The timeline development of female canine germ cells
Abstract
During the sex differentiation, the primordial germ cells (PGCs) pass through a differentiation, becoming spermatogonial cells in males and oocytes in females. In this phase, there is difference in gene expression and differentiation potency between males and females. Specific cell markers have been essential in the PGC meiosis beginning and become oocyte cells. However, there are few studies about germline in domestic animals. The domestic dog (Canis lupus familiaris) is an interesting animal model to be used in the investigation about the mammal development because it has several biochemical and physiological similarities to humans. In addition, some additional investigations about dogs may contribute to a better understanding of the biology and genetic components, improving clinical veterinary and zoological sciences. Here, we elucidated by immunofluorescence and quantitative reverse transcriptase-polymerase chain reaction (RT-qPCR), the dynamics of the expression of pluripotent (POU5F1 and NANOG) and germline (DDX4, DAZL and DPPA3) markers that are very important in the development of female canine germ cells during 35–50 days post-fertilization (dpf). The female canine germ cells were positive for pluripotent markers during middle developmental period. The number of DDX4, DAZL and DPPA3 cells increased along the germ cell maturation from 45 to 50 dpf. We provided an expression analysis of the pluripotent and germline markers in paraffin sections using the middle and later periods in female canine germ cells. The results can contribute the understanding about the timeline of each marker along the maturation of female canine germ cells. These results have a great significance to demonstrate the germ cell profile changes because it may allow the development of protocols about in vitro germ cell derivation.
1 INTRODUCTION
Since 1977, researches about germ cells have been conducted and new findings are published every year for a better understanding about the behaviour and production of primordial germ cells (PGCs). It is established that PGCs are specified as extragonadal and migrate to develop in the gonadal ridges (McLaren, 2003). The gonadal originate from the elongated area of mesoderm, over the mesonephros ventromedial border (Calson, 2014). In mammals, when the PGCs reach the gonadal ridges, some morphological transformations occur, increasing the cellular numbers until it reaches the sexual differentiation by the formation of seminiferous cords in males and ovarian cords in females (Fujimoto, Miyayama, & Fuyuta, 1977; Møllgård et al., 2010).
In females, the germ cells enter in the meiotic prophase as oocytes and pass through leptotene, zygotene and pachytene stages before arriving in diplotene near the time of birth (McLaren, 2003; Virant-Klun, 2015). After entering in meiosis, oocytes become primordial follicles surrounded by one or more layers of cubic or cylindric cells, and when developing a secondary or antral follicle, there is presence of multiple layers of follicular (granulosa) cells which an antrum can be seen. The signalling between the oocyte and granulosa cells is essential for the folliculogenesis success (Albertini, 2015). The female mice PGCs enter in meiosis activation at E13.5 stage, and to human female PGCs, this occurs near the twentieth pregnancy week (Wilhelm, Palmer, & Koopman, 2007).
Three main phases constitute the characterization of PGCs, first: the beginning of development, which constitutes the pluripotency phase and the germ cell formation; second: the increase in the germ cell number; and third: the differentiation phase of gametes in males and females (Cooke & Nanjappa, 2015; De Felici, 2000). Usually, the OCT4 (or POU5F1), DDX4 (or VASA), DAZL and DPPA3 (or STELLA) markers have been used for characterizing male and female germ cells of different species (Anderson, Fulton, Cowan, Coutts, & Saunders, 2007; Gaskell, Esnal, Robinson, Anderson, & Saunders, 2004).
There are few studies about domestic canine species (Canis lupus familiaris) in this area. Gier and Marion (1969) demonstrated that canine PGCs at 20–21 days of gestation migrate through the mesenchyme between the coelomic epithelium. Martins et al. (2011) showed that male canine PGCs are positive by the OCT4 marker, and recently, De Souza et al. (2018) performed a profile of pluripotent, germinative and epigenetic markers throughout the embryonic and foetal development of canine male gonads.
Dogs can be used as models in biomedical and clinical applications, also for modelling of human diseases and researches, because of their physiological and genetics similarity (Kirkness, 2003; Parker & Ostrander, 2005; Starkey, Scase, Mellersh, & Murphy, 2005). Also, additional investigations in dogs may contribute to a better understanding about the biology and genetic components, improving the veterinary and zoological clinics. However, most of studies about female canine germ cells had been conducted in adult in vitro oocytes (Otoi, Wilingham, Shin, Kraemer, & Westhusin, 2002) and no research about canine germ cells in foetal females was published. Though, to understand the routes and the initial mechanisms of the female sex differentiation, it is relevant to identify the foetal gonadal morphology and the profile of markers during the germ cell development. With this, the comprehension about germline may become an important source in the reproductive and regenerative areas of the veterinary medicine and its biological development. Herein, we presented the profile of pluripotent and germline markers in female canine foetus germ cells.
2 MATERIALS AND METHODS
2.1 Sample collection
All analysis and procedures involving animals were conducted following the ethical principles of animal studies in accordance with the Committee of Ethics of the Faculty of Food Engineering and Animals Sciences, University of São Paulo, Pirassununga-SP, Brazil (process number 7043240215). The female canine foetus gonadal samples (n = 16) were obtained from healthy pregnant female mongrel dogs with age between 1 and 5 years. The pregnant dogs (n = 20) were obtained from a dog population control campaign in São Paulo and Pirassununga (São Paulo state—Brazil), underwent to ovary-salpingo-hysterectomy surgery. Before performing any surgical procedure, the animals were food restricted for 12 hr and water restricted for 8 hr. Anaesthesia was applied as previously described (Miglino et al., 2006). During the surgery, the uterus and foetus were removed according to the Duke University Animal Care & Use Program Policy. All the foetuses were washed twice in saline phosphate solution (pH 7.0), and the gestational age was estimated using the crown-rump (CR) length, morphogenesis and organogenesis. It was used the Pieri et al. (2015) manuscript too that was based on histological and macroscopic characteristics during the embryological and foetal development in dogs. The foetuses were collected between 35 and 40 days post-fertilization (dpf; middle period, n = 8), which corresponds to the beginning of sexual differentiation, and between 45 and 50 days (later period, n = 8), which corresponds the period during the gonadal development into testis precursors.
2.2 Histological assay
Each stage of female canine foetuses (middle, n = 6 – later, n = 6) was carefully dissected to collect the gonadal ridge. The gonadal ridge was fixed in a 4% paraformaldehyde solution (PFA) during 24 hr, after dehydrated and embedded in paraffin (Behmer, Tolosa, & Neto, 1976). Serial sections of 5 µm were stained with haematoxylin–eosin and analysed under a Leica-DMS300 digital microscope systems and the Leica Acquire software version 3.4.1 2006-2013.
2.3 Immunofluorescence assay
The immunofluorescence staining was performed as previously described (Heeren et al., 2015) using 0.01 M citrate buffer at pH 6.0 for the antigen retrieval. Prior applying the primary antibodies, the slides were blocked with 1% bovine serum albumin (BSA, Sigma-Aldrich Corp.) and 0.1% PBS Tween-20 (PBS-T) during 1 hr at room temperature. The primary antibodies (Table 1) were applied on the slides resting overnight. Negative controls were performed using PBS instead of the primary antibody solution.
| Antibodies | Host | Dilution | Catalog/Company |
|---|---|---|---|
| OCT4 | anti-Rabbit | 1:100 | ab18976/ABCAM |
| OCT4 | anti-Goat | 1:100 | sc8629/SANTA CRUZ |
| DDX4 | anti-Rabbit | 1:500 | ab13840/ABCAM |
| DAZL | anti-Goat | 1:200 | sc27333/SANTA CRUZ |
| DPPA3 | anti-Rabbit | 1:500 | sc67249/SANTA CRUZ |
The secondary antibodies (Table 2) were applied and rest during 1 hr in room temperature. The nuclei were stained with a Hoechst dye (trihydrochloride, trihydrate, cat# 33342, Invitrogen), mounted with Prolong Gold antifade (cat# P36930, Life technology) and stored at 4°C. All data were acquired with a fluorescence light microscope (FLM; Nikon Eclipse 80i) at 60× and 100× magnification. ImageJ (National Institutes of Health, USA) was used for the quantification analyses. The images were converted to 8-bit greyscale images, and the colour inversion was applied to selected images that required more sensitivity.
| Antibodies | Host | Dilution | Catalog/Company |
|---|---|---|---|
| Alexa Fluor 488 | Donkey anti-Rabbit | 1:500 | A-11058, Life Technologies |
| Alexa Fluor 594 | Donkey anti-Goat | 1:500 | A-21203, Life Technologies |
2.4 RNA extraction, cDNA conversion and RT-qPCR analysis
Total RNA was isolated from female canine foetus for each stage (middle, n = 3 – later, n = 3) using the TRIzol reagent (Life Technologies) according to the manufacturer's recommended protocol. The RNA was quantified according to the 260/280 ratio using a spectrophotometer (DS-11-B, DeNovix). The cDNA synthesis was performed with 1,000 ng/µl per sample using a High-Capacity Reverse Transcription Kit (Applied Biosystems) according to the manufacturer's recommended protocol. The primer sequences (Table 3) used for the glyceraldehyde 3-phosphate dehydrogenase (GADPH) analyses have been previously described (Lee et al., 2014). Another specific gene primer was designed using Primer 3 (Koressaar & Remm, 2007). The PCR amplification of the selected pluripotent and germinative genes (Table 3) was performed using an ABI-7500 instrument with SYBR Green Master Mix (Applied Biosystems).
| Genes (Accession no) | Forward primer sequence | Reverse primer sequence | Annealing (°C) |
|---|---|---|---|
| GADPH (NM_001003142.2) | CTTCACCACCATGGAGAAGC | CAGCTCAGGGATGACCTTGC | 60 |
| POU5F1 (XM_538830.3) | ATATGTGTAAGCTGCGGCCC | CAATGTGGCTGATCTGCTGC | 62 |
| NANOG (XM_022411387.1) | TCTGCCACCACGGAATATGC | TCTGACTGTTCCAGGAGTGG | 60 |
| DDX4 (XM_005617393.2) | CATACCACCTCCTCCACCTG | TGTCTGACAGAGATTAGCTTCCTC | 59.60 |
| DAZL (XM_005634380.2) | ACTGGTGTGTCCAAAGGCTAT | GGACGAGGCTGCACATGATA | 59.90 |
| DPPA3 (XM_022411213.1) | AACTCCCTTCCCCTCTACCA | CGCTGGTACTGAATCAATCG | 59.60 |
RT-qPCR was performed as follows: 95°C during 15 min and 45 cycles at 95°C during 15 s, 60°C during 5 s, 72°C during 30 s and finally 72°C during 2 min. A melting curve analysis was performed to verify the amplification of the specific products, and all reactions were performed in triplicate. The transcripts levels were determined by RT-qPCR and analysed using a LinReg PCR software (Version 2015.0). The cycle threshold (Ct) values of the target genes were normalized to the Ct value of GAPDH, and then, the fold changes were calculated using the Pfaffl equation (Pfaffl, 2001). The statistical analyses included a variance analysis (ANOVA, p < 0.1) followed by Tukey's test to determine the differences in gene expression and the group means (p < 0.1) using R software (R Development Core Team, 2013).
3 RESULTS
3.1 Morphology and characterization of female germ cells
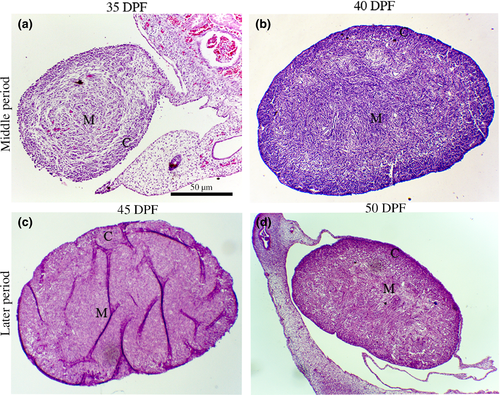
The histological analysis demonstrated the development of the gonadal ridge along the periods. On the middle periods, at 35 dpf the gonadal ridge could be seen very developed, and with the advancement of foetal periods at 40 dpf, it was possible to observe a reorganization between medullar (M) and cortical areas (C; Figure 1a,b). The immunofluorescence assay at the middle period exhibited some specific germ cell markers DAZL+ (red) and OCT4+ (green; Figure 2). However, cytoplasmic staining of OCT4 (green) was also visualized in germ cells.
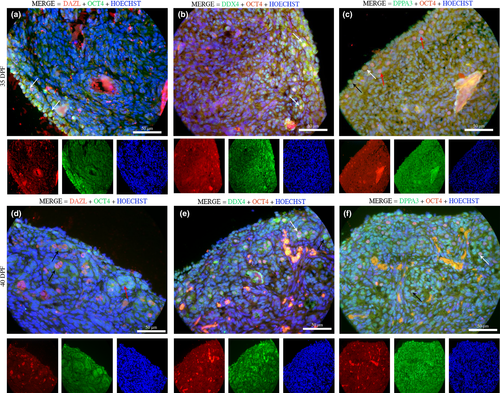
Positive germ cells were detected by DDX4+ (green) markers, and almost all germ cells were positive for DXX4 with co-localization of the nuclear markers with OCT4 (red; Figure 2). These positive cells showed a spherical shape and exhibited morphologically a large nucleus that resembled that seen in germ cells. The nuclear protein DPPA3 (green) and/or co-localization of OCT4 (yellow/orange) are emphasized in the gonadal ridge (Figure 2).
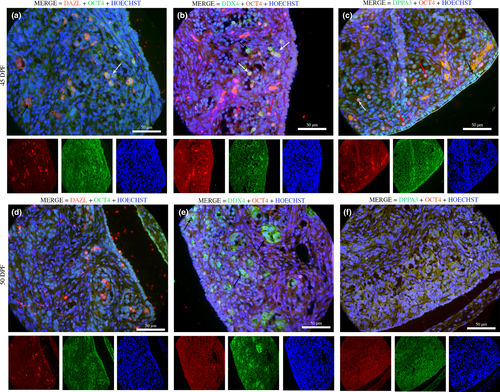
Morphologically, the later periods (45–50 dpf) were marked by clear cortical–medullar delimitation and gonadal vascularization (Figure 1c,d). During this stage of gestation, the OCT4 marker was few detected in the gonads, while most of germ cells were DDX4+ (green), DPPA3+ (green) and DAZL+ (red; Figure 3). There was co-localization with cytoplasm DDX4+ (green) and nuclear OCT4+ (red); and nuclear DPPA3/OCT4 (yellow/orange; Figure 3). On 50 dpf, positive germ cells could not be detected with DPPA3 and OCT4 markers (Figure 3).
3.2 Transcript levels of pluripotent and germline genes
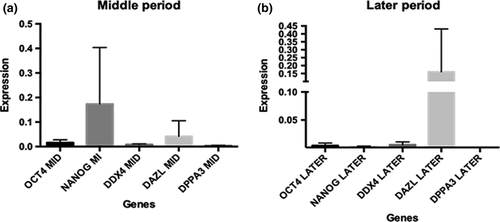
The transcripts levels of pluripotency (POU5F1 and NANOG), germline (DDX4, DAZL and DPPA3) genes in the middle and later periods were measured (Figure 4). It was possible to detect a modification in the parameters of the transcripts according to the germ cell development. On the middle period, the presence of pluripotent genes occurs, and on the later period, there was more abundance of germinative genes, such as DAZL gene. However, the statistical analysis not showed a significant difference between the genes among the periods.
4 DISCUSSION
A wide range of markers is expressed during the gonadal development (in vivo) and helps to recognize the developmental stage in germ cells. These markers are shared between species but the time of expression of some proteins could differ among species strains and antibodies used. In this study, it is important to cite that was performed a first step expression profile of some germ cell markers during foetal development in female canines. Specifically, it was combined morphological examinations, immunofluorescence characterization and gene expression analyses to elucidate each period in the female gonadal formation.
In canine species, the gestational period is variable between 60 and 63 days (Pretzner, 2008) and the embryo sex is genetically determined since the fertilization moment. However, this is only morphologically determined out of 30 dpf, when the morphological organogenesis begin in foetal gonadal onset the differentiation process to turn into male or female individuals (De Souza et al., 2018). The histological results showed that on 35 dpf was possible to identify different characteristics of each sex, like the male gonads that started the testes cord development and in female not happens. According to Hyttel, Sinowatz, and Vejlsted (2010), the female gonadal ridge could be divided into two parts, medulla (inside of the gonad) and cortex (outside of the gonad). The results demonstrated that in female canine gonadal ridge was possible to visualize the division at 40 dpf. In humans, this separation is evident in the sixth week of pregnancy (Biason-Lauber, 2010). However, in mice gonadal ridge at E11.5 stages, the distinction between the cortex and the medulla is not so evident (Jiménez, 2009).
In the current study, a pluripotency profile was tested in germline markers in middle and later periods in the female canine gonadal ridge that in humans corresponds the 2nd trimester of pregnancy and mouse from E13.5 to E16.5 stages. Using OCT4 markers, it was possible to identify germ cells during middle and later (45 dpf) periods. Two different OCT4 markers were used and just one expression in nuclear cells, but the other marker demonstrated more cytoplasmic than nuclear expression. According to Gkountela et al. (2015) and Pieri et al. (2016), it is possible to visualize both markers. The absence of OCT4 marker on 50 dpf can be associated with germ cell maturation during the transformation in primary oocytes. The OCT4 and NANOG gene expression was decreased in humans during the 2nd trimester of pregnancy in both sexes when the germ cells enter in the meiotic wave (Anderson et al., 2007; Stoop et al., 2005). However, in mice, there is a sexual difference in the expression of OCT4 and NANOG; in males, both markers are reduced at E13.5–E16.5 stages, but in females, only the NANOG marker declined at E16.5 until newborn oocytes (Ovitt & Schöler, 1998; Yamaguchi, Kimura, Tada, Nakatsuji, & Tada, 2005).
The markers and genes DDX4, DAZL and DPPA3 were used to characterize in vivo and in vitro germ cells. The classic cytoplasm germ cell marker DDX4 was positive in all periods, indicating the presence of mature germ cells, which prepares the cell development into primordial follicles. Similar results were found in humans during the 2nd trimester of pregnancy ( Heeren et al., 2016; Hummitzsch et al., 2013; Rosario, Adams, & Anderson, 2016). In mice, DDX4 was observed from E10.5 and continues its expression up to E12.5 in both sexes (Fujiwara et al., 1994).
Female canine germ cells also were positive for DAZL marker and genes, which is the same results compared to humans during the 2nd trimester of pregnancy and mouse in the E12.5 stages (Rosario et al., 2016). DAZL marker is conserved in all vertebrates, and it has been associated with normal development to germ cells, besides being related to spermatogonial differentiation or in the meiosis beginning. In mice, the non-expression of this gene results in infertility in both sexes (Chen et al., 2014, 2011).
The DPPA3 showed intense positive reactions on the middle period and decreased as the gonadal development occurred. Comparable with human germ cells, DPPA3 marker also decreases during germline maturation (Payer et al., 2003). However, in mice, DPPA3 was identified from E7.5 stage and continues until E15.5 stage, then remains restricted to oocytes (Sato et al., 2002).
During their development, germ cells undergo extensive reprogramming that regulates their development. The global transcriptome and the cell cycle kinetics maintain germ cell-related characteristics and block the expression of genes related to somatic cell lineage. Nonetheless, epigenetic adjustments aid in the specification and maintenance of the germinal lineage.
The study by Ohta et al. (2017) observed that a partial activation of the major germ cell genes occurred concurrently with the erasure of DNA methylation and reorganization of the histone modification landscape. Such epigenetic reorganization leads to a basal activation of some germline genes and might encourage the germ cells for an eventual reception of the sex determination signals. Other modifications that aid and control the germline expression are the non-coding RNAs. They control the gene expression by various means, ranging from degradation of microRNA-induced mRNAs to long-term ncRNA-mediated chromatin modification. Many aspects of germline appear to be controlled by ncRNAs, including the maintenance of pluripotency, specification and differentiation in functional oocytes (Hayashi et al., 2008).
Based on previous studies, it is concluded that the complex interaction of germ cells with other cell subtypes demonstrates other underlying post-transcriptional mechanisms that may be involved in their development. This is the first study that reveals the specificity of a panel of pluripotent and germline markers that aid in the identification and reorganization of female canine germ cells. In the meantime, future studies that relate the epigenetic profile of these cells may be essential for a better understanding of the germline.
5 CONCLUSION
This study provides new and important findings about the morphology and expression of female canine primordial germ cells. We had demonstrated the timeline expression in several markers during the germ cell development. These results open new ways for studying canine reproductive physiology, for example, the implementation of germ cell in vitro that could be provided an elucidation of genetic and epigenetic during mammalian germ cell development.
ACKNOWLEDGEMENTS
This research was supported by São Paulo Research Foundation (FAPESP)—Process numbers 2013/10542-5 and 2016/15260-6.
CONFLICT OF INTEREST
The authors have no conflict of interests to declare.
AUTHOR CONTRIBUTIONS
A.F. de Souza was responsible for conception, design, collection and assembly of data, data analysis, interpretation and manuscript writing. E.C. Ramos and N.C.G. Pieri accomplished data analysis interpretation. F.S. Cury was responsible for manuscript writing. D.S. Martins contributed to the experimental design and coordination.




