---|---|
| Barn 1 | Yes | +1.9a | 70 ± 31a | 73 ± 5.2a,b |
| Barn 2 | Yes | +1.9a | 182 ± 14a | 83 ± 5.3a |
| Barn 3 | Yes | +2.0a | 185 ± 90a | 76 ± 3.6a,b |
| Barn 4 | Yes | +4.6b | 245 ± 8a | 69.7 ± 3.6b |
| Barn 5 | Yes | −0.5c | 1,891 ± 595b | 58 ± 5.3c |
| Barn 6 | No | +2.6b | 160 ± 30a | 132.5 ± 13.4d |
Note
- Different superscript indicates significant differences between the values within the same column.
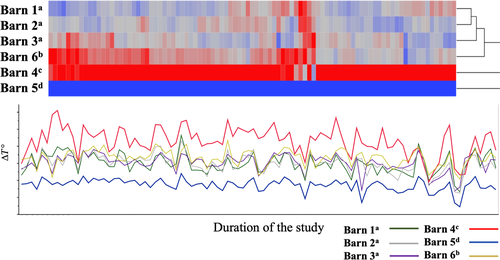
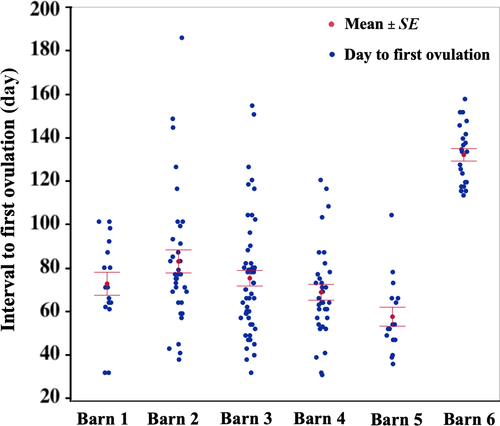
Mares under artificial photoperiod (Barns 1–5) had a significantly shorter interval to first ovulation than mares kept under natural photoperiod (Barn 6) (74.4 ± 25.9 days and 132.5 ± 13.4 days; mean ± standard error, respectively, p < 0.001) (Figure 2, Table 2). Mares in Barn 5 (with the lowest ambient temperature; ∆T° = −0.5°C) ovulated significantly earlier (58 ± 4.3 days) than all other groups, with intervals to ovulation for mares in Barns 1, 2, 3 and 4 being 73 ± 5.2, 83 ± 5.3, 76 ± 3.6 and 69.7 ± 3.6 days [mean ± standard error], respectively (Figure 2). Mares in Barn 4 (highest ∆T°) ovulated significantly earlier than mares in Barn 2 (p = 0.03). There was no significant difference in the duration of anestrus between other barns.
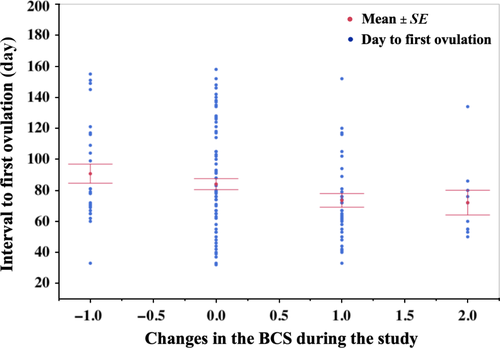
The BCS at the start and end of the experiment had no effect on the interval to first ovulation. However, changes in BCS during the study had an effect on the interval to first ovulation (p = 0.001) and were negatively correlated, with mares that had an increase in BCS ovulating earlier than mares with a constant or decreasing BCS (p = −3.0, p = 0.002) (Figure 3). No difference was detected in the change in BCS between the barns. Moreover, there was no effect of the body weight at start or the end of the experiment nor of changes in weight on the interval to first ovulation.
To identify other possible factors that may influence the interval to first ovulation, we measured the light intensity at midday in each barn (Table 2). Daylight intensity in Barns 1–4 was similar, and light intensity was significantly higher in Barn 5 (1,891 ± 595 Lux) compared to Barns 1–4 (171 ± 74 Lux) (p = 0.0006).
4 DISCUSSION
The aim of present study was to evaluate the effect of ambient temperature on the onset of first ovulation in the spring. In order to separate the effect of ambient temperature from other possible influences, mares were housed under strictly similar conditions of photoperiod, nutrition and management. In addition, we also analysed the potential impact of changes in BCS over the winter and the effect of exposure to natural daylight during daytime on the interval to first ovulation.
In previous studies, it has been repeatedly demonstrated that artificial photoperiod is the dominant factor in stimulating the early onset of reproductive activity in the spring (Daels, 2006; Kooistra & Ginther, 1975; Oxender et al., 1977; Palmer et al., 1982). In this study, we confirmed the effect of artificial photoperiod treatment in mares kept under artificial photoperiod. Mares placed under a light schedule of natural daylight during daytime supplemented with two hours (2 hr) of light approximately 9 hr after dusk ovulated significantly earlier than control mares kept under natural photoperiod. In fact, the mean interval to first ovulation of all mares under artificial photoperiod was 58 days shorter than for mares under natural photoperiod as has been previously reported (Palmer et al., 1982).
In the literature, there are ample references describing the role of additional factors that can modulate the response of the mare to photoperiodic manipulation. The proposed factors include, but are likely not limited to, ambient temperature, availability and quality of food, age, breed, lactation, body condition, contact with other (cyclic) mares and stallions and stress (Carnevale et al., 1997; Guerin & Wang, 1994). Several studies have attributed a role to BCS in the regulation of reproductive efficiency. Ginther reported that mares that gained weight during the winter had a shorter interval to first ovulation than mares that lost weight (Ginther, 1974). Also, maiden mares with a BCS ≤ 4 tend to ovulate 3–4 weeks later in the spring than mares with a BCS ≥ 5. Others have reported that body condition also appears to modulate the effect of photoperiod, and good BCS has been reported to have a positive effect on the early onset of the breeding season (Henneke, Potter, & Kreider, 1984; Kubiak et al., 1987; Salazar-Ortiz et al., 2011; Vecchi et al., 2010). Ball (2005) reported that mares in poor body condition (BCS < 5/9) have a longer interval to first ovulation than mares with higher body condition (Ball, 2005). It has been reported that a high-energy intake shortened the interval to first ovulation in mares with a poor BCS but did not in mares with a moderate or fat BCS (Kubiak et al., 1987). In the present study, the vast majority of the mares had a fair to good BCS at the beginning and end of the experiment, and therefore, an effect of poor BCS at those two time-points on the interval to first ovulation could not be demonstrated. The mares in the current study were housed inside in group pens. Although the management during the winter was aimed at avoiding changes in BCS, we did see fluctuations (up and down) of BCS between individuals. Changes in BCS were negatively correlated with the interval to first ovulation with those mares that improved their BCS during the winter ovulating earlier. These observations are in accordance with previous reports on the role of body condition and changes in body condition on the onset to cyclicity in the spring (Ginther, 1974; Salazar-Ortiz et al., 2011). Ginther reported that mares that gain weight during the winter have a shorter interval to first ovulation then mares that lose weight (Ginther, 1974). Salazar-Ortiz et al. concluded that the annual rhythm driven by melatonin secretion is primarily responsible for the timing of the breeding season, whereas the occurrence and length of winter ovarian inactivity are defined by metabolic hormones such as GH (growth hormone), IGF-1 and leptin that are regulated by food-intake and body condition. These observations highlight once again the importance of high-quality nutrition and good feeding systems that allow equal access to food for all animals in the group.
In the present study, we calculated the differences between ambient temperature inside the barn and the outside temperature as a measurement of comfort. We evaluated the effect of these differences in ambient temperature on the onset of ovarian activity. In contrast to our starting hypotheses, we were not able to confirm that increased ambient temperature results in an earlier onset of the breeding season. Our observations are in contrast with what has been previously reported by Guerin and Wang (1994) who reported a negative correlation (r = 0.67; p < 0.01) between the mean weekly minimum (outside) temperature and the first spring ovulation (Guerin & Wang, 1994). But the observations of these authors were based on anecdotal environmental conditions which appeared to coincide with an early onset of breeding season. Minimum and maximum environmental spring temperatures were closely related to onset of reproductive activity (Guerin & Wang, 1994). Undoubtedly, these climatic conditions coincided with changes in other potential influencing factors such as availability of green grass and bright sunlight, and thus, it is difficult in this study to separate the role of each of these factors. It is also noteworthy to mention that in this study the temperature differences were on average about 5.1°C between the warmest and coldest barns. Therefore, it cannot be excluded that higher ambient temperatures or larger differences between ambient and outside temperatures (than what was recorded in the current study) might have influenced the onset of the breeding season.
It was intriguing to us that the mares in the barn with the coldest ambient temperatures (Barn 5) had the shortest interval to first ovulation. This difference could not be attributed to a disproportionate number of mares with an increase in BCS or an exceptionally high BCS at the start or end of the study. Further examination of the environmental conditions in Barn 5 revealed a significant higher light intensity during the daytime (due to exposure to direct sunlight) compared with all the other barns. In fact, the mares that were housed in this “cold”, open-front location (Barn 5) had the highest exposure to direct sunlight, whereas the mares in the other barns were exposed to indirect light through partially opaque windows and roof panels. We were able to demonstrate a significant relationship between intensity of light during the daytime and interval to first ovulation. This observation suggests that open barns, in which mares are more exposed to direct sunlight, might be beneficial for an early onset of reproductive activity, even though they have lower ambient temperature and are more exposed to weather conditions than closed barns.
In recent studies on the impact of circadian rhythm on athletic performance in racehorses, researchers reported that both the quantity and quality of light during the natural daytime has a significant impact on the body composition and on the circadian clock gene expression in horses in training (Murphy & O'Brien, 2018). The authors suggested that exposure of young horses in training to additional bright white LED light during the daytime has a positive impact on their metabolism, resulting in a more efficient development of muscle mass during training. In a second experiment, they observed that supplemental short wavelength (blue) LED lights added to the white LED light induced significant changes in gene expression. It was suggested that changes in body composition may occur as a result of strengthened circadian rhythmicity of peripheral clocks and better internal synchronization that were the result of the abundance of (blue) light during daytime (Murphy & O'Brien, 2018). Moreover, in recent years, it has been demonstrated that blue light is the essential signal for entrainment of circadian rhythm in mares (Murphy et al., 2014; Nolan et al., 2017; Walsh et al., 2013, 2014). Manipulation of the photoperiod using artificial blue light results in an earlier onset of cyclicity in the spring, reduced duration of pregnancy, increased birthweight of foals and changes in hair coat at birth (Murphy et al., 2014; Nolan et al., 2017). The spectral qualities of different light sources (incandescent light, fluorescent light and direct natural sunlight) are significantly different from each other with natural daylight having the highest proportion of biologically effective short wavelength blue light (Murphy, 2019). We hypothesize that in our experimental groups, the exposure to high intensity, direct sunlight (Barn 5), presumably with the highest proportion of short wavelength blue light, during the daytime (as opposed to indirect light) further strengthened the perception of the light/dark cycle resulting in a more robust response to the artificial photoperiod to which the mares were exposed. Further studies will be needed to confirm the role of daytime lighting conditions in the response to artificial photoperiod in winter anestrous mares.
In conclusion, the results of our study suggest that a moderate increase in the ambient temperature does not significantly accelerate the return to reproductive cyclicity in mares under artificial photoperiod. More importantly, it would appear that artificial photoperiod combined with abundant, direct sunlight or artificial light provided by light sources with a spectrum similar to sunlight during the daytime may work synergistically in the activation of reproductive activity in early spring. Based on these observations, it appears that an optimal strategy to induce early cyclicity consists of a 2 hr exposure to artificial light, preferably short wavelength blue light, at 9 hr after dusk, combined with daytime exposure to direct sunlight or artificial light again with a strong short wavelength blue light component. Given the recent published information on the spectral quality of the light sources, further studies should also address the potential merits of blue-enriched lights, for example LEDs instead of the traditional fluorescent lights, during the nightly 2 hr light pulse to determine whether this could achieve additional efficiencies.
ACKNOWLEDGEMENTS
The authors are grateful to Dr. Micaela Vita, Dr. Osvaldo Bogado and all Keros personnel for their help during the sample collection. Mares were provided by Keros AI and ET center, Passendale, Belgium. We would also like to thank Crijns energy controlling to provide the temperature measurement devices.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
PD1 preformed sample collection, data collection and statistical analyses, and manuscript preparation; KD performed animal work and sample collection, temperature data collection, farm data collection and manuscript editing. IL performed progesterone assays; WW assisted with weight and BCS determinations, HV participated in study design, sample collection, mare monitoring, and PD2 performed study design, oversight the project, funding acquisition, animal work, statistics, manuscript writing and submission. All authors read and approved the final manuscript.
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author on request.




