Expression of aquaporin 4 in the chicken ovary in relation to follicle development
Contents
In the mammalian ovary, aquaporins (AQPs) are thought to be involved in the regulation of fluid transport within the follicular wall and antrum formation. Data concerning the AQPs in the avian ovary is very limited. Therefore, the present study was designed to examine whether the AQP4 is present in the chicken ovary, and if so, what is its distribution in the ovarian compartment of the laying hen. Localization of AQP4 in the ovarian follicles at different stage of development was also investigated. After decapitation of hens the stroma with primordial follicles and white (1–4 mm), yellowish (4–8 mm), small yellow and the three largest yellow pre-ovulatory follicles F3-F1 (F3 < F2 < F1; 20–36 mm) were isolated from the ovary. The granulosa and theca layers were separated from the pre-ovulatory follicles. The AQP4 mRNA and protein were detected in all examined ovarian compartments by the real-time PCR and Western blot analyses, respectively. The relative expression of AQP4 was depended on follicular size and the layer of follicular wall. It was the lowest in the granulosa layer of pre-ovulatory follicles and the highest in the ovarian stroma as well as white and yellowish follicles. Along with approaching of the largest follicle to ovulation the gradual decrease in AQP4 protein level in the granulosa layer was observed. Immunoreactivity for AQP4 was present in the granulosa and theca cells (theca interna ≥ theca externa > granulosa). The obtained results suggest that AQP4 may take part in the regulation of water transport required for follicle development in the chicken ovary.
1 INTRODUCTION
In the domestic hen (Gallus gallus domesticus), a mature ovary includes follicles of various size and stage of development. There are numerous pre-hierarchical follicles (the cortical follicles embedded in the ovarian stroma with <1 mm in diameter, white follicles > 1–4 mm and yellowish follicles > 4–8 mm), the pre-ovulatory yellow follicles (>8–36 mm; Fn-F1), which are organized in a characteristic hierarchy (typically the largest five to seven follicles), and several post-ovulatory follicles (POF1-POFn). The largest follicle (F1) is the most developed and ovulates on an almost each day. At the same time one of the pre-hierarchical follicles is recruited into a pre-ovulatory hierarchy (Bahr & Johnson, 1984). The growth and development of ovarian follicle can be divided into three phases. In the initial one, which lasts from a few weeks to several years, the slow growing follicles increase in diameter from 10 μm to 1 mm. During the next stage of growth, the subpopulation of white follicles begins to accumulate yolk and increases in diameter to 6–8 mm within a few weeks. Approximately every 24–26 hr, one 6–8 mm follicle enters into the rapid growth phase. This recruited follicle in 5–9 days increases in diameter up to 36 mm and becomes part of the pre-ovulatory hierarchy (Johnson, 1998).
The growth and development of the follicle are accompanied by a series of events such as selection into pre-ovulatory hierarchy or atresia, reorganization of follicular wall with differentiation of cells and deposition of large amount of the yolk. These processes are regulated by numerous hormones and growth factors. It seems plausible that quick deposition of the yolk in the chicken follicles is similar to rapid accumulation of fluid in the antral cavity of mammalian follicles. In the large pre-ovulatory yellow follicle of chicken, yolk contains approximately 48% of water (9.1–9.3 g) (Etches, 1995). Therefore, a large amount of water passing through a follicular wall is required during the growth of the follicle. It is known that in mammals the fluid accumulation and transcellular water transport into follicular antrum are mediated via aquaporins (Grzesiak, Knapczyk-Stwora, Luck, Mobasheri, & Słomczyńska, 2016; McConnell et al., 2002; Rodgers & Irving-Rodgers, 2010; Starowicz, Grzesiak, Mobasheri, & Szoltys, 2014). However, the mechanisms of water movement into avian ovarian follicles are largely unknown.
Aquaporins (AQPs) are group of integral plasma membrane proteins, which transport water and other small, uncharged solutes across the membrane under an osmotic gradient (Agre, Sasaki, & Chrispeels, 1993; Alleva, Chara, & Amodeo,2012). There are 13 mammalian AQPs of ~ 25–35 kDa (AQP0-AQP12) that are classified into three subfamilies: channels selective only for water (AQP0, 1, 2, 4, 5, 6, 8), aquaglyceroporins (AQP3, 7, 9, 10) and superaquaporins (AQP11, 12) (Ishibashi, Hara, & Kondo, 2009; Murata et al., 2000). Most tissues and cells have a specific pattern of AQPs expression (Agre & Kozono, 2003; Verkman, 2012). The AQPs can be found in many mammalian organs including female and male reproductive system where they can play a key role in a water movement between tissue components (for review see Huang et al., 2006; Zhang, Tan, Qu, Sheng, & Huang, 2012). It was demonstrated that different AQPs present in mammalian ovary beside involvement in follicle maturation, especially in antral expansion, and participation in the transition of the follicles from pre-antral to antral stage (Grzesiak et al., 2016; McConnell et al., 2002; Thoroddsen et al., 2011) may mediate water loss needed for apoptotic changes in mitochondria of granulosa cells (Jablonski, Webb, McConnell, Riley, & Hughes, 2004).
Very limited studies have reported the AQPs in the chicken reproductive system. So far, Tiwari, Hadley, and Ramachandran (2014) found differential expression of AQP5 in the cancerous ovary and suggested potential involvement of AQP5 in ovarian tumourigenesis in the chicken model of ovarian tumour. Yang, Lim, Bae, and Song (2016) revealed the involvement of AQP3 in the development of the chicken oviduct, which is regulated by oestrogen and increased expression of AQP3 in the cancerous ovary of laying hens. As the expression and the role of AQPs in the ovary of birds were not investigated in details, the present study was designed to examine whether the AQP4 is present in the chicken ovary, and if so, what is its distribution in the ovarian compartments of the laying hen. Additionally, localization of AQP4 in the ovarian follicles at a different stage of development was determined.
2 MATERIALS AND METHODS
2.1 Birds and tissue preparation
The animal protocol for the study was performed in accordance with the research protocol, under the licence given by the Local Animal Ethics Committee in Krakow, Poland (No. 30/2010). Hy-Line Brown laying hens were obtained from the commercial farm H&P2 s.c. (Czarków, Poland). Birds were housed individually under a photoperiod of 14-hr light:10-hr dark with free access to commercial food and water. Hens (n = 9) at the age of 42 weeks were killed approximately 22 hr after ovulation, that is, 3 hr before the next ovulation of F1 follicle, and the ovaries were collected rapidly, placed on ice and the following ovarian compartments were isolated: stroma with primordial follicles <1 mm in diameter (Str), white follicles (>1–4 mm; WF), yellowish follicles (>4–8 mm; YF), small yellow follicles (>8–14 mm; SYF) and the three largest yellow pre-ovulatory follicles F3 to F1 (F3 < F2 < F1; 20–36 mm). From the pre-ovulatory follicles the granulosa (G) and theca (T) layers were separated. Tissues from each bird (n = 6) were divided into two parts, placed into RNAlater (Sigma-Aldrich, Saint Louis, MO, USA) for later quantitative Real-time PCR or snap frozen in liquid nitrogen and stored at −80°C for Western blot analysis. Moreover, ovarian stroma, follicles, cerebellum and fragments of kidney (n = 3 birds) were fixed in 10% buffered formalin, dehydrated through graded ethanol solutions, cleared in xylene and embedded in Paraplast (Sigma-Aldrich). Microtome sections (6 μm thickness) were mounted onto microscope slides covered with (3-aminopropyl) triethoxysilane (APES; Sigma-Aldrich) and used for immunohistochemical analysis.
2.2 RNA isolation and RT-PCR analysis
Total RNA was extracted from collected tissues with the TRI Reagent (Sigma-Aldrich) following the manufacturer's protocol. Total RNAs (2 μg) were reverse-transcribed with a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Reverse transcriptase reaction mixtures were performed in 20 μl volume including the random primers, dNTP mix and MultiScribe Reverse Transcriptase. The reaction was conducted in a thermocycler (Mastercycler Gradient; Eppendorf, Hamburg, Germany) and performed at 25°C for 5 min, 37°C for 120 min and 85°C for 5 min. The cDNA was stored at –20°C. The multiplex real-time qPCRs were carried out as described recently (Hrabia, Leśniak-Walentyn, Ocłoń, & Sechman, 2016) using a 96-well StepOne Plus thermocycler (Applied Biosystems) in a 10 μl volume containing 5 μl of TaqMan Gene Expression Master Mix (Applied Biosystems), 0.5 μl TaqMan Gene Expression Assays with specific TaqMan MGB-probe and one pair of primers (AQP4, assay ID: Gg03346640_m1, Genbank accession no. NM_001004765.1, amplicon size: 87 bp; Applied Biosystems), 0.5 μl of Eucaryotic 18S rRNA Endogenous Control (pair of primers and TaqMan probe-labelled VIC/TAMRA, cat # 4310893E, amplicon size: 187 bp; Applied Biosystems), 2 μl of water and 2 μl of cDNA (10× diluted samples after the RT). Amplifications included initial denaturation step at 50°C for 2 min and 95°C for 10 min and 40 PCR cycles at 95°C for 15 s and 60°C for 1 min. Each sample was run in duplicate. Water as a negative control was used in all reactions. The 2−ΔΔCt method (Livak & Schmittgen, 2001) was used to calculate relative expression levels of AQP4 gene after normalization to 18S rRNA, and calibration to expression in the stroma samples.
2.3 Protein extraction and Western blot analysis
Collected tissues (examined sample separately for each tissue was pulled from two birds; n = 6 chickens) were homogenized in cold lysis buffer (BioVision, Milpitas, Calif., USA), sonicated and centrifuged at 4°C for 20 min at 10,000 g. The protein concentration was estimated by Bradford protein assay (Bio-Rad, Hercules, CA, USA) with bovine serum albumin (BSA) as the standard. According to Grzesiak et al. (2016), supernatants containing 20 μg of total protein were mixed with loading buffer (62.5 mm Tris–HCl pH 6.8, 2% SDS, 25% glycerol, 0.01% bromophenol blue, 5% β-mercaptoethanol) and warmed at 99.9°C for 3 min. After denaturation, samples were loaded into 12% SDS-polyacrylamide gel, and proteins were separated by electrophoresis under reducing conditions. Resolved proteins were transferred from the gel to a nitrocellulose membrane using a wet blotter in Genie Transfer Buffer (20 mm Tris, 150 mm glycine in 20% methanol) for 90 min at a constant voltage of 135V. Membranes were blocked overnight at 4°C with 5% non-fat milk in Tris-buffered saline (TBS, pH 7.6). After washing, the membranes were incubate with rabbit polyclonal anti-chicken AQP4 primary antibody (custom-made by Operon Biotechnologies, Tokyo, Japan, and its specificity confirmed in the chicken tissues as described previously by Yoshimura, Sugiura, Ohmori, Aste, & Saito, 2011) diluted 1: 2,500 at room temperature for 90 min. Membranes were then washed and treated with secondary horseradish peroxidase-conjugated anti-rabbit antibody (1: 3,000, 60 min, RT; cat # PI-1000, Vector Laboratories, Burlingame, USA). Next, to control for variable amounts of protein, the membranes were stripped and reprobed with mouse monoclonal anti-β-actin antibody (1: 3,000; cat # A2228, Sigma-Aldrich) and with HRP-conjugated anti-mouse IgG (1: 3,000; cat # PI-2000, Vector Laboratories). The sites of antibody-antigen reaction were detected by chemiluminescence using Western Blotting Luminol Reagent (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and visualized using ChemiDoc-It 410 Imaging system and visionworks Life Science software. The bands representing each sample were densitometrically scanned to obtain the semiquantitative results. Relative levels of AQP4 protein were normalized to the β-actin in each corresponding data point. Semiquantitative analysis was performed for three separately repeated determinations from each ovarian tissue.
2.4 Immunolocalization of AQP4
The tissue sections were dewaxed in xylene and rehydrated in a graded ethanol solution. Endogenous peroxidase activity was blocked by 0.5% H2O2 in methanol for 10 min at room temperature. After washing in TBS, to retrieve antigenicity the sections were submerged in citric acid buffered solution (pH 6.0) and heated in a microwave oven (3 × 1 min, 750 W). The slides were then left in the buffer for cooling. Non-specific binding sides were prevented by incubation of sections for 10 min in 5% normal goat serum in TBST. After that, the sections were incubated overnight at 4°C in a humidified chamber with primary rabbit polyclonal anti-chicken AQP4 antibody (the same as for Western blot) diluted 1: 250 in TBST. The slides were rinsed two times for 5 min in TBS, before incubation with secondary biotin-labelled goat anti-rabbit IgG (1: 300, 40 min, room temperature; cat # BA-1000, Vector Laboratories), followed by an avidin-biotin-horseradish peroxidase complex —Vectastain ABC kit (30 min; Vector Laboratories). Immunoreaction was visualized by diaminobenzidine and H2O2 solution and sections were counterstained with haematoxylin (Vector Laboratories). Finally, the slides were washed for 5 min in running water, dehydrated and mounted in DPX (Sigma-Aldrich). Negative control was performed by replacement of the primary antibody with normal rabbit serum or TBST buffer. For positive control, section of cerebellum and kidney of chicken was used. Slides were examined under a light microscope Axio Scope. A1 with Axiocam 503 colour camera and zen 2.3 pro software (Carl Zeiss, Germany). The intensity of the immunoreactivity was estimated subjectively as strong, moderate, weak and very weak.
2.5 Statistical analysis
Data were statistically analysed by one-way ANOVA followed by Bonferroni t test. Log transformations were performed as needed to maintain homogeneity of variance and normality. Differences of values were considered to be significant at p < .05. Calculations were performed with sigmaplot_V_13 (Systat Software Inc., USA). Results are presented as means ± SEM.
3 RESULTS
3.1 Messenger RNA and protein expression of AQP4 in the chicken ovary
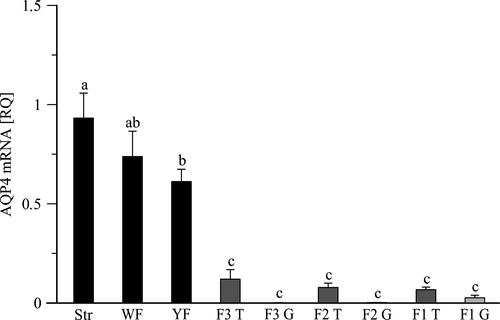
RT-PCR analysis showed the presence of AQP4 mRNA in all examined ovarian compartments. The highest relative expression (RQ) of AQP4 mRNA was observed in the ovarian stroma with primordial follicles (0.932 ± 0.126) and the lowest in the granulosa layer of pre-ovulatory follicles (0.001 ± 0.0007–0.026 ± 0.013), where was less detectable (Figure 1). Along with follicle development the relative expression of AQP4 mRNA gradually decreased. In yellowish follicles (4–8 mm) were lower (p < .05) by 34.3% than in the ovarian stroma. Further, in the pre-ovulatory follicles F3-F1 expression of AQP4 mRNA was significantly lower (p < .001) than in the pre-hierarchical follicles. There were no differences (p > .05) in AQP4 mRNA expression among F3-F1 follicles and between examined layers of the hierarchical follicles’ wall (Figure 1).
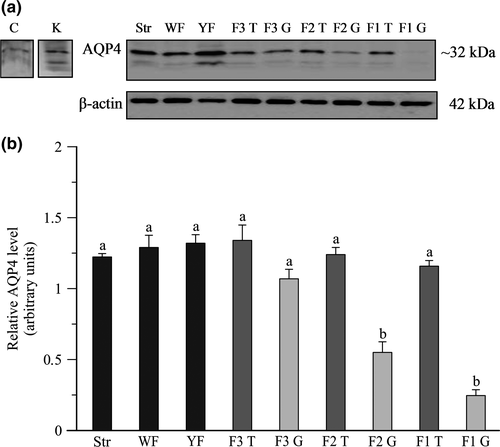
The presence of AQP4 protein in the chicken ovary was detected by Western blot using rabbit polyclonal anti-chicken antibody. In all examined tissues, a band of approximately 32 kDa was seen, and additional weak bands (~22 kDa and ~20 kDa) were also observed (Figure 2a). The expression of AQP4 protein did not differ among the stroma, white and yellowish follicles and theca layer of pre-ovulatory follicles F3-F1, whereas in the granulosa layer of F2-F1 follicles was lower (p < .001) than in other tissues. When compared to the theca the relative levels of AQP4 protein in the granulosa layer were lower (p < .001) by 56% and 79%, respectively, in F2 and F1 (Figure 2b).
3.2 Immunohistochemical localization of AQP4 in the chicken ovary
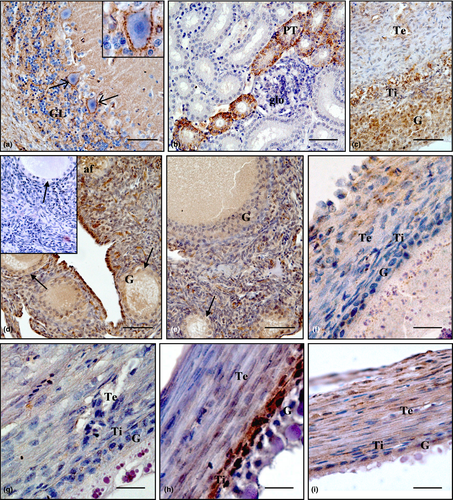
The localization of AQP4 protein in the ovarian compartments and control tissues, that is cerebellum and kidney, was examined by immunohistochemistry and is shown in Figure 3. As predicted, strong positive staining was found in the membrane and dendrites of Purkinje cells as well as in fibres in granular layer of the cerebellum (Figure 3a). In the kidney, positive staining was present in some collecting ducts and proximal tubules (Figure 3b). Within the ovary, the intensity of the immunoreaction depended on the stage of follicle development and the layer of follicular wall. Strong or moderate immunoreactivity was observed in the atretic follicles (Figure 3c) and the stroma with primordial follicles (Figure 3d, e). Moderate staining for AQP4 was found in the wall of white follicles (Figure 3f). Weak or very weak immunoreaction was in the yellowish follicles (Figure 3g), whereas in small yellow follicles (Figure 3h) medium immunostaining was noted. The intensity of AQP4 staining was moderate or weak in the wall of large hierarchical follicles (Figure 3i) and was arranged as follows: theca interna ≥ theca externa > granulosa. Replacing the primary antibody with normal rabbit serum or TBST buffer abolished the staining (Figure 3d insert).
4 DISCUSSION
The present study demonstrated the expression of AQP4 mRNA and protein in different compartments of the laying hen ovary, that is, stroma and follicles: white, yellowish, small yellow and yellow pre-ovulatory (F3-F1). Moreover, this AQP was present in the granulosa and theca cells of follicular wall throughout the period of development until ovulation. Thus, this finding implies a possible involvement of AQP4 in folliculogenesis in the hen ovary similarly as it was strongly suggested for AQPs in mammalian ovaries (Grzesiak et al., 2016; McConnell et al., 2002; Sales, Lobo, Carvalho, Moura, & Rodrigues, 2013; Skowronska, Mlotkowska, Eliszewski, Nielsen, & Skowronski, 2015; Starowicz et al., 2014; Sun et al., 2007, 2009). These results, obtained for the first time in birds, are consistent with those previously reported in the human (Thoroddsen et al., 2011) and bovine (Williams, 2012) ovary, and extend the number of avian tissues in which presence of AQP4 has been shown, that is, the brain—a main place of AQP4 presence, proventriculus, muscle, kidney, ureter and testis (Goren, Adorján, & Kálmán, 2006; Nishimura & Yang, 2013; Saito, Ikegami, & Shimada, 2005; Skowronski, Leska, Robak, & Nielsen, 2009; Sugiura, Aste, Fujii, Shimada, & Saito, 2008; Yoshimura et al., 2011).
Using real-time PCR the highest amount of AQP4 mRNA was found in the ovarian stroma. Along with follicle maturation, the relative mRNA expression of AQP4 gradually decreased and very low mRNA expression was observed in the granulosa layer of pre-ovulatory follicles. In contrary, AQP4 protein level determined by Western blot did not differ among stroma, pre-hierarchical follicles and theca layer of yellow pre-ovulatory follicles. In the granulosa layer of pre-ovulatory follicles, especially in F2 and F1, similarly as mRNA, the protein level was lower than in other ovarian compartments. Interestingly, immunohistochemical localization of AQP4 protein showed differences among the type of cells and stages of follicle development. Intense immunostaining was in the stroma, much weaker in pre-hierarchical follicles and increased again in smaller yellow follicles. In the largest pre-ovulatory follicles, the intensity of immunoreaction was slightly decreased. Observed discrepancies in mRNA and protein amounts may be due to regulatory mechanisms at the post-transcriptional and translational levels, stability of mRNA and different sensitivity of each method. Notably, a mammalian cell produces two copies of the given mRNA per hour, while it produces dozens of copies of suitable protein per mRNA per hour. Furthermore, proteins are more stable than mRNAs (Vogel & Marcotte, 2012). Similar discrepancy in the amount of mRNA and protein was revealed previously for AQP5 in the ovary of chickens. Namely, Tiwari et al. (2014) observed approximately threefold lower relative AQP5 mRNA expression in tumour ovarian tissue than in the healthy one and approximately twofold greater AQP5 protein level in cancerous compared to that in normal ovaries.
Taking into consideration that AQP4 is responsible not only for high water permeability but has also multifunctional potentiality such as potassium buffering (Amiry-Moghaddam et al., 2003), cell migration (Saadoun et al., 2005), adhesion (Hiroaki et al., 2006) and apoptosis (Chu et al., 2014). The relatively high expression of AQP4 found in the ovarian stroma with primordial follicles and pre-hierarchical follicles may be related to the participation of AQP4 in the growth of these follicles and, on the other hand, to their atresia. The atresia, initiated in the granulosa cells (Johnson, 2002), mediated mainly via the process of apoptosis, is the most abundant in the group of avian pre-hierarchical follicles, ensures proper functioning of the ovary and guarantees a required number of the developing follicles (Gilbert, Perry, Waddington, & Hardie, 1983). It was suggested that AQPs may mediate water loss needed for downstream apoptotic events in granulosa cells, and the water permeability of the plasma membrane can control the rate of cell apoptosis in the rat ovarian follicles (Jablonski et al., 2004). Moreover, overexpression of AQP1 in Chinese hamster ovary cells enhanced the rate of apoptosis (Jablonski et al., 2004). Thus, it cannot be ruled out that AQP4 present in the granulosa cells of chicken ovarian follicles participate in alteration of apoptosis and may contribute to regulation of proliferation-apoptosis balance within chicken follicles. This assumption may be supported by strong immunoreaction for AQP4 found in pre-hierarchical atretic follicles.
In turn, the marked level of AQP4, and even elevated, as demonstrated by immunostaining in the yellow follicles may be related to the involvement of AQP4 in water transport across follicular wall during the transition of the follicle to pre-ovulatory stage. Follicle from a group of follicles in diameter 6–8 mm, selected into pre-ovulatory hierarchy starts to grow very fast. Within 7–11 days it reaches diameter about 36 mm. This enlargement of the follicle is accompanied by rapid accumulation of huge amount of yolk which contains approximately 48%, that is, 9.2 g of water per egg. Such huge accumulation of water requires its rapid movement. Therefore, obtained data may indicate possible engagement of AQP4 in water transport into the yolk in the interior of follicle.
Furthermore, within the wall of ovarian follicles the mRNA and protein of AQP4 were detected in the theca (externa and interna) and granulosa cells; therefore, there is the reason to believe that AQP4 could be important in the regulation of water homeostasis and/or function of these cells in the wall of chicken follicles. Whereas the level of AQP4 evidenced by real-time PCR, Western blot and immunohistochemistry were high in the theca layer of pre-ovulatory follicles, it was low in the granulosa one. The obtained results are largely consistent with those demonstrated by Thoroddsen et al. (2011) for the theca and granulosa cells of human ovarian follicles. It should be noted that the theca layer in large part is composed of extracellular matrix, which contains dermatan sulphate, heparan sulphate and hyaluronic acid (Jackson, Friberg, & Bahr, 1991). The hyaluronan is strongly hydrated in biological environment (Hunger, Bernecker, Bakker, Bonn, & Richter, 2012); thus, the most probable role of AQP4 in the theca layer is supplying the water for hyaluronan and other glycosaminoglycans hydration similarly as was suggested for AQP5 in the rat ovary (Starowicz et al., 2014; ). It seems possible that AQP4 present in the theca layer of chicken ovarian follicles may also be implicated in the regulation of nerve cell formation. Such speculation may be supported by suggestion of Zheng et al. (2010) that AQP4 has potential role in neurogenesis, and ovarian follicles of chicken have extensive adrenergic and cholinergic innervation which is confined primarily to the theca rather than the granulosa layer. Moreover, innervation increased with progressive growth and maturation of the follicle (Gilbert, 1969).
Other roles of AQP4 in the theca and granulosa cells of chicken ovarian follicles may be related to these cell differentiation as well as specific function such steroid production. On the other hand, growing evidence in mammals suggest that AQPs expression in reproductive system is regulated by ovarian steroids (Zhang et al., 2012). For instance, the expression of AQP2 was elevated in the mouse uterus in response to estradiol (Jablonski, McConnell, Hughes, & Huet-Hudson, 2003) and regulatory role of estradiol and/or progesterone in AQP2 control in human endometrium was suggested (He et al., 2006). An augmented AQP5 level in the rat uterine luminal epithelium after progesterone alone or in combination with estradiol treatment was also observed (Lindsay & Murphy, 2006). Recently, Grzesiak et al. (2016) revealed that maternal and neonatal exposure to anti-androgen flutamide leads to decreased AQP5 expression in ovarian follicles of adult pigs. Moreover, presence of the oestrogen-response element was confirmed in the promoter region of the AQP2 (Zou et al., 2011) and AQP5 (Kobayashi, Takahashi, Miyagawa, Watanabe, & Iguchi, 2006) genes, and androgen-response element in the promoter of AQP5 gene (Moehren, Denayer, Podvinec, Verrijdt, & Claessens, 2008). Our results demonstrating the follicle size-dependent expression of AQP4 could also indicate that AQP4 expression is under steroid control. The growth and development of avian follicles until ovulation are accompanied by changes in steroid hormone production and their receptor expression (Hrabia, Wilk, & Rzasa, 2008; Huang, Kao, & Nalbandov, 1979; Yoshimura & Bahr, 1991; Yoshimura, Chang, Okamoto, & Tamura, 1993). It is well established in birds that secretion of estradiol decreases, while progesterone increases during maturation of ovarian follicles. Thus steroids, as the key regulators of ovarian processes, may control AQPs expression in birds as well.
A lack of spectacular differences in the expression of AQP4 in the granulosa, and especially theca layers among the three largest pre-ovulatory follicles in the chicken ovary may suggest that this AQP is not the only crucial factor involved in the regulation of water movement within the follicle before ovulation or the AQP4 expression is constant at the last hours preceded this event. These results are in accordance with blood flow through the largest chicken ovarian follicles, which also does not differ among them, although is higher in pre-ovulatory than in pre-hierarchical follicles and the stroma (Hrabia, Paczoska-Eliasiewicz, Niezgoda, & Rząsa, 2005; Scanes, Mozelic, Kavanagh, Merrill, & Rabii, 1982). On the other hand, it should be noted that in the current investigation, the mRNA and protein levels of AQP4 were examined at one stage of the ovulatory cycle (approximately 3 hr before ovulation of F1 follicle), so it may be a reason why the likely changes were not found. It seems important to examine in further study the expression of AQP4 at different stages of the ovulatory cycle.
5 CONCLUSIONS
In conclusion, our results provide evidence that the AQP4 mRNA and protein are present in the chicken ovary. The expression of AQP4, dependent on the stage of follicle development and the layer of follicular wall, suggests participation of this channel protein in water movement required for follicle growth and maturation. Further studies clarifying the regulation of AQP4 expression as well as exact mechanisms of this AQP action in the avian ovary are necessary.
ACKNOWLEDGEMENT
The study was financially supported by DS-3243/KFiEZ.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
MN, MG and MK performed the research. MN and AH wrote the manuscript. AS and NS revised the manuscript. AH designed the research study.




