Gene and protein expression in the reproductive tract of Brazilian Somalis rams
Contents
Brazilian Somalis is a locally-adapted breed of rams raised in tropical climate and native pastures. The present study was conducted to evaluate gene expression and proteome of the reproductive tract of such rams. Samples were collected from testes, epididymides, seminal vesicles and bulbourethral glands of four rams. Expression of clusterin (CLU), osteopontin (OPN) and prostaglandin D2 synthase (PGDS) genes were evaluated in all samples by real-time PCR. Shotgun proteomic analysis was performed using samples from the head, corpus and cauda epididymides and from all other structures as well. Gene ontology terms and protein interactions were obtained from UniProtKB databases and MetaCore v.6.8 platform. CLU trasncripts were detected in the testes, epididymides, seminal vesicles and bulbourethral glands of the Somalis rams. The initial region and body of the epididymis had the greatest CLU expression. OPN mRNA was localized in all tissues of the ram reproductive tract. PGDS mRNA was detected in the testes and epididymides. Lable-free mass spectrometry allowed the identification of 137 proteins in all samples. Proteins of the epididymis head mainly participate in cellular processes and response to stimulus, participating in catalityc activity and binding. Proteins of epididymis body acted as regulatory proteins and in cellular processes, with binding and catalytic activity. Cauda epididymis molecules were associated with cellular processes and regulation, with binding function and catalytic activity as well. Testis proteins were mainly linked to cell processes and response to stimuli, and had catalytic function. Seminal vesicle proteins were involved in regulation and mainly with binding functions. Most bulbourethral gland proteins participated in cellular processes. The present study is the first to evaluate the proteome and gene expressions in the reproductive tract of Brazilian Somalis rams. Such pieces of information bring significant cointribution for the understanding of the reproductive physiology of locally-adapted livestock.
1 INTRODUCTION
The Brazilian Somalis rams are small animals with a body surface favorable to heat dissipation and possess a considerable reserve of fat at the base of the tail, which can be used as a source of energy when food supply is low. A study conducted by Silva, Araújo, and Figueiredo (1998) evaluated body weight gains from birth to 112 days of age (weaning) and mortality rates in Brazilian Somalis sheep and concluded that these animals have remarkable adaptability to native pasture conditions of the Brazilian semi-arid region. The Somalis is able to produce meat and skin of good quality, being a viable option for crossbreeding with local breeds and as a paternal line with breeds of high meat production, such as Dorper. In this regard, dissemination of locally-adapted breeds such as the Somalis has been recommend due to its genetic value and adaptation to the tropical climateand native pastures (Barros, Vasconcelos, Araújo, & Martins, 2003).
Seminal plasma contains secretions from epidydimides, testes and accessory sex glands that influence survival of sperm in the female tract, protect sperm from oxidative damage and modulate the fertilizing capacity of the sperm (Evans & Maxwell, 1987; Yanagimachi, 1994). Studies show that proteins of seminal plasma act during sperm capacitation and protects spermatozoa of rams of European breeds (Barrios, Fernández-Juan, Muiño-Blanco, & Cebrián-Pérez, 2005; Fernández et al., 2006). The expression of many proteins inseminal plasma changes during the reproductive development of sheep as well, being directly linked to the growth of the gonads and evolution of sperm production (Souza et al., 2010). The fluid to which the spermatozoa are exposed in the epididymis undergoes substantial changes in composition, including changes in osmolarity, ionic ratio, energy reserves and protein types (Zenick, Blackburn, Hope, Richdale, & Smith, 1984). Also, the expression of several proteins shows remarkable changes throughout the epididymal tubule while the expression of other proteins appear be consititute in all epididymal regions (Dacheux & Dacheux, 2014; Dacheux, Dacheux, & Druart, 2016). Clusterin, for example, is involved in sperm maturation (Hermo, Wright, Oko, & Morales, 1991), protection of sperm against oxidative processes (Reyes-Moreno, Boilard, Sullivan, & Sirard, 2002) and toxic effects of protein precipitation (Meri & Jarva, 2001; Wilson & Easterbrook-Smith, 2000). Osteopontin (OPN), in turn, is a phosphorylated, calcium-binding glycoprotein, capable of interacting with the cellular surface integrins and its content in the seminal plasma and fluid of the accessory sex glands is associated with fertility of dairy bulls (Killian, Chapman, & Rogowski, 1993; Moura, Koc, Chapman, & Killian, 2006). Osteopontin and its transcripts were detected in the epididymis, seminal vesicle and ampulla of bulls (Cancel, Chapman, & Killian, 1997; Rodriguez, Day, & Killian, 2000a). Prostaglandin D2 synthase (PGDS) is found in epithelial cells of the epididymis and represents, about 8% of all proteins secreted by the epididymis of rams and horses (Fouchécourt, Charpigny, Reinaud, Dumont, & Dacheux, 2002; Rodriguez, Day, & Killian, 2000b; Urade & Hayaishi, 2000). PGDS appears in the epidydimal fluid of the ram when the first sperm enter the anterior section of the organ, at early age (Fouchécourt et al., 2002). However, the precise function played by PGDS in the male reproductive tract has not been clarified yet, but it is possible that PGDS may act in transport of retinol and steroids in the epididymal fluid and seminal plasma (Leone, Haq, & Saso, 2002; Moura et al., 2006). Given this scenario, the present study was conducted to evaluate gene expression and proteome of the reproductive tract of Brazilian Somalis rams.
2 MATERIALS AND METHODS
2.1 Experimental procedure
Brazilian Somalis rams (n = 4) were selected for the present study were at the age of 5 months and weighed 20.6 kg, after they had been fed for 65 days with Tifton 85 hay and concentrate. Tissue samples were collected from testes, epididymides, seminal vesicles and bulbourethral glands for evaluation of gene expression by quantitative PCR. Also, shotgun, label-free proteomics analysis was performed using samples from the head, corpus and cauda epididymides, as well as from the testes, seminal vesicles and bulbourethral glands of the rams.
2.2 Tissue collection and sample preparation for the study of gene expression
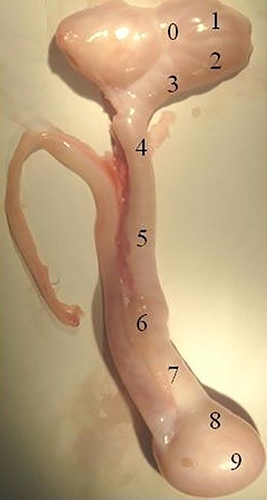
The animals were slaughtered at a commercial abattoir following all legal regulations required by the Ministry of Agriculture of Brazil (RIISPOA, ) and guidelines defined by the National Council for Control of Animal Experimentation (Law # 11,794; October 8th, 2008; Brazil), as described by Oliveira et al (2015). Epididymis, testicles, seminal vesicles and bulbourethral glands of the four animals were collected, transported to the laboratory on ice and immediately dissected. The epididymides of rams were sectioned in ten parts, as shown in Figure 1. We collected approximately 100 mg fragments from each part, of all animals. Right after the dissection, samples were frozen in liquid nitrogen and stored at −80°C until further analysis.
2.3 PCR analysis
Only head (Epi 2), body (Epi 5) and tail (Epi 8) of the epididymides (as shown in Figure 1) were used for PCR analyses. Samples of epididymides (Epi 2, Epi 5 and Epi 8), testes, seminal vesicles and bulbourethral glands (100 mg) stored at −80°C were placed in 1.5 ml tubes and macerated with autoclaved mortar and pestle, using 1 ml Trizol for RNA extraction. Then, each sample was vortexed and allowed to rest for 3 min at room temperature. Then, 200 μL chloroform was added, vigorously mixed for 15 s. After 3 min, the material was centrifuged for 15 min (12,000 g, at 4°C). After centrifugation, the supernatant was pipeted out and an equal volume of ethanol (70%) was added and lightly mixed. From this mixture, total RNA was purified using the PureLink™ RNA Mini Kit (Anbion®, USA) according to the manufacturer's instructions and as described by Souza et al (2012). In order to avoid contamination with genomic DNA, samples were treated with DNAse (Promega, EUA), with 1 μL DNA diluted 10-fold in 0.1% DEPC water, for 40 min at 37°C and 15 min at 70°C for inactivation of the enzyme. Total RNA concentration was measured in a NanoDrop 2000 spectrophotometer (ThermoScientific,USA). We considered that one unit of absorbance at 260 nm corresponded to 40 μg/mL RNA. When necessary, samples were stored at −80°C. Total RNA (1 μg) and oligo-dT were used for cDNA synthesis using SuperScript III RT-PCR methodology (Invitrogen, USA). Sequences of primers forthe gene study were obtained from the NCBI database and the primers were designed using the Primer 3 program (Untergasser et al., 2012; Table 1).
| Gene | Product size (bp) | Primers (5′-3′) | Genbank accession |
|---|---|---|---|
| Clusterin | 225 |
S-TGTTGGAACCCCTCAACTTC A-TCCTGGCACTTCTCACACTG |
XM_004004431.1 |
| Osteopontin | 200 |
S-GATGGCCGAGGTGATAGTGT A-TCTTTGGGAAGCTCGTCACT |
AF152416.1 |
| Prostaglandin D2 Synthase | 183 |
S-ACTGCTGACTGTCACCCCCATC A-GACGGATGTCCGCATCGGGAC |
XM_002700881.1 |
| GAPDH | 76 |
S-ATGCCTCCTGCACCACCA A-AGTCCCTCCACGATGCCAA |
NM_001190390.1 |
Note
- A: antisense; S: sense.
2.4 Transcript analysis
Prior to qPCR, standart-curves were prepared using a 10-fold cDNA dilution series associated with three different concentrations for all genes (0.9, 0.5 and 0.3 μM; Supplemental Figure S1). Amplification detection used the fluorophore Power SYBR® Green PCR Master Mix (Applied Biosystems, USA) and the iQ5 Real-Time PCR Detection System (Bio-Rad, USA). qPCR was performed in a 20 μL reaction solution containing 10 μL 1x SYBR Green PCR Master Mix, 0.8 μL primer, 1.0 μL templante cDNA, and 7.4 μL DEPC-treated water.
The amplification conditions were: 95°C (2 min), followed by 40 amplification cycles at 95°C (10 s), 60°C (30 s) and 72°C (30 s). Fluorescence data were acquired at the 72°C step and during the melting-curve program. The reactions were performed in quadruplet using control without cDNA to avoid contamination. For all amplifications, one dissociation curve (melting curve) was done for verification of unspecific amplifications. Quantification of the transcripts of target genes was calculated from the difference of Ct values in relation to transcripts of the endogenous gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH). First, mean Cts of the three readings for each sample was determined (both the target and the endogenous gene). From each sample, the subtraction of the mean value of the Ctgene-target to Ctgene-endogene provided the ΔCt. Subsequently, one ΔCt corresponding to a calibrator was chosen, normalizing all values by subtracting the resulting ΔCt chosen. The final value of relative quantification was given by 2-ΔΔCt, where the calibrator or standard sample was equal to one (Livak & Schmittgen, 2001). According to standard curve assays, the following concentrations were used in the experiment: 1 μM GAPDH primers; 1 μg cDNA; 1 μM clusterin primers and 1 μg cDNA; 0.1 μM osteopontin primers and 1 μg cDNA; Primers of 0.1 μM prostaglandin D2 synthase and 1 μg cDNA (Supplemental Figure S1). We selected the concentration of the genes in which the graph of the standard curve presented a slope next to −3.32.
2.5 Statistical analysis of gene expression
The mRNA expression level evaluated in technical replicates weresubjected to Shapiro-Wilk normality test (UNIVARIATE procedure; SAS 9.0 software, SAS Institute Inc., USA). Statistical significances of variables determined in the tissues were assessed with Student-Newman-Keuls (SNK) multiple comparisons test (p < 0.05) (Sousa et al., 2014).
2.6 Protein extraction
For protein extraction and analysis, we used all ten parts of the ram epididymides (Figure 1), testes, seminal vesicles and bulbourethral glands of the four rams. After thawing, samples were macerated, homogenized and incubated for 2 hr under moderate agitation with a lysis buffer containing 20 ml PBS (1x), 200 μl Triton X100, 20μL of the 500μL protease inhibitor (Sigma Aldrich, EUA). Subsequently, the material was centrifuged (4°C, 10,000 g) and the supernatant, transferred to new tubes (Souza et al., 2014). Then acetone (1:9) was added, centrifuged (5 min., 1,000 g, 4°C) and the pellet was solubilized in 0.5 ml PBS (1x). Soluble protein concentration was measured in all samples (Bradford, 1976).
2.7 Protein digestion
Protein extracted from samples of epididymides, testes, seminal vesicles and bulbourethral glands were subjected to tryptic digestion as described by Shevchenko, Wilm, Vorm, and Mann (1996) and Lobo et al (2017). A protein aliquot (50 μg) was resuspended in 50μL ammonia bicarbonate (50 mM) and added 25μL 0.2% RapiGest SF (Waters, USA). The solution was heated at 80°C for 15 min. Then, proteins were denatured with the addition of 2.5 μL 100 mM dithiothreitol (DTT), incubated at 60°C for 30 min. After cooling to room temperature, proteins were alkylated with the addition of 2.5 μL 300 mM iodoacetamide. After 30 min, 400 ng trypsin (Promega, USA) were added, vortexed, and incubated overnight at 37°C. Digestion was stopped with 10 μL 5% trifluoroacetic acid (ThermoScientific, USA) for 90 min at 37°C. Then, the samples were centrifuged (14, 000 g, 6°C, 30 min) and the supernatant, transferred to new microtubes. Extracts were dried, resuspended in 10 μL acetonitrile (5%) containing formic acid (0.1%), and transferred to the injection tubes (Waters, USA).
2.8 Identification of proteins by electrosprayionization-quadrupole-time offlight (ESI-Q-TOF)
Tryptic digests were analyzed by capillary liquid chromatography/nanoelectrospray ionization tandem mass spectrometry (nanoUPLC-MS/MS), using a Synapt G1 HDMS mass spectrometer (Waters Corp., USA; Lobo et al., 2017). Peptides were injected into solvent A (acetonitrile/water/formic acid, 5/95/0.1) supplied by the auxiliary pump of the capillary high-performance liquid chromatography unit and trapped in a Waters Symmetry 300 column (C-18, 5-mm film; 0.3 mm × 5 mm) for on-line desalting and preconcentration. After washing for 3 min. with solvent A at 5 ml/min, trapped peptides were backflushed with the gradient solvent flow into the analytical column, an HSS T3 fused silica capillary column (C-18, 5 mm, 0.075 mm × 150 mm), using a 10-port switching valve. The analytical column was run with a gradient (5%–80% solvent B; acetonitrile/water/formic acid; 95/5/0.2; in 40 min). The mass spectrometer was calibrated using Glu-Fib product ion fragments to maintain mass accuracy within 10 parts per million.
The ESI-Q-TOF mass spectrometer was operated to acquire tandem mass spectrometry of tryptic peptides in a data-dependent acquisition mode for precursor ion selection using charge-state recognition and intensity threshold as selection criteria (MassLynx 4.0 software; MicroMass, UK). For data acquisition, a survey scan (1.5 s) over the m/z of 400 to 1,500 was performed. From each survey scan, up to three most intense precursor ions based on the selection criteria were chosen to obtain the production spectra resulting from collision-induced dissociation in the presence of argon. The product ion spectra (6–8 s) collected were processed using Protein Lynx Global Server v. 2.1. Software (Waters, USA) and were converted to peak-list text files. For protein identification, MS/MS ion searches were performed on the processed spectra against the NCBInr and SwissProt databases using MASCOT Daemon and search engine (Matrix Science Inc., USA). Searches were made given there was maximum one missed trypsin cleavage, that peptides were monoisotopic and using partially oxidized methionine residues and carbamidomethylated cysteine residues. Peptide mass tolerance and fragment mass tolerance were initially set to 0.3 and 0.1 Da, respectively, for MS/MS ion searching. Candidate peptide IDs were only accepted if m/z values were within 0.1 Da of the theoretical mass of the candidate ID, when manually reviewing MASCOT search results. The false-positive rate was determined by searching the same parameters against a decoy database.
2.9 Gene ontology analysis
Data about proteins identified in samples of testes, epididymides, seminal vesicles and bulbourethral glands of the rams were analyzed using the software for researching annotations of proteins (STRAP), an open-source application (Bathia, Perlman, Costello, & Mccomb, 2009). Gene ontology terms for biological process, cellular component, and molecular function was obtained from UniProtKB databases.
2.10 Protein interaction analysis
Interactions associated with proteins present in the testes, epididymideshead (Epi 2), body (Epi 5) and tail (Epi 8), seminal vesiclesand bulbourethral glands (Figure 1) were evaluated with MetaCore v.6.8 network (https://portal.genego.com/; GeneGo, USA). These types of in silico analysis include annotated databases and metabolic and signaling pathways gathered from publications. Protein names were manually inserted in the system and all interactions processed considering canonical paths (Landiet al., 2013).
3 RESULTS
3.1 Gene expression
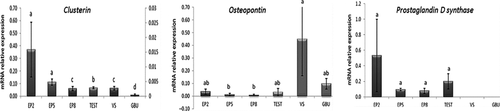
Clusterin trasncripts were detected in the Epididymides, testicles, seminal vesicles and bulbourethral glands. The initial region (Ep2) and body (Ep5) of the epididymis had the greatest expression of clusterin, different from the cauda epididymis (Ep8), testes and bulbourethral glands (Figure 2). Osteopontin mRNA was localized in all tissues of the ram reproductive tract. However, the body and cauda epididymis differed (p < 0.05) from the seminal vesicles as regard to expression of OPN gene (Figure 2). Prostaglandin D2 synthase mRNA was detected in the testes and epididymides, but without differences among the epididymal regions (p > 0.05). PGDS mRNA was not detected in the ram seminal vesicles and bulbourethral glands (Figure 2).
3.2 Proteomics
The use of lable-free mass spectrometry allowed the identification of 137 proteins in the testes, epididymides, seminal vesicles and bulbourethral glands of Brazilian Somalis rams (Supplemental Table S1). Gene ontology annotations of proteins are represented in Figure 3. Proteins identified in the epididymis head mainly participate in cellular processes and response to stimulus. These proteins are mainly extracellular components, from nucleus and mitochondria, participating in catalityc activity and binding (Figure 3a). Proteins found in the epididymis body acted as regulatory proteins and in cellular processes. These molecules are mainly from the nucleus and cytoplasm, with binding and catalytic activity (Figure 3a). Molecules that were exclusive to the cauda epididymis were associated with cellular processes and regulation. Most of them are from the nucleus and cytoplasm, with binding function and catalytic activity (Figure 3a).
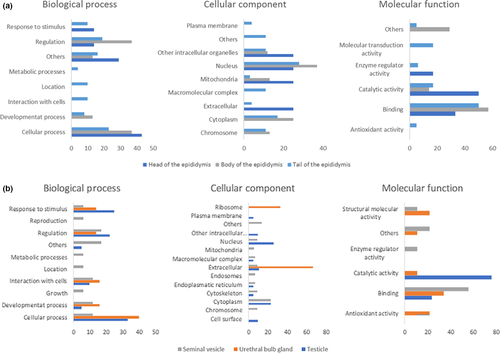
Proteins specific to the ram testis were mainly associated with cell processes and response to stimuli, came from the nucleus and cytoplasm and most of them had catalytic function (Figure 3b). Proteins unique to seminal vesicles were involved in regulation and mainly from cytoplasm, with functions related to binding (Figure 3b). Proteins detected only in the bulbourethral glands participated in cellular processes and most of them were extracellular (Figure 3b).
3.3 Protein interactions
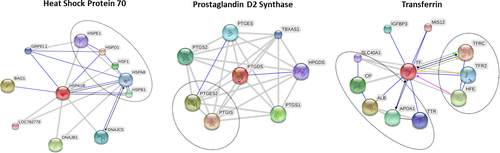
According to in silico analyses (Figure 4), Heat Shock Protein 70 have links with chaperones that bind to the heat shock factor (HSF1) and with other heat shock proteins (HSPE1, HSPD1, HSPA8, HSPB1). Prostaglandin D2 synthase interacts with proteins responsible for the regulation or promotion of cell proliferation (PTGS1), with catalytic proteins (PTGIS, PTGES2), proteins that mediate inflammation and participate in signaling activity (PTGS2). Transferrin networks with proteins involved in iron absorption (TFRC, TFR2, HFE), component transport (TTR, APOA1, CP, SLC40A1), albumin (ALB) and protein necessary for chromosome alignment (MISS12).
4 DISCUSSION
The present study includes a comprehensive evaluation of molecular aspects of the reproductive tract of Brazilian Somalis rams, a tropically-adapted breed of the Brazilian Northeast. In this regard, we provide valuable pieces of information about the expression of clusterin, osteopontin and prostaglandin d-synthetase genes, and about the major proteome profile of the epididymis, seminal vesicle and bulbourethral glands of the locally-adapted rams.
Clusterin, osteopontin and prostaglandin D2 synthase transcripts were evaluated in the head, body, and tail of the epididymis, testes, seminal vesicles and bulb urethral glands of the Brazilian Somalis rams. The patter of clusterin mRNA occurrence is in agreenment with studies describing the expression of the same gene in the reproductive tract of bulls, mice and other breeds of rams (Sensibar et al., 1993). In fact, the expression of clusterin in the testis and epididymis of mice is indicative of a complete differentiation of germ cells (Grima, Pineau, Bardin, & Cheng, 1992; Hermo et al., 1991). CLU apparently promotes sperm aggregation in vitro (Robaire & Viger, 1995), sperm protection against attacks of the immune system (Meri & Jarva, 2001), reabsorption of defective spermatozoa in the tail epididymis and modulation of cell lysis (Akerlof et al., 1989; Matsuoka, Imai, Kohno, & Fukui, 2006). In rats, CLU appears to participate in sperm maturation, lipid transport (Tenniswoodet al., 1992) and membrane remodeling (Humphreys, Carver, Easterbrook-Smith, & Wilson, 1999). In rat testis, CLU is in the cytoplasm of Sertoli cells at all stages of spermatogenesis (Parvinen, 1982). Clusterin is present along the entire epididymis of the bovine (Ibrahim et al., 1999) and testicular fluid of sheep (Blaschuk, Burdzy, & Fritz, 1983). Other authors report the distribution of clusterin in the efferent ducts and in the head of the mouse epididymis (Oliveira et al., 2010). The complex secretion and reabsorption of clusterin suggest its functional role during sperm maturation, in the sense that spermatozoa should be eliminated from the tail of the epididymis without CLU. This could explain the low expression of clusterin in the cauda epididymis of the rams, as well as in the seminal vesicles and bulbourethral glands, as also described by Hermo et al. (1991) in the mouse.
The highest expression of osteopontin was detected in the seminal vesicles although some OPN was also found in the epididymis of the Somalis rams. These pieces of information agree with results obtained in cattle, where OPN was expressed in the seminal vesicles, in the entire epididymis and seminiferous tubules, but not in the interstitial tissue (Rodriguez et al., 2000a). Osteopontin is typically involved in cell adhesion, tissue and extracellular remodeling, inflammation and immune-mediated events (Bouleftour et al., 2017; Denhardt, 2002; Wai & Kuo, 2004). OPN was associated with Sertoli and germ cells in the seminiferous tubules and ejaculated sperm of humans (D'Cruz, 1996) and OPN content in seminal plasma and accessory sex gland fluid related to bull fertility (Killian et al., 1993; Moura et al., 2006). Moreover, OPN is involved in post-fertilization events as addition of OPN to sperm and/or fertilization media has positive effects on early development of bovine (Gonçalves, Chapman, Bertolla, Eder, & Killian, 2008), swine (Hao et al., 2008) and mouse embryos (Liu et al., 2015).
PGDS was expressed in the testis and in all parts of the ram epididymis, but without statistical difference between them. Such results are in agreement with a study reported by Fouchécourt et al. (2002), in which PGDS was present in the ram testis and epididymis during puberty. The expression of PGDS was confirmed in the epithelial cells of the head, body, and tail of the bovine epididymis as well (Rodriguez et al., 2000b) and in the ram epididymis (Gerena et al., 1998). Prostaglandin D synthase is a major component of the bovine cauda epididymal fluid proteome and also a marker of high fertility in bulls (Gerena et al., 1998; Gerena, Irikura, Eguchi, Urade, & Killian, 2000; Killian et al., 1993; Moura, Souza, Chapman, Stanley, & Killian, 2010). However, the exact function that PGDS exerts on spermatozoa has not been clarified yet (Moura et al., 2006). Epididymis PGDS may contribute with the testis hematopoietic barrier, transport of thyroid and retinoic hormones (Leone et al., 2002). Treatment of bovine sperm and/or oocytes with antibodies against PGDS decreases in vitro fertilizationand embryo development (Gonçalves, Staros, & Killian, 2008), indicating that PGDS, a typical epididmal protein, also participates in post-ejaculation events. In silico analysis shows that PGDS interacts with catalytic proteins, with a protein responsible for regulation of cell proliferation and with a mediator of inflammation and a factor acting on cell signaling (PTGS2). PTGS2 is expressed in the endothelium, kidney, brain and in cancer cells. In such cell type, PTGS2 is keyfor production of prostaglandin E2, which plays roles in cell proliferation and resistance to apoptosis (Kim, Huri, & Snyder, 2005). This array of interactions linked to PGDS indicates how complex this protein can be, as well as its functions in male reproduction.
Most of the 17 proteins exclusively identified in the testis are associated with cellular processes, response to stimuli and regulation with respect to biological processes. In relation to molecular function, components of the nucleus and cytoplasm prevailed. As for the molecular function, catalytic proteins were highlighted. In this scenario, HSP70 was one of the proteins present only in the testis. HSPs are expressed through stress and participate in assembly and transport of proteins. In addition, HSPs protect cells against harmful conditions (Georgopoulos & Welch, 1993) and modulate immune reactions (Calderwood, Khaleque, Sawyer, & Ciocca, 2006). HSP70 plays an important role in spermatogenesis (Bohring, Krause, Habermann, & Krause, 2001; Huszar, Stone, Dix, & Vigue, 2000) and sperm maturation, influencing mouse fertility (Dix et al., 1996). Based on String in silico analysis, HSP70 showed interactions with proteins with binding function, resistance to fatigue or acting as chaperones. Such connections amplify the importance of HSP70, which potentially help to establish the conditions to maintain integrity of the reproductive tract and prevent negative effects of stress on male fertility.
Five proteins were identified in the head epididymis of Somalis rams, including transferrin and glutamine synthetase (GS). Transferrin acquires iron in the majority of cells in the organismin a pH-dependent process, and one of its domains contains a high-affinity Fe(III)-binding site (Ponka, Beaumont, & Richardson, 1998; Richardson & Ponka, 1997). The major source of plasma transferrin in adults is the liver but the synthesis of transferrin has already been observed many tissues, including lymph nodes and circulating lymphocytes, macrophages, bone marrow, spleen, thymus, salivar glands, mammary glands, and Sertoli cells of testis (Morgan, 1981; Ponka et al., 1998). Based on the analyses of protein networks, transferrin interacts with other proteins involved in iron absorption, binding proteins and with a protein required for chromosome alignment. Activity of GS in the epididymis head may help to maintain an ideal microenviroment for sperm maturation, and to reestablish acid-base balance in the lumen. Homeobox protein Hox-A11 is one of the seven proteins identified in the body of the ram epididymis. Studies reported that the loss of homeobox protein Hox-A11 in rats causes “homeotic transformation events” in which one segment acquires the appearance of another segment in the epididymis (Robaire & Hinton, 2002), suggesting that Hox-A11 is involved in the developmentof the epididymis (Maclean, Hayashi, Turner, & Wilkinson, 2012). Several factors are important for the regionalization, elongation and coiling of the developing reproductive tract. For example, animals lacking homeobox A10 (Hoxa10) or Hoxa11 have homeotic or partial homeotic transformations of the vas deferens to the epididymis (Hsieh-Li et al., 1995; Podlasek et al., 1999), linking the expression of Hox genes to regionalization of the Wolffian duct (Snyder et al., 2010). Twenty proteins were present exclusively in the tail of the ram epididymis, including histones (H1-1, H1-2, H1-3 and H2A). This group of proteins makes up a family of basic molecules that bind to DNA and help to condense it into chromatin. Studies point out that sperm histone phosphorylation is involved in chromatin condensation during spermatogenesis (Marushige, Marushige, & Wong, 1976; Ward, Kimura, & Yanagirnachi, 1999).
Seventeen proteins were identified in the seminal vesicle of the Somalis sheep, including Histone H4. The core histone is composed of four proteins (H2A, H2B, H3 and H4) and their N-terminal ends can be chemically modified by methylation, acetylation, and phosphorylation (Berger, 2002). These modifications change the chromatin structure, influencing gene expression (Dion, Altschuler, Wu, & Rando, 2005). Histone H4 and also H2A, H2B, H3 are related to nucleosome compaction, which can affect gene regulation (Griffiths et al., 2011). Shirakata, Hiradate, Inoue, Sato, and Tanemura (2014) demonstrated that the individual N-terminal sites of H4 exhibit different patterns of modification during differentiation of male germ cells, suggesting that histone H4 modification plays an important role during spermatogenesis. In the present work, ten proteins were exclusive to the bulbourethral glands, including myoglobin, a cytoplasmic hemoprotein that is expressed primarily in cardiomyocytes, skeletal muscle fibers and various non-muscle tissues (Flogel, Godecke, Klotz, & Schrader, 2004). Myoglobin facilitates oxygen transport and acts as a reservoir for oxygen in muscle (Kanatous & Mammen, 2010) and its function may relate to overall secretory activity of the accessory sex glands, such as the bulbourethral glands.
In summary, this is the first study to identify proteins and evaluate the expression of key genes in the testis, epididymis, seminal vesicle and bulbourethral gland of Brazilian Somalis rams. The use of a shotgun proteomics approach allowed the identification of 137 proteins in those samples, which present potential functions and association with several aspects of reproduction in male. Our study brings meaningful cointribution for future studies designed to understand the reproductive physiology of locally-adapted livestock.
ACKNOWLEDGMENTS
This study was funded by the Brazilian Research Councils (CAPES, CNPq and FUNCAP).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
M. J. Bezerra, M. Silva and F. Vasconcelos conducted the proteomics analyses, gene ontology, interactome analyses. Samples were collected by M. Silva. Mass spectrometry was carried out by M. Lobo and qPCR, by C. H. Lobo. A. C. Monteiro-Moreira and R. Moreira are head of the “Proteomics Laboratory” and J. R. Figueiredo leads the “Laboratory of Manipulation of Pre-Antral Ovarian Oocytes and Follicles”. All three professors provided financial support for the study. The article was prepared and written by M. J. Bezerra, M. Silva, C. H. Lobo, M. Machado-Neves and A. Moura. The experiment was designed by C. H. Lobo, M. Machado-Neves and A. Moura. PI of the project is A. Moura.




