The development and integrity of equine pre-antral follicles cultured in vitro with follicle-stimulating hormone (FSH) supplementation
Contents
This study investigated the effects of different concentrations of FSH (10, 50, 100 and 200 ng/ml) in supplemented MEM+ on the development of equine pre-antral follicles that were cultured in vitro for 2 or 6 days. The ovaries (n = 5) from mares in seasonal anoestrus were collected from a local abattoir. Ten ovarian tissue fragments of approximately 3 × 3 × 1 mm were obtained from each animal. The fragments were cultured in situ for 2 days (D2) or 6 days (D6) in MEM+ or MEM+ supplemented with FSH at four different concentrations, establishing the following 11 groups: control (D0); MEM + (D2); MEM + (D6); MEM + 10 ng/ml of FSH (D2); MEM + 10 ng/ml of FSH (D6); MEM + 50 ng/ml of FSH (D2); MEM + 50 ng/ml of FSH (D6); MEM + 100 ng/ml of FSH (D2); MEM + 100 ng/ml of FSH (D6); MEM + 200 ng/ml of FSH (D2); and MEM + 200 ng/ml of FSH (D6). Follicles were observed in only 9.65% (388 of 4,018) of the histological sections. Of the 861 follicles evaluated, 488 were in the primordial stage, and 373 were in various developmental stages; 59.7% were morphologically normal. Regarding the integrity of the pre-antral follicles, the groups with 100 ng/ml FSH of 2-days culture as well as 50, 100 and 200 ng/ml FSH of 6-days culture provided the best results. In conclusion, the in vitro culture of abattoir-derived equine ovarian fragments presented better morphological integrity when supplemented with FSH for 6 days, in comparison with the MEM culture group. However, no clear effects were observed with FSH regarding the promotion of activation from a primordial to a developing follicle.
1 INTRODUCTION
The development of new reproductive biotechnologies for horses faces the obstacle of massive follicle loss, a process that occurs naturally in the physiology of mammalian females. Additionally, mare ovaries have a low follicle density, which creates a challenge for any reproductive technique (Gomes et al., 2015). Approximately 99% of the follicles present in the reserve pool undergo follicular atresia, and the majority of the population does not reach follicular ovulation (Hickey et al., 2005; Haag et al., 2013a).
In vitro culture is a technique that enables the recovery of pre-antral follicles and promotes follicular maturation in vitro (Figueiredo, Rodrigues, Silva, & Santos, 2011; Andrade et al., 2012). Pre-antral follicles represent 90% of the follicular population in the ovary and are responsible for providing the structures recruited for each follicular wave (Saumande, 1991; Gomes et al., 2015). Several components have been considered for use to improve the in vitro culture of follicles. For example, follicle-stimulating hormone (FSH) is a crucial factor required for the in vitro development and survival of pre-antral follicles. Additionally, FSH has an important role in follicular activation in several species (Gutierrez, Ralph, Telfer, Wilmut, & Webb, 2000; McLaughlin, Bromfield, Albertini, & Telfer, 2010).
The action of FSH is mainly stimulatory, and it affects follicular growth through specific receptors known as G protein-coupled receptors (Almeida et al., 2011). These receptors use cyclic adenosine monophosphate (cAMP), and a signalling cascade that stimulates the cellular response through the junction with the receptor is triggered (Saraiva et al., 2011). Although the action of FSH on the receptor is restricted to granulosa cells and has been detected in developing pre-antral follicles, some evidence has demonstrated the presence of these receptors in the oocytes as well, suggesting an additional effect.
The actions of FSH have been evaluated in some species, and these studies have shown that FSH maintains follicular viability and promotes follicle growth in in vitro culture (Saraiva et al., 2011; Barros et al., 2013). However, studies concerning the influence of FSH on the in vitro culture of equine pre-antral follicles are scarce.
Therefore, the objective of this study was to evaluate the effect of different concentrations of FSH (0, 10, 50, 100 and 200 ng/ml) on the in vitro culture of pre-antral follicles derived from the fragments of equine ovaries for two different culture durations (i.e., 2 or 6 days).
2 MATERIALS AND METHODS
This study was performed in accordance with the Animal Experimentation Ethics Committee at the State University of Londrina based on Federal Law 11.794. Unless otherwise mentioned, the culture media and other chemicals used in the present study were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
2.1 Collection of ovaries
The ovaries (n = 5) were collected from a local abattoir from mares of unknown breed, age and body condition scores in seasonal anoestrus. At the abattoir, the ovaries were washed with 70% ethanol, which was followed by a wash in PBS. At the time that the ovaries were harvested, large (maturing/growing) or small (regressing) CL and pre-ovulatory (>30 mm) follicles in one or both ovaries were present and use to classify them (CL or antral follicles in abundance were discarded). Immediately after slaughter, the ovaries were placed into PBS supplemented with penicillin (200 UI/ml) and streptomycin (200 mg/ml) following a standard protocol (Gomes et al., 2012). The ovaries were maintained at 4°C for 1 hour (i.e., during the transportation period to the laboratory). In the laboratory, the ovaries of each animal were stripped of the surrounding fat tissue and ligaments. The ovaries were sectioned in the sagittal plane, and a portion of the parenchyma (internal) of the five selected ovaries was cut into approximately 3 × 3 × 1 mm fragments. One fragment of each ovary was randomly selected and immediately fixed for histological analysis in Bouin solution (Control group; Day 0).
2.2 Experimental protocol
In the laboratory, successive ovarian fragments from each animal were cultured in 24-well culture dishes containing the base medium (1 ml/well; control) or the base medium supplemented with 10, 50, 100 or 200 ng/ml FSH (Folltropin®; Bioniche, Inc., Ontario, Canada). The base medium was minimum essential medium (MEM; product code M 7278; osmolarity 300 mOsm/L, pH 7.2–7.4; Silva et al., 2004; Haag et al., 2013a) supplemented with penicillin (200 UI/ml), streptomycin (200 mg/ml), BSA (1.25 mg/ml), hypoxanthine (2 mM), glutamine (2 mM), pyruvate (0.23 mM), insulin (6.25 mg/ml), transferrin (6.25 mg/ml) and selenium (6.25 mg/ml). This medium will be referred to as MEM+.
In vitro culture was performed at 39°C in an atmosphere of 5% CO2 for 2 or 6 days. The medium was changed every 2 days. Each treatment was repeated five times using the ten ovarian fragments from each mare. At the end of the culture period, the ovarian fragments were evaluated using histological techniques. All samples were fixed in Bouin's fixative for 24 hours. The ovarian fragments were dehydrated in ethanol, diaphanized in xylene, embedded in paraffin, cut serially at intervals of 5 μm and mounted on slides (Gomes et al., 2012). The ovaries were stained with periodic acid-Schiff (PAS) stain and counterstained with haematoxylin.
The follicles were classified according to the following developmental stages: (i) primordial follicles (the oocyte was surrounded by a single layer of flattened granulosa cells); (ii) developing follicles composed of primary follicles (the oocyte was surrounded by a single layer of cuboidal granulosa cells); and (iii) secondary follicles (the oocyte was surrounded by more than one complete layer of cuboidal granulosa cells). The activation and growth of the primordial follicles in the various developmental stages and the number of primordial and growing follicles were determined in the control group and in each in vitro-cultured treatment group.
The follicles were also classified as normal or degenerated, according to the morphology of the oocyte and the granulosa cells, to determine the rate of follicular atresia in the control group and each in vitro-cultured treatment group (Figure 1). Morphologically normal follicles had intact oocytes and granulosa cells organized in discrete layers without pyknotic nuclei. Degenerated follicles were defined as follicles that contained an oocyte with a pyknotic nucleus and/or an oocyte surrounded by disorganized granulosa cells that had detached from the basement membrane with retracted cytoplasm (Andrade et al., 2012; Haag et al., 2013a).
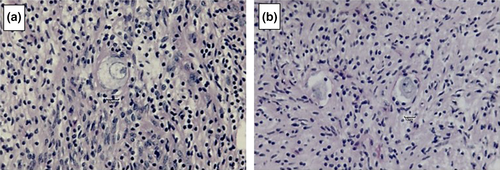
2.3 Statistical analyses
The percentages of the primordial follicles, developing follicles and morphologically normal pre-antral follicles in the control group replicates and at 2 and 6 days of culture in the various treatment groups were subjected to a Kolmogorov–Smirnov test for a normal distribution. The statistical model was non-parametric, and Fisher's exact test was used to compare the percentage of primordial follicles and development among the control group, MEM+ and the FSH-treated groups. The level of significance needed to reject the null hypothesis was p ≤ .05. All statistical analyses were performed using the statistical software Minitab® 16.1.1.
3 RESULTS
In this investigation, we evaluated 1,187 slides, which included 4,018 histological sections. Follicles were found in only 9.7% (388 of 4,018) of the fragments assessed, and they were in various stages of development. No follicles were found in 90.3% of the histological sections (3,630 of 4,018) in either the parenchyma or in the inner portion of the ovarian tissue. Of the 861 follicles evaluated, 488 (56.7%) were primordial follicles, 373 (43.7%) were developing follicles, and 59.7% were morphologically normal.
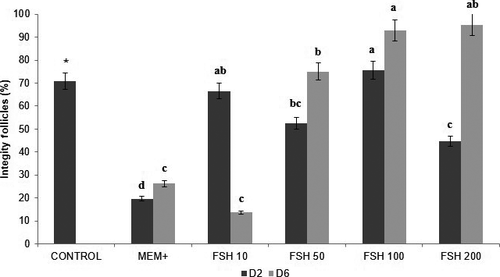
The control group had 86.8% (223 of 257) primordial follicles and 13.2% (34/257) in development, of which 70.8% (182 of 257) were classified as intact. The percentages of primordial and developing follicles for 2, 6 days and intact follicles are shown in Figures 2, 3 and 4, respectively. It was observed that the percentages of developing follicles at 2 days of culture remained the same at concentrations of 10, 50 and 100 ng/ml FSH (33.3%, 45.0%, and 34.1%, respectively). The developing follicles at Day 2 in the 200 ng/ml FSH treatment (64.9%) were different from the other treatments (p < .05), as were the percentages of primordial follicles compared to developing follicles.
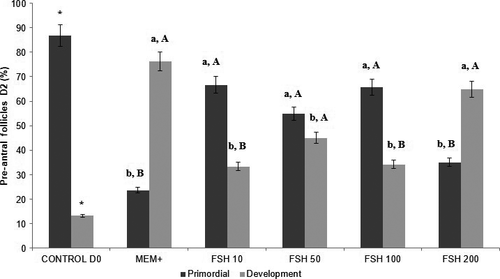
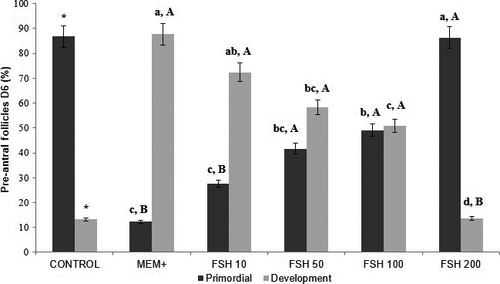
The 6-days in vitro culture had a higher proportion of developing follicles in the ten ng/ml FSH treatment (72.4%) compared to the other treatments (p < .05). Considering only the follicular categories within the same treatment, the 10 ng/ml treatment after 6 days of culture showed the best results, that is, the proportion of developing follicles was higher than the percentage of primordial follicles.
However, regarding the integrity and survival of pre-antral follicles, 100 ng/ml of FSH (75.6%) in cultivation for 2 days and 50, 100 and 200 ng/ml of FSH (75.0%,92.9% and 95.4%, respectively) in cultivation for 6 days provided the viability of follicles compared to the other groups (p < .05).
4 DISCUSSION
In this study, we showed that the addition of 50, 100 or 200 ng/ml FSH for 6 days preserved follicular integrity, as compared to 10 ng/ml FSH and MEM. In the 2-days in vitro culture, primordial follicles of equine ovaries had better morphological characteristics when cultured with 10, 50 or 100 ng/ml compared to 200 ng/ml FSH. In general, all FSH treatments preserved follicular integrity compared to MEM+. However, no clear effect of FSH on promoting the transition of follicles from the primordial to developing stages was noted. The proportion of primordial and developing follicles after 6 days of culture indicates that a higher concentration of FSH seemed to negatively affect the transition of a follicle from the primordial to the developing stages. This contrast was clearly evident according to the proportion of primordial and developing follicles in the MEM and FSH 200 ng/ml treatments. While a high percentage of developing follicles occurred in the treatment without FSH with only a few primordial follicles, the 200 ng/ml FSH treatment showed exactly the contrary. Our data are not in agreement with those reported by Aguiar et al. (2016), who reported that 50 ng/ml of FSH was the best treatment for promoting the growth of equine pre-antral follicles. Yet, the culture time was not the same. Additionally, those authors worked with mares from areas close to the Ecuadorean border where no seasonal anoestrous occurs, while our work was performed in the south of South America at a latitude at which mares have distinct reproductive seasons. Perhaps, these differences may have contributed to the differences between our data and the results from Aguiar et al. (2016). In other words, the in vitro development of follicles in ovarian fragments could be influenced by the origin of the samples. We formed this hypothesis based on the recent data from Alves et al. (2016), who showed changes in follicular activity and distribution according to the phase of the mare's oestrous cycle. In this way, we can hypothesize that the previous stage of ovarian activity may interfere with the in vitro follicular growth, including the response to FSH, which might explain the different results for the in vitro culture of the follicles in mares and other species with reproductive seasonality.
After 2 days of culture, it was not possible to identify a clear pattern of FSH in promoting primordial follicles to subsequent developmental stages. The same unclear pattern of FSH in the short term of the culture of equine primordial follicles was also described by Aguiar et al. (2016). Perhaps, the initial activity of equine primordial follicles may require other factors besides FSH.
Despite the unclear effect of FSH on the initial steps of follicular growth, it is interesting to consider the positive influence of FSH for retaining the morphological integrity of the equine primordial follicles. Many explanations are possible. For example, mare ovaries may need other growth factors to promote the activation of primordial follicles.
Despite some unclear aspects of the application of FSH on equine primordial follicles, we believe that this and other recent studies have shown interesting perspectives for using this gonadotropin. Perhaps the addition of other factors is necessary. In other species, FSH is involved in inducing the release of growth factors, proliferating granulosa cells and increasing the expression of paracrine factors, such as IGF-I, Activin, GDF-9 and BMP-15 (Thomas, Ethier, Shimasaki, & Vanderhyden, 2005; Van Den Hurk & Zhao, 2005; Aguiar et al., 2016).
Interestingly, equine follicles cultured only in MEM+ for two and 6 days also demonstrated in vitro development. Some studies have shown that in vitro culture stimulates most of the primordial follicles to spontaneously initiate development (Wandji, Srsen, Vossm, Eppig, & Fortune, 1996; Braw-Tal & Yossefi, 1997). This is probably due to the activation of primordial follicles as a consequence of the fragmentation of the ovarian cortex (Cushman, Wahl, & Fortune, 2002). In murine females, ovarian fragmentation increases actin polymerization and disrupts the signalling pathway, which leads to the increased expression of growth factors (Kawamura et al., 2013). It is not clear why the primordial follicles enclosed in fragments obtained from dead mares become spontaneously activated. However, this property seems to have been considered in all strategies of artificial protocols for promoting follicular growth in the laboratory. Yet, despite the possibility of developing of follicles without FSH, the MEM+ culture did not maintain the integrity of the developing pre-antral follicles.
According to Duarte et al. (2013), the addition of FSH to the culture medium is essential to promote the activation of pre-antral follicles, in addition to its role in maintaining follicular integrity. The same study also found that along with FSH, the addition of a growth factor similar to Insulin-II (IGF-II) benefits goat follicles, whereas the lack of FSH resulted in increased resonated follicular degeneration. In our study, it was observed that the FSH added to the in vitro culture medium promoted a gradual increase in the number of normal developing follicles.
Only FSH concentrations of 100 ng/ml (D2), 50 ng/ml (D6), 100 ng/ml (D6) and 200 ng/ml (D6) preserved the follicular integrity. Not all concentrations used in this study had favourable effects on the in vitro culture. Barros et al. (2013) achieved good results with the development and integrity of goat pre-antral follicles in in vitro culture using an FSH concentration of 100 ng/ml for 6 days. Similar studies were performed by Behl and Pandey (2002) and Costa et al. (2010) with sheep, and 100 ng/ml and 200 ng/ml FSH also enabled the development and preservation of follicular integrity. However, results from other species must be considered with caution because of the many differences in the morphology and the physiology of equine ovaries.
The number of follicles varied in each treatment in this study, which is common in experiments with equine ovarian follicles (Haag et al., 2013b; Alves et al., 2017). Several teams have described the challenges in obtaining a better understanding of the ovarian reserve of mares. A common restriction is related to the high individual variation in the equine pre-antral follicle population (Driancourt, Paris, Roux, Mariana, & Palmer, 1982). Some of the difficulties seem to be very specific to equine ovaries, such as variability of the density and distribution of follicles with the area of the ovary and the phase of the oestrous cycle (Alves et al., 2016). The small number of slaughterhouses for horses worldwide causes another critical restriction in their study. Given all of these aspects, we believe the number of follicles in our treatment groups was quite acceptable, providing enough follicles to ensure the accuracy of our results.
During the histological procedures of our work, we identified many sections without follicles, a clear demonstration of the variation in the follicular distribution in the ovary, which was also a situation previously reported by Gomes et al. (2015). This aspect was also described by Alves et al. (2015), and it was related to the influence of the regulatory genes that controlled the survival, proliferation, colonization and inclusion of primordial germ cells.
In conclusion, the in vitro culture of abattoir-derived equine ovarian fragments retained their morphological integrity when supplemented with FSH for 6 days. However, no clear effects were observed with FSH regarding activation from primordial to developing follicles.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
MCM, RGG, LAL and MMS did the research design. CBS, SMG and TRRB worked in the experiments. AGL, IB, FM and CBC prepared the acquisition and data analysis and interpretation.




