Disorder of sex development in a cat with chromosome mosaicism 37,X/38,X,r(Y)
Contents
An 18-month-old European shorthair cat was subjected to genetic studies due to ambiguous external genitalia (underdeveloped both penis and scrotum). Further anatomic and histopathological studies revealed the presence of abdominal, atrophic testes and uterus. Cytogenetic analysis showed two cell lines, one with X monosomy—37,X [90% of the analysed metaphase spreads], and other line had 38 chromosomes with normal X chromosome and abnormally small Y-derived chromosome—38,X,der(Y) [10%]. Further fluorescence in situ hybridization study with telomeric probe revealed a ring structure of the der(Y). Eight Y chromosome-specific genes, SRY, TETY1, TETY2, CUL4BY, CYORF15, HSFY, FLJ36031Y and ZFY, were detected. We conclude that the described abnormality of the reproductive system, leading to sterility, was caused by a very rare type of chromosomal mosaicism—37,X/38,X,r(Y).
1 Introduction
Disorders of sex development (DSD), defined as a condition where chromosomal, gonadal or phenotypic sex is atypical, are a well-known problem in domestic animal reproduction (Szczerbal & Switonski, 2016). Three main categories of DSD are distinguished as follows: sex chromosome DSD, XX DSD and XY DSD (Meyers-Wallen, 2012). The most common chromosome mutation causing DSD found in cats is XXY trisomy (Pedersen, Berg, Almstrup, & Thomsen, 2014). However, other abnormalities, such as X monosomy (Szczerbal, Nizanski, et al., 2015), 37,X/38,XY mosaicism (Balogh et al., 2015) or X/Y translocation (Szczerbal, Stachowiak, Dzimira, Sliwa, & Switonski, 2015), have also been reported recently. It is known from human studies that structural rearrangements of the Y chromosome are associated with male infertility or sterility (Dhanoa, Mukhopadhyay, & Arora, 2016).
In this report, we describe a very rare case of DSD in a cat with two cell lines detected in lymphocytes, both carrying sex chromosome abnormalities.
2 Case Report
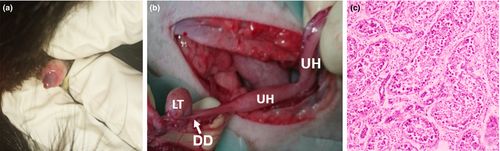
An 18-month-old European shorthair cat (4.5 kg) with male appearance was subjected to clinical, histological, cytogenetic and molecular studies due to an ambiguous phenotypic sex. The physical examination revealed the presence of undeveloped both penis (Figure 1a) and scrotum. The B-mode ultrasonography in dorsal recumbence was applied. Left testicle was found in the scrotal sac, while the right gonad was abdominally located. A sagittal sonogram of the abdominal cavity visualized a longitudinal structure resembling the uterus. The cat was neutered (Figure 1b) by a veterinary surgeon according to standard Polish veterinary protocols under the consent of the owner and the approval of the local Bioethical Commission for Animal Care and Use in Poznan (Poland).
2.1 Histological analysis
Gonads obtained after surgery were fixed in formalin. Tissue sections of 5 μm in thickness were stained with haematoxylin and eosin and analysed under Olympus CX41 light microscope equipped with Olympus DP-20 camera (Olympus Corporation, Tokyo, Japan). Both gonads were classified as testes without spermatogonia, with chaotically arranged Sertoli cells and a single cluster of interstitial cells. Histology of the uterus was not typical, that is the wall of the organ consisted of the thick myometrium and a very thin mucosa without uterine glands (Figure 1c).
2.2 Cytogenetic analysis
Chromosome preparations were obtained from a short-term lymphocyte culture according to a standard procedure. Fluorescence in situ hybridization (FISH) with feline X painting probe (kindly provided by Prof. M. A. Ferguson-Smith, Cambridge University, UK), SRY-specific probe (BAC clone RP86-278G21) and telomeric probe (Chrombios GmbH) was performed as described by Szczerbal, Stachowiak, et al. (2015). Hybridization with the SRY-specific probe was also performed on a normal male cat as a reference for the SRY gene location on the Y chromosome. Microscopic evaluation was carried out under Nikon E600 Eclipse fluorescent microscope (Melville, NY, USA) equipped with a cooled CCD digital camera and lucia software (Laboratory Imaging, Praha, Czech Republic).
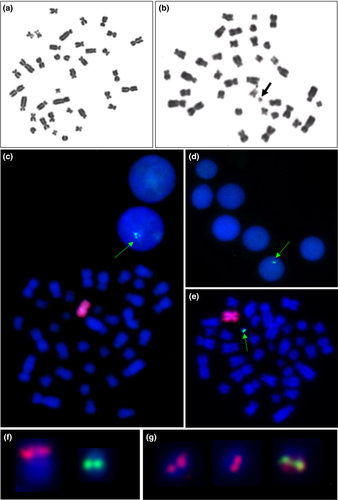
Conventional cytogenetic analysis including Giemsa staining performed on 100 metaphase spreads revealed the presence of two abnormal cell lines. The first cell line carried X chromosome monosomy (37,X) in 90% (95% confidence interval was 84.1–95.8%) of the analysed cells, and the second had 38 chromosomes, including one X and a small marker chromosome (38,X,+mar) in 10% of the analysed cells (Figure 2a,b). Fluorescence in situ hybridization procedure using the X chromosome-specific probe confirmed the presence of a cell line with X monosomy. The use of the SRY-specific probe revealed that the marker chromosome is derived from the Y chromosome—der(Y) (Figure 2c–e). Therefore, the karyotype of the studied cat was described as 37,X/38,X,+mar.ish der(Y)(SRY+). As the location of the SRY gene was different from a normal, feline Y chromosome (Figure 2f), it is suggested that der(Y) can be a ring chromosome. To verify this hypothesis, a telomeric probe was applied. We observed a single hybridization signal, instead of two expected on both ends of der(Y); thus, we have assumed its ring (r) structure (Figure 2g).
2.3 Molecular analysis
Genomic DNA was isolated from leukocytes, buccal mucosa and hair follicles, using Blood Mini, Swab and Genomic Mini kits (A&A Biotechnology, Gdansk, Poland). Eight Y chromosome-specific genes (SRY, TETY1, TETY2, CUL4BY, CYORF15, HSFY, FLJ36031Y and ZFY) were detected by PCR and RFLP (only for the ZFY gene). The primer sequences and amplification details are shown in Table S1. The entire coding region of the SRY gene was sequenced by the Sanger method. The RFLP test (BsmI) was applied to distinguish between ZFX and ZFY genes derived from the X and Y chromosomes, respectively. The obtained products were separated on 1.5% agarose gel.
The studied genes were detected in the DNA isolated from leucocytes (Figure S1). Moreover, two of these genes (CUL4BY and CYORF15) were used to search for the Y chromosome sequences in other tissues (buccal mucosa and hair follicles), and their presence was confirmed (Figure S2). The SRY coding sequence was normal in comparison with the reference (NCBI, NM_001009240.1).
3 Discussion
The DSD are an important breeding issue, as they affect reproduction, and in case of their hereditary forms, a wide spreading of the undesired mutations may occur. Thus, the extensive studies of the genetic background of such disorders have been conducted in domestic animals (Meyers-Wallen, 2012; Parma, Veyrunes, & Pailhoux, 2016; Raudsepp & Chowdhary, 2016; Szczerbal & Switonski, 2016). Nevertheless, structural rearrangements involving sex chromosomes were rarely reported in companion species. Until now, only a single case of DSD cat (phenotypically tortoiseshell female with an underdeveloped penis without spines and no scrotum), caused by X/Y translocation, was reported (Szczerbal, Stachowiak, et al., 2015). In the present study, we have described for the first time a DSD cat carrying two abnormal cell lines—37,X/38,X,r(Y). The application of FISH technique with the telomeric probe indicated a ring morphology of this der(Y). It is known that telomeres play a crucial role in the stabilization of the derivative chromosomes during cell division (Guilherme et al., 2012). Probably, due to mitotic instability of the der(Y), the monosomic cell line was found in the studied case. Such situation was reported in human patients carrying ring Y chromosome, leading to a mosaic karyotype 45,X/46,X,r(Y) and DSD, for example the presence of ambiguous genitalia, gonadal dysgenesis or complete masculinization (Bertini, Canale, Bicocchi, Simi, & Valetto, 2005).
In case of the studied cat, we have observed a high proportion of the X monosomy cell line, despite the presence of testes. A similar case was reported by Balogh et al. (2015), who described a cryptorchid cat with a mosaic karyotype—37,X[96%]/38,XY[4%]. In both cats, a low proportion of cell lines carrying the Y or der(Y) chromosome was observed, but it may not be excluded that in other tissues and organs, including gonads, the proportion of these cell lines was higher and sufficient to drive gonad development into testes.
To the best of our knowledge, it is the first report describing a small marker Y chromosome, having a ring structure, which is associated with DSD and/or infertility in domestic animals. Identification of such cases is also important from the oncological point of view, as the presence of the sequences derived from the Y chromosome in patients with dysgenetic gonads may cause development of gonadal tumours (Oliveira et al., 2009).
Acknowledgement
This study was financed by the statutory fund (No. 508.534.00.0), Poznan University of Life Sciences, Poland.
Conflict of Interest
None of the authors have any conflict of interest to declare.
Author contributions
Szczerbal, Stachowiak and Nowacka-Woszuk contributed to cytogenetic and molecular analyses. Dzimira and Szczepanska performed clinical examinations and histological studies. Switonski developed the research design and supervised the study. Szczerbal and Switonski were responsible for manuscript preparation and submission.




