Cell cycle synchronization and analysis of apoptosis-related gene in skin fibroblasts from domestic cat (Felis silvestris catus) and kodkod (Leopardus guigna)
Funding information
This work was supported in part by CONICYT Ministerio de Educación: CONICYT-PCHA/Doctorado Nacional/2014 – 21140320
Contents
The kodkod population is in constant decrease and the somatic cell nuclear transfer (SCNT) might help to preserve the genetic pool of this species. The cell cycle synchronization of donor cells plays a crucial role in SCNT. The objective of this research was to evaluate two different methods for quiescence induction, serum starvation (SS) and contact inhibition (CI), both for 1, 3 and 5 days, on skin fibroblast from domestic cat and kodkod. Flow cytometry analysis revealed that in domestic cat, SS and CI, both at 3 and 5 days, increased the percentage of fibroblasts in G0/G1 compared to growing cells (GC) (p < .05). In kodkod, only SS for 3 and 5 days and CI for 1 and 3 days increased the percentage of fibroblasts in G0/G1 compared to GC (p < .05). Viability analysis by differential staining revealed that SS for 5 days decreased the proportion of live fibroblasts in domestic cat and kodkod (p < .05). Regarding gene expression analysis, in domestic cat fibroblasts, no differences were found in the BAX/BCL2 ratio in SS and CI (both at 1, 3 and 5 days) compared to GC. In kodkod fibroblasts, BAX/BCL2 ratio was increased in CI at 3 and 5 days compared to SS at 3 and 5 days (p < .05). In conclusion, in kodkod fibroblasts SS for 5 days and CI after 3 days might have a negative impact on cellular viability. According to these results, we suggest SS for 3 days for cell cycle synchronization in kodkod fibroblasts.
1 INTRODUCTION
Actually, five different species of endemic wild felids inhabit in Chile; among these species, the kodkod (Leopardus guigna) is the only one classified as vulnerable according to the Red List of Threatened Species of the International Union for Conservation of Nature (IUCN 2015). The geographical distribution of the kodkod is the most reduced of any other feline in America (Napolitano, Gálvez, Bennett, Acosta-Jamett, & Sanderson, 2015). Because of their restricted distribution, the kodkod is very susceptible to the loss of habitat, which along with the hunting is the principal reason of its reduced population (Napolitano et al., 2015). At present, the interspecies somatic cell nuclear transfer (iSCNT) is considered a valuable tool that might help to preserve the genetic pool of endangered felids without the necessity of use their gametes (Gómez et al., 2003). Through iSCNT, matured oocytes from a domestic cat can be used as recipient cytoplasts for somatic cells from different felid species, enabling the in vitro embryo production and production of offspring of endangered felids (Gómez et al., 2004, 2008). However, reprogramming a differentiated cell by SCNT is still an inefficient process, which is associated with aberrant epigenetic modifications and a low developmental competence of cloned embryos (Blelloch et al., 2006). It has been reported that the cell cycle phase of donor cells might affect the ploidy, the chromosome integrity and therefore the development of cloned embryos (Campbell, Loi, Otaegui, & Wilmut, 1996). The nuclear transfer of cells in G0/G1 to metaphase II (MII) oocytes with high MPF levels allows a correct ploidy and an improvement of the developmental competence of cloned embryos (Campbell, 1999). Serum starvation (SS) is the most used method for cell cycle synchronization in different species, because it generates a high percentage of cells in G0/G1 phase (Gómez et al., 2003; Kues et al., 2000). Yet, the induction of quiescence by SS during a prolonged period increases significantly the proportion of apoptotic cells and DNA fragmentation (Koo, Hossein, Hong, Martinez-Conejero, & Lee, 2009; Kues et al., 2000). On the other hand, the contact inhibition (CI) method generates a proportion of cells in G0/G1 similar to SS without the high apoptosis incidence (Hayes et al., 2005). Furthermore, no statistical differences have been found in the blastocyst rate generated by SCNT using somatic cell synchronized in G0/G1 by SS or CI (Hayes et al., 2005).
Regarding the cell cycle synchronization in felids, Wittayarat et al. (2013) evaluated the cell cycle synchronization by SS, CI and roscovitine treatment in fibroblast from domestic cat, Asian golden cat (Pardofelis temminckii), marbled cat (Pardofelis marmorata) and leopard cat (Prionailurus bengalensis), concluding that the fibroblasts from these species respond differently to each treatment. For this reason, it is essential to establish a specific protocol for cell cycle synchronization for each particular species. No previous information has been reported concerning to the cell cycle synchronization in somatic cells from kodkod. The objective of this research was to evaluate the cell cycle synchronization of kodkod fibroblast by SS and CI and perform a viability and apoptosis analysis after each treatment. The final purpose was selecting the best treatment for cell cycle synchronization in the kodkod.
2 MATERIALS AND METHODS
All chemical reagents were purchased from Sigma-Aldrich Chemicals Company (St. Louis, MO, USA), except for those otherwise indicated.
2.1 Experimental design
Skin samples from domestic cat and kodkod were processed for fibroblasts isolation, which were in vitro-cultured and expanded until the passage five. The fibroblasts of domestic cat and kodkod were subjected to six different treatments for quiescence induction, CI (for 1, 3 and 5 days), SS (for 1, 3 and 5 days) and growing cell reached 60%–80% confluence (GC) were used like negative control. For cell cycle synchronization analysis, the fibroblasts subjected to each treatment were fixed and then stained with propidium iodide for flow cytometry analysis, and the percentage of cells in apoptosis, G0/G1, S and G2/M were estimated. For the cellular viability assessment, after each treatment, the cells were stained with propidium iodide and acridine orange and were counted using a dual fluorescence cell counter. Finally, an apoptosis analysis was performed by RT-qPCR evaluating the relative gene expression of the apoptotic gen BAX and the anti-apoptotic gene BCL2, in fibroblasts from domestic cat and kodkod subjected to each treatment. According to these results, the treatment that generated the highest proportion of cells in G0/G1 phase along with a reduced apoptosis incidence was selected like the optimum treatment for cell cycle synchronization in kodkod fibroblasts.
2.2 Animals and ethics statement
All animal experiments were approved by the Ethics Committee of the Faculty of Veterinary Sciences, Universidad de Concepcion. In the case of domestic cats, the skin sample collection was permitted by previous consent of their owners. The kodkod samples were taken from animals from the wildlife rehabilitation centre of the Universidad de Concepcion (Centro de Rehabilitación De Fauna Silvestre UdeC) with previous authorization of their managers.
2.3 Establishment of cell lines from domestic cat and kodkod
For the establishment of cell lines, skin samples from domestic cat were taken from the abdominal region of females subjected to ovariohysterectomy. In the case of kodkod, skin samples were taken by ear puncturing from previously anaesthetized animals. The samples were transported to the laboratory in 1x PBS with 1% of antibiotic–antimycotic solution (10,000 U/ml of penicillin, 10,000 μg/ml of streptomycin and 25 μg/ml of amphotericin B, HyClone; GE Healthcare Inc., Logan, UT, USA). Once in the laboratory, the remaining hair and fat were removed and the samples were cut into small pieces. The small pieces of tissue were washed in 1x PBS with 1% of antibiotic–antimycotic solution three to five times and then were digested in 1–2 ml of DMEM:F12 (Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA) supplemented with 10% of FBS (HyClone; GE Healthcare Inc.) and 1 mg/ml of collagenase A, for 18 hr to 38.5°C (Tovar, Navarrete, Rodríguez, Skewes, & Castro, 2008). After the incubation, the solution was vigorously pipetted and seeded in T25 culture flask in a final volume of 4 ml of DMEM: F12 medium supplemented with 30% of FBS, 1 mM pyruvate, 2 mM L-glutamine and 1% of antibiotic–antimycotic solution and were incubated in a 5% CO2 humidified atmosphere, to 38.5°C.
Once the cultures reached approximately 80% of confluence, the fibroblasts were disaggregated using 0.25% trypsin–EDTA solution (Gibco; Thermo Fisher Scientific Inc.) during 4 min. The pelleted fibroblasts were resuspended in frozen medium consisting of 8% DMSO, 22% FBS and 70% DMEM:F12 and placed in cryogenic vials. The vials were frozen at 1°C/min using a freezing container (Mr. Frosty; Nalgene, Rochester, NY, USA) placed inside a −80°C freezer for 3 days and then were transferred to liquid N2. Subsequently, the vials were thawed and the fibroblasts were expanded in 100-mm2 culture dishes, under the same conditions described previously but reducing the FBS concentration to 10%. The fibroblasts were expanded and frozen continually until the passage five. Three cell lines of domestic cat and two cell lines of kodkod fibroblasts were established.
2.4 Cell treatments for quiescence induction
Fibroblasts of domestic cat and kodkod between the passages 5 and 6 were subjected to the different treatments. In both species, seven experimental groups were made, and SS (for 1, 3 and 5 days), CI (for 1, 3 and 5 days) and growing cells reached 60%–80% of confluence (GC) were used like negative control. For the SS treatments, growing cells with 60%–80% of confluence were used, the normal culture medium containing 10% of FBS was changed for culture medium containing 0.5% of FBS and was changed every 1–2 days if it was required. For CI, it was allowed that the cells reached 100% of confluence and these were kept in culture for 1, 3 and 5 additional days, once reached the confluence, the medium was changed every 1–2 days.
2.5 Cell cycle synchronization analysis
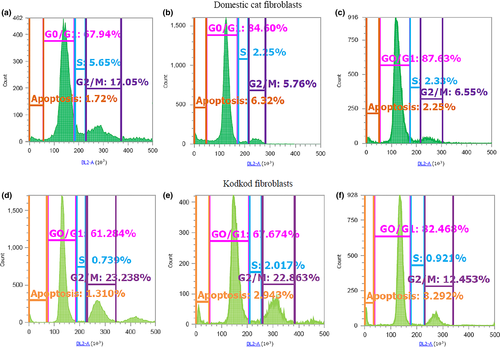
For this experiment, the fibroblasts were cultured in T25 flask. Once finished each quiescence induction treatment, the cells were trypsinized and then centrifuged. The pellet was washed two to three times with 1x PBS to eliminate the remaining culture medium. The cells were fixed with 90% methanol to −20°C for 10 min. Subsequently, the cells were washed two times with 1x PBS and then were incubated in a solution containing 50 μg/ml of propidium iodide and 100 μg/ml of RNAsa A, for 30 min to 37°C, in the dark. After the incubation, the cells were centrifuged and the pellet was resuspended in 1–1.5 ml of 1x Focussing fluid (Thermo Fisher Scientific Inc., Waltham, MA, USA). Flow cytometry analysis was conducted using the Attune® NxT Acoustic Focusing Cytometer (Thermo Fisher Scientific Inc.); in each run, at least 10,000 events were collected to 200 μl/min speed, using the channel BL2-A (574/26 nm filter). The histograms, dot plots and density plots were made using the Attune® NxT SW v1.1 software. The percentage of fibroblasts in the different phases of cells cycle (G0/G1, S and G2/M) were estimated (Figure 1). Moreover, according to previous report from Wittayarat et al. (2013), the fluorescence peak before the G0/G1 peak in the histogram was used to determine the percentage of apoptotic cells in each treatment.
2.6 Cellular viability assessment
For this experiment, the domestic cat and kodkod fibroblasts were cultured in 35-mm culture dishes. Three technical replicates were made for each treatment. At the end of each treatment, the cells were trypsinized, centrifuged and then resuspended in 100 μl of culture medium. After that, 18 μl of the cell suspension was stained with 2 μl of a solution of acridine orange and propidium iodide (Logos Biosystems, Annandale, VA, USA) during 2 min to 37°C. The live cells were stained green by the acridine orange meanwhile the apoptotic cells were stained red by the propidium iodide. Subsequently, 10 μl of the stained cells were loaded in Photon slides™ (Ultra-low fluorescence counting slides; Logos Biosystems) and were placed in an automated dual fluorescence cell counter (Luna-FL™, Logos Biosystems) for the cell viability analysis. Additionally, the mean diameter of the total cells was calculated by the Luna-FL cell counter.
2.7 Gene expression analysis of BAX and BCL2
The remaining proportion of the cells used in the cellular viability assessment was used for the relative expression analysis of the apoptotic gene BAX and the anti-apoptotic gene BCL2, by RT-qPCR. The cells were washed with 1x PBS and centrifuged in a 1.5-ml tube and stored to −80°C until the RNA extraction procedure.
2.7.1 RNA extraction and reverse transcription reaction
For the RNA extraction, a total of 21 samples of each species (three replicates for each treatment) were used. The procedure was performed using the E.Z.N.A RNA extraction kit (Omega Bio-Tek, Norcross, GA, USA) according to the manufacturer's instruction. Once RNA extraction was completed, the RNA concentration of each sample was quantified using a spectrophotometer (Epoch; BioTek Instruments Inc., Winooski, VT, USA) and then the samples were stored at −80°C until the reverse transcription (RT) procedure. Prior to RT procedure, the samples were treated with DNAsa I (0.04 U/μl) and incubated at 37°C for 30 min, for the removal of genomic DNA. Subsequently, the reaction was stopped adding 25 Mm of EDTA (1 μl) to the samples and were incubated at 65°C for 10 min. The RT reaction was made in a final volume of 20 μl, using 100 ng of the extracted RNA, 5 μM of random primers, 100 mM of each dNTPs and incubated to 65°C for 5 min. After that, the samples were placed in ice and 4 μl of 5X first-strand buffer, 2 μl of DTT and 10 U of RNase out were added to the samples, which were incubated at 37°C for 2 min. Lastly, the samples were incubated with 200 U of M-MLV-Reverse transcriptase (Invitrogen; Thermo Fisher Scientific Inc., Waltham, MA, USA) at 25°C for 10 min, 37°C for 50 min and finally at 70°C for 15 min. The samples were stored at −20°C until they were required for PCR.
2.7.2 Real-time quantitative polymerase chain reaction
The gene expression analysis was made by real-time quantitative polymerase chain reaction (qPCR) using the standard curve method similar to that previously described by Veraguas, Gallegos, Velasquez, Castro, and Rodriguez-Alvarez (2017). The reaction was run on a MX3000P Real-Time PCR device (Agilent, Santa Clara, CA, USA). Melting curves and threshold (CP) values were calculated with built-in software for all the runs. The housekeeping gene SDHA was used like internal control. Only those PCR experiments with an efficiency (including standard curves and samples) whiting the range of 90%–110% and a correlation coefficient of at least 0.9 were used for gene expression analysis. Only samples within the quantification range of the standard curve were considered for the analysis. The primers used and PCR conditions for each gene are presented in Table 1.
| Gene name | Primer sequences (5′-3′) | Annealing temperature (°C) | Product length (bp) | Accession number (NCBI) |
|---|---|---|---|---|
| SDHA |
F: GCAGCAGAAGAAGCCATTTG R: GTCATTGACGGGTCTGTACTC |
58 | 103 | XM_003981595.1 |
| BAX |
F: GTCGTTGCCCTCTTCTACTTT R: TCTCGAAGGAAGTCCAGTGT |
55 | 110 | NM_001009282.1 |
| BCL2 |
F: GTGGATGACTGAGTACCTGAAC R: GGACAGCCAGGAGAAATCAA |
55 | 124 | NM_001009340.1 |
2.8 Statistical analysis
For cell cycle synchronization analysis and cellular viability assessment, the percentage of data were subjected to arcsine transformation. The percentage of transformed data and the data of cellular diameter measurement were analysed using the general lineal model (GLM) procedure, and mean comparison was made by Tukey's test, all using the SAS software (Cary, NC, USA). RT-qPCR results were analysed using the Kruskal–Wallis nonparametric test in the statistical software InfoStat (2014 Version; University of Cordoba, Argentina). Statistical differences were considered at p < .05.
3 RESULTS
3.1 Cell cycle synchronization analysis of domestic cat and kodkod fibroblasts
The results of this experiment demonstrated that domestic cat and kodkod fibroblasts respond differently to each method of cell cycle synchronization. Both SS and CI increased the proportion of domestic cat and kodkod fibroblasts in G0/G1 phase, but with differences between these species.
In the domestic cat, SS and CI, both for 3 and 5 days, increased significantly the proportion of fibroblasts in G0/G1 phase and reduced the proportion in S and G2/M phases, compared to GC (p < .05). No differences were found in the proportion of fibroblasts in the phases G0/G1, S and G2/M among SS for 3 and 5 days and CI for 3 and 5 days (p > .05). Furthermore, no differences were found in the proportion of apoptotic cells among SS (for 1, 3 and 5 days), CI (for 1, 3 and 5 days) and GC (p > .05) (Table 2).
| Domestic cat | Kodkod | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | % Apoptosis | % G0/G1 | % S | % G2/M | % Apoptosis | % G0/G1 | % S | % G2/M |
| GC | 1.2 ± 1a | 63 ± 8.7a | 5.7 ± 0.3a | 21 ± 6.9a | 1 ± 0.5a | 53.6 ± 8.8a | 3.1 ± 1.8a | 29.1 ± 5.5a |
| SS 1 day | 2.7 ± 1.4a | 79.1 ± 6.4ab | 2.7 ± 0.8b | 12.1 ± 3.9ab | 1.9 ± 1.7a | 66 ± 4.4ab | 2.4 ± 0.2a | 24.3 ± 6.2ab |
| SS 3 days | 2.5 ± 0.6a | 85.2 ± 2.4b | 2.2 ± 0.2b | 8.5 ± 2.2b | 2 ± 2.3a | 75.2 ± 7.2bc | 3.4 ± 2.5a | 16 ± 9.2ab |
| SS 5 days | 1.5 ± 0.9a | 87.2 ± 3.6b | 2.7 ± 1b | 7.1 ± 1.7b | 2.1 ± 1.7a | 82.5 ± 4.8c | 2.9 ± 1.6a | 10.5 ± 5.7b |
| CI 1 day | 4 ± 1.7a | 79.6 ± 6.1ab | 3.3 ± 1ab | 10.5 ± 3.2ab | 1.7 ± 1.4a | 71.1 ± 3.5bc | 3 ± 1.8a | 19.5 ± 3ab |
| CI 3 days | 3.1 ± 1.9a | 85.5 ± 7b | 2.5 ± 1.2b | 7.3 ± 4.9b | 1.8 ± 1.6a | 71.1 ± 4.8bc | 3.7 ± 1.5a | 18.5 ± 4.3ab |
| CI 5 days | 4.7 ± 1.6a | 85.4 ± 2b | 2.6 ± 0.3b | 6 ± 0.8b | 4.1 ± 3.6a | 66.4 ± 5.1ab | 3.6 ± 2a | 19.8 ± 5.2ab |
- a, b, c: different superscripts within a column indicate significant difference (p < .05).
- CI, contact inhibition; GC, growing cells; SS, serum starvation.
In the kodkod, SS for 3 and 5 days, and CI for 1 and 3 days increased significantly the proportion of fibroblasts in G0/G1 phase compared to GC (p < .05). The SS method for 3 and 5 days generated the higher proportion of kodkod fibroblasts in G0/G1 phase. On the other hand, differently to domestic cat fibroblasts, CI for 5 days did not increase the proportion of kodkod fibroblasts in G0/G1 phase compared to GC (p > .05). Furthermore, CI for 5 days had the higher proportion of apoptotic cells compared to the other treatments, although no statistical difference was found (p > .05). Only SS for 5 days reduced significantly the proportion of kodkod fibroblasts in G2/M phase (p < .05) (Table 2).
3.2 Viability assessment and measurement of domestic cat and kodkod fibroblasts after quiescence induction
According to the viability analysis by differential staining, in both the domestic cat and kodkod, only SS for 5 days reduced significantly the proportion of live fibroblasts compared to GC (p < .05). Additionally, the measurement of domestic cat fibroblasts revealed that SS for 3 and 5 days and CI for 1, 3 and 5 days reduced significantly the size of the cells compared to GC (p < .05). On the other hand, in kodkod fibroblasts, only SS for 5 days and CI for 3 and 5 days reduced significantly the cellular size compared to GC (p < .05) (Table 3).
| Treatment | Domestic cat | Kodkod | ||
|---|---|---|---|---|
| % Viability | Cellular size (mm) | % Viability | Cellular size (mm) | |
| GC | 94.5 ± 0.5a | 14.2 ± 0.4a | 97.3 ± 1.8a | 16.8 ± 0.2a |
| SS 1 day | 90.2 ± 5.2a | 13.2 ± 0.8ab | 96.3 ± 1.2a | 15.7 ± 0.5ab |
| SS 3 days | 86.8 ± 5.5ab | 10.9 ± 1.6bc | 94.3 ± 1.7a | 14.8 ± 1.9ab |
| SS 5 days | 74.7 ± 3.6b | 10.2 ± 0.6c | 83.5 ± 7.3b | 13.5 ± 1.2b |
| CI 1 day | 95.9 ± 2.6a | 10.8 ± 0.8bc | 94.8 ± 3a | 14.1 ± 0.9ab |
| CI 3 days | 94.1 ± 1.5a | 11.4 ± 1.2bc | 95.4 ± 2.7a | 13.4 ± 0.2b |
| CI 5 days | 89.6 ± 3.2a | 9.8 ± 1c | 97.2 ± 0.2a | 13 ± 0.6b |
- a, b, c: different superscripts within a column indicate significant difference (p < .05).
- CI, contact inhibition; GC, growing cells; SS, serum starvation.
3.3 Relative expression of BAX and BCL2 in domestic cat and kodkod fibroblasts after quiescence induction
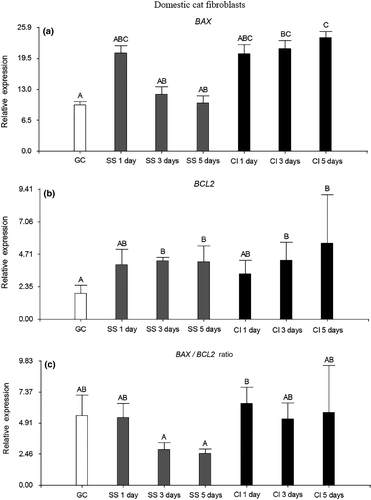
In the domestic cat fibroblasts, only CI for 3 and 5 days increased significantly the relative expression of the apoptotic gene BAX compared to GC (p < .05). Besides, the relative expression of BAX was higher in CI for 5 days than in SS for 3 and 5 days (p < .05). On the other hand, SS and CI, both after 3 and 5 days of treatment, increased significantly the relative expression of the anti-apoptotic gene BCL2 compared to GC (p < .05). Finally, when the BAX/BCL2 ratio was assessed, no statistical differences were found among all the treatments compared to GC (p > .05). This could be explained because in CI for 3 and 5 days, the increased relative expression of BCL2 might have counteracted the high levels of BAX, which was reflected in a BAX/BCL2 ratio similar to GC (Figure 2).
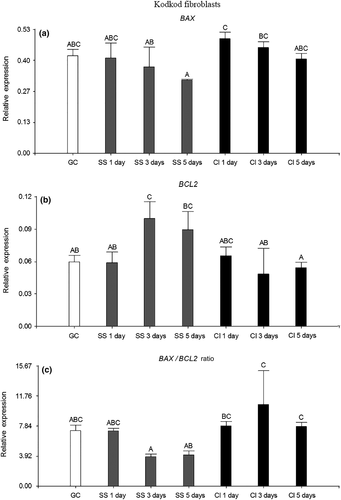
Regarding to kodkod fibroblasts, no statistical differences were found in the relative expression of BAX among all the treatments compared to GC (p > .05). However, the relative expression of BAX was significantly higher in CI for 1 and 3 days than in SS for 5 days (p < .05). In contrast, SS for 3 days increased significantly the relative expression of BCL2 compared to GC (p < .05). Furthermore, the relative expression of BCL2 was higher in SS for 3 and 5 days than in CI for 5 days (p < .05). As a result, no statistical differences were found in the BAX/BCL2 ratio among all the treatments compared to GC (p > .05). However, CI for 3 and 5 days had an elevated BAX/BCL2 ratio, which was significantly higher than in SS for 3 and 5 days (p < .05). This might be caused because in the SS method, the relative expression of BCL2 increased along with time of treatment, decreasing the levels of BAX. Instead, in CI, the relative expression of BCL2 remained almost constant through the time of treatments, which was reflected in an elevated BAX/BCL2 ratio at 3 and 5 days (Figure 3).
4 DISCUSSION
The correct interaction between the cell cycle phase of the donor nucleus and the recipient cytoplast is essential in the SCNT (Campbell, 1999; Campbell et al., 1996). Quiescent cells in G0 stage undergo chromatin condensation, have a reduced transcriptional and translational activity and suffer an active degradation of mRNA (Campbell, 1999). For these reasons, it has been postulated that the chromatin of quiescent cells could be more amenable to reprogramming by SCNT when a MII oocyte is used like recipient cytoplast (Campbell, 1999; Wilmut, Schnieke, McWhir, Kind, & Campbell, 1997). Dolly, the first cloned mammal, was generated from a selected mammary gland cell synchronized in G0/G1 phase after SS for 5 days (Wilmut et al., 1997). Since then, SS has been widely used for cell cycle synchronization in different species, including sheep, bovine, porcine, dogs and cats (Gómez et al., 2003; Hayes et al., 2005; Koo et al., 2009; Kues et al., 2000; Wilmut et al., 1997). According to Gómez et al. (2003), in the domestic cat and African wildcat, SS for 5 days generates a higher proportion of skin fibroblasts arrested in G0/G1 phase than CI and roscovitine treatment. However, SS also induces higher rates of DNA fragmentation in fibroblasts from domestic cat and African wildcat (Gómez et al., 2003). This is in accordance with a previous report, which indicated that after a prolonged SS, some domestic cat cells start detaching and undergo apoptosis, which increase along with the time of treatment (Bochenek et al., 2001). On the other hand, de Barros et al. (2010) described that CI and the association of CI and SS are more efficient to synchronize foetal cat fibroblast in G0/G1 phase than SS alone. Regarding to our results, in the domestic cat, SS and CI, both for 3 and 5 days, increased similarly the proportion of fibroblasts arrested in G0/G1 phase. However, SS for 5 days reduced significantly the viability of domestic cat fibroblasts.
Furthermore, we observed dissimilar responses between domestic cat and kodkod fibroblasts after been exposed to the different treatments of quiescence induction. This was in agreement with results reported by Wittayarat et al. (2013), which described differences in the cell cycle synchronization among different species of the Felidae family. In our research, SS for 3 and 5 days produced the highest proportion of kodkod fibroblasts arrested in G0/G1 phase. However, after viability assessment, similar to that observed in the domestic cat, only SS for 5 days reduced significantly the viability of kodkod fibroblasts. On the other hand, only CI for 1 and 3 days increased the proportion of kodkod fibroblast in G0/G1 compared to GC, which was not observed after CI for 5 days. This might be related to the increased proportion of apoptotic cells detected after CI for 5 days in the flow cytometry analysis. This was similar to a previous report from Wittayarat et al. (2013), which described that in the marbled cat, no differences were found in the proportion of fibroblasts in G0/G1 phase after CI for 5 days compared to GC at 60%–70% of confluence. These results might indicate that both SS and CI, after 5 days of treatment, could have a negative effect in kodkod fibroblasts viability.
The Bcl2 protein family plays a crucial role in apoptosis regulation, protection against pathogens, development and homoeostasis (Adams & Cory, 1998). BCL2 is an anti-apoptotic and antiproliferative gene, and its overexpression prolongs cell survival (Chao & Korsmeyer, 1998). BAX is a pro-apoptotic gene homolog to BCL2, and its overexpression accelerates the apoptosis rate in response to a death signal (Oltvai, Milliman, & Korsmeyer, 1993). It has been described that BAX heterodimerizes with BCL2 and homodimerizes with itself (Chao & Korsmeyer, 1998; Oltvai et al., 1993). When BCL2 is overexpressed, it heterodimerizes with BAX reducing the apoptosis incidence. Therefore, the BAX/BCL2 ratio has been established like an important indicator of apoptosis susceptibility (Chao & Korsmeyer, 1998; Oltvai et al., 1993). In our research, in the domestic cat fibroblasts, no differences were found in the BAX/BCL2 ratio among all the quiescence induction treatments and GC. However, in the kodkod fibroblasts, CI for 3 and 5 days had the highest BAX/BCL2 ratios, which were significantly different compared to SS for 3 and 5 days. This was in agreement with the increased proportion of apoptotic cells and the low proportion of cells in G0/G1 phase observed after CI for 5 days in the flow cytometry analysis, which could indicate a negative effect after a long period of cell confluence in kodkod fibroblasts. On the other hand, the Bcl2 protein family members also participate in the cell cycle control, and the anti-apoptotic genes BCL2 and BCL-XL have an antiproliferative role and kept the cells arrested in G0 phase, while BAX is a proliferative gene which accelerates the progression to S phase (Zinkel, Gross, & Yang, 2006). This indicates that the high relative expression of BCL2 observed in kodkod fibroblasts after SS for 3 and 5 days might also be related to the high proportion of cells arrested in G0/G1 phase. Finally, in the in vitro embryo production systems, a reduced Bax/Bcl2 and Bax/Bcl-xl protein ratios have been related to an apoptosis reduction and an enhanced embryo development (Cui et al., 2011; Gupta, Uhm, Han, & Lee, 2007; Kölle, Stojkovic, Boie, Wolf, & Sinowatz, 2002; Yang & Rajamahendran, 2002). Therefore, the use of kodkod fibroblasts with a reduced BAX/BCL2 ratio for SCNT might have a positive effect in the development of reconstructed embryos compared to used fibroblasts with a high BAX/BCL2 ratio.
In conclusion, domestic cat and kodkod fibroblasts respond differently to the methods of cell cycle synchronization. In kodkod fibroblasts, the proportion of cells arrested in G0/G1 phase did not increase after CI for 5 days, which was in agreement with an increased BAX/BCL2 ratio and might be related to a high apoptosis incidence. According to these results, we suggest SS for 3 days for cell synchronization in kodkod fibroblasts, because it generated a high proportion of cells in G0/G1 phase along with a reduced impact in cellular viability and apoptosis. The results of this research could be relevant for the generation of kodkod embryos by iSCNT.
ACKNOWLEDGEMENTS
We express our appreciation to the Centro de Rehabilitación De Fauna Silvestre UdeC for allowing us to collect the skin samples from kodkod. In addition, we are grateful to Dr. Claudio Zuñiga Olivares and Dr. Daniela Doussang Ortíz for their collaboration in the collection of skin samples from domestic cats.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
D. Veraguas performed cell culture, cell cytometry analysis, RT-qPCR, statistical analysis and manuscript writing. P.F. Gallegos performed cell culture, cell cytometry analysis, viability analysis and RT-qPCR. F.O. Castro collaborated in the experimental design and critical reading. L. Rodriguez-Alvarez conceived the original idea and collaborated in the experimental design and critical reading.




