Reduced Theta Inter-Trial Phase Coherence in Error Processing: A Marker of Neural Dysfunction in Attention Deficit Hyperactivity Disorder
Funding: This work was supported by Israel Science Foundation (Grants 1058/16, 756/98-01, and 869-01).
Tali Devor and Tzlil Einziger contributed equally to this study.
ABSTRACT
Cognitive control deficits and increased intra-subject variability have been well established as core characteristics of attention deficit hyperactivity disorder (ADHD), and there is a growing interest in their expression at the neural level. We aimed to study neural variability in ADHD, as reflected in theta inter-trial phase coherence (ITC) during error processing, a process that involves cognitive control. We examined both traditional event-related potential (ERP) measures of error processing (i.e., error-related negativity [ERN] and error-positivity [Pe]) and theta ITC within a prospective longitudinal study of children at familial risk for ADHD. The participants were 63 male adolescents who were followed since birth. At the age of 17 years old, they performed the stop-signal task (SST) while an electroencephalogram (EEG) recording was continuously carried out. The EEG data from the trials in which the subjects failed to inhibit their response were used to calculate three different neurophysiological measures (i.e., ERN, Pe, and theta ITC). Consistent with our hypotheses, theta ITC during error processing predicted ADHD symptomatology above and beyond the traditional ERP measures. Moreover, we found that ADHD symptoms throughout childhood were uniquely associated with theta ITC, beyond ADHD symptomatology during adolescence. Overall, our findings strengthen the view of increased neural variability (as reflected by theta ITC) as a neurophysiological characteristic of a core neural dysfunction in ADHD.
1 Introduction
Attention deficit hyperactivity disorder (ADHD) is one of the most common neurodevelopmental disorders, with an estimated worldwide prevalence of 5.9% in youths (Faraone et al. 2021). Individuals with ADHD deal with core symptoms of inattention, hyperactivity, and impulsivity, which persist throughout the lifespan (American Psychiatric Association 2013; Kieling and Rohde 2012). Moreover, they are likely to face additional negative impacts on their life such as emotional and social impairments (Beheshti, Chavanon, and Christiansen 2020; Ros and Graziano 2018), poorer academic performance (Daley and Birchwood 2010; Korrel et al. 2017), and involvement in delinquency (Mohr-Jensen et al. 2019).
1.1 Cognitive Control Deficits in ADHD
Individuals with ADHD exhibit deficits in cognitive control (e.g., Cai et al. 2021; de Zeeuw and Durston 2017), which refers to a high-level neural network that supports the management of executive functions, including goal-oriented processes (Friedman and Robbins 2022; Gratton et al. 2018; Niendam et al. 2012). Certain theoretical frameworks of ADHD propose that deficits in cognitive control underlie the symptomatology of the disorder and contribute to its associated adverse outcomes (Nigg 2010; Nigg et al. 2020). Individuals with ADHD struggle with maintaining high cognitive control demands, as evident both in behavioral methods and in neuroimaging approaches (King et al. 2007; Kumar, Arya, and Agarwal 2022). From a behavioral perspective, it has been found that individuals with ADHD exhibit lower performance on tasks that require the implementation of cognitive control (e.g., the stop-signal task [SST], the go/no-go task, and dichotic listening) compared to typically developed individuals (e.g., Dramsdahl et al. 2011; Lipszyc and Schachar 2010; Randall, Brocki, and Kerns 2009). For example, in the SST, individuals with ADHD present deficits in inhibitory control, reflected by longer stop-signal reaction times (SSRT; Lipszyc and Schachar 2010; Senkowski et al. 2024). However, the extent of this phenomenon varies by additional parameters such as gender, age, and the individual's type of ADHD symptomatology (DeRonda et al. 2021; Øie et al. 2014). From a neurophysiological perspective, neuroimaging studies indicate that individuals with ADHD are characterized by abnormal neural characteristics in connectivity and activation in the cognitive control network both in resting state (de Zeeuw and Durston 2017; Sutcubasi et al. 2020) and during cognitive demand (Cai et al. 2021; Michelini et al. 2019; Mulder et al. 2011).
Cognitive control deficits in ADHD have been studied within the framework of error processing. Specifically, abnormalities in different neural measures related to error processing have been documented among individuals with ADHD, including the event-related potential (ERP) components of error-related negativity (ERN) and error-positivity (Pe; Czobor et al. 2017; Groom et al. 2010). The ERN is a negative deflection of the ERP that peaks in the frontocentral scalp area around 50 ms after the commission of an error and is followed by the Pe, a larger positive deflection that peaks between 150 and 300 ms (Falkenstein et al. 2000; LoTemplio et al. 2023; Overbeek, Nieuwenhuis, and Ridderinkhof 2005). These two components dissociate from each other and are assumed to reflect distinct aspects of the error processing mechanism (Overbeek, Nieuwenhuis, and Ridderinkhof 2005). Specifically, the ERN has been suggested to reflect response conflict monitoring (Larson, Clayson, and Clawson 2014; LoTemplio et al. 2023), while the Pe is thought to reflect error awareness (O'Connell et al. 2007). Reduced amplitudes of these components have been documented in ADHD (Kaiser et al. 2020; Lutz et al. 2021). Such discrepancies can be detected already in early childhood (Janssen, van Atteveldt, and Oosterlaan 2020; Liao et al. 2018; Liu et al. 2020). In addition, the reduction in the Pe amplitude was found to be more consistently associated with ADHD, as compared to the reduction in the ERN (Kaiser et al. 2020; McLoughlin, Gyurkovics, and Aydin 2022).
Neural oscillations of theta frequencies (i.e., 4–8 Hz) at midfrontal scalp areas, probably originating in the anterior cingulate cortex (ACC), are also commonly used as a measure for cognitive control efficiency (Cohen 2014; Womelsdorf et al. 2010). These midfrontal theta oscillations are considered to reflect the computations needed for cognitive control, monitoring, error processing, and adjusting of temporally sequenced actions (Cavanagh and Frank 2014; Cohen 2014; Trujillo and Allen 2007; Valadez and Simons 2017; Womelsdorf et al. 2010). Specifically, during error processing, better error awareness (i.e., more successful cognitive control implementation) has been associated with greater midfrontal theta power (Beatty et al. 2020; Valadez and Simons 2017; Wang et al. 2020). In ADHD, the theta power increase during error processing is generally weaker compared to control, both in childhood and adulthood (Groom et al. 2010; Keute et al. 2019; Michelini et al. 2022).
1.2 Increased Intra-Subject Variability in ADHD
Another core deficit associated with ADHD is increased reaction time (RT) variability, which reflects high inconsistency in the individual's response to a cognitive task (Klein et al. 2006; Kofler et al. 2013; Kuntsi and Klein 2012). There is some evidence showing that such variability in RT is linked to less effective cognitive control, for example, higher rates of commission errors (Kuntsi et al. 2010). In recent years, there has been a growing interest in the manifestation of this increased variability at the neural level; this has been studied within different cognitive processes such as sensory processing (Einziger et al. 2023; Gonen-Yaacovi et al. 2016), response execution (Saville et al. 2015), and cognitive control (Aydin et al. 2023; Groom et al. 2010; McLoughlin et al. 2014). Within the context of cognitive control, neural variability can be assessed using theta inter-trial phase coherence (ITC), a measure of the degree to which the phase of the theta frequencies signal aligns equally across trials. Theta ITC is thought to reflect the consistency of temporal coordination of neural activity relative to a specific external stimulus (Sauseng and Klimesch 2008). Dysregulation of the phase alignment is representative of failure to comply with task-relevant demands, which means failure in cognitive control implementation (Cavanagh and Frank 2014). Consistently, in the general population, theta ITC is enhanced during high cognitive control demands (Papenberg et al. 2013).
McLoughlin et al. (2014) found that high variability in theta phase coherence during the flanker task was associated with an ADHD diagnosis. They proposed that the observed variability in theta oscillations underlies deficits in cognitive control processes, which consequently impair the individual's ability to regulate task-related responses. A recent study supported the significance of theta ITC dysfunctions in ADHD by showing that theta ITC was lower in individuals with persistent ADHD compared to those who experienced remission in symptoms from childhood to adolescence. This suggests that such deficits may reflect a core dysfunction in ADHD (Vainieri et al. 2022).
Within the research on increased neural variability in ADHD (e.g., Einziger et al. 2023; Gonen-Yaacovi et al. 2016; Saville et al. 2015), only two studies assessed theta ITC during error processing. Specifically, Groom et al. (2010) found that, compared to the control group, the ADHD group exhibited a reduction in theta ITC in the time windows that corresponded both to the ERN and to the Pe. This finding was substantial, even though compared to the control group, no difference was found in the ADHD group's ERN amplitude, and only a statistical trend was found for their reduced Pe amplitude. Thus, Groom et al. implied that theta ITC might be a more sensitive measure of neural abnormalities in error processing compared to traditional ERP measurements. A recent study by Aydin et al. (2023) demonstrated that theta ITC is significantly associated with ADHD both genetically and phenotypically in mid-childhood and young adulthood. Therefore, theta ITC was suggested as a neurophysiological marker for a potentially innate core neural dysfunction in the cognitive control network; this dysfunction might impair the regulation of task-relevant responses in individuals with ADHD.
To summarize, while cognitive control deficits and increased neural variability in ADHD are suggested to be core deficits of ADHD (de Zeeuw and Durston 2017; Kofler et al. 2013; Kuntsi and Klein 2012; Nigg et al. 2020), the potential link between them has received limited attention in the literature (McLoughlin, Gyurkovics, and Aydin 2022). However, theta ITC measured in the context of error processing might act as a specific neural marker of ADHD (Aydin et al. 2023) and therefore might contribute to the understanding of the disorder's core neural dysfunctions. It should be noticed that the existing research on the relationship between theta ITC and ADHD has primarily focused on adolescents and young adults. In light of Aydin et al.'s (2023) finding that theta oscillations reflect, at least in part, an inherited genetic trait underlying ADHD symptomatology, early manifestations of ADHD symptoms might longitudinally predict such neural abnormality. Therefore, investigating this relationship across development could improve the understanding of the etiology of the disorder.
1.3 The Present Research
The current study is part of a large prospective longitudinal study on the development of ADHD and its associated neurocognitive deficits (e.g., Auerbach et al. 2004; Berger et al. 2013; Einziger et al. 2023). We aimed to examine neurophysiological markers of error processing, specifically focusing on theta ITC and its contribution to ADHD symptomatology, beyond traditional measures of error processing (i.e., ERN and Pe). To achieve this, EEG data were recorded in response to inhibition errors during the SST (Logan 1994) in a sample of adolescents displaying varying levels of ADHD symptoms. Examining the relation between theta ITC and ADHD symptomatology across a continuum allows the inclusion of subclinical cases, rather than solely relying on the more severe cases typically found in clinical samples.
We hypothesized that: (1) reduced amplitude of the ERN and Pe and reduced theta ITC would be associated with higher concurrent ADHD symptomatology; (2) theta ITC would have a unique contribution to ADHD symptomatology beyond the traditional ERP measures; and (3) childhood ADHD symptomatology would have a unique association with theta ITC, beyond the expected link with concurrent ADHD symptomatology.
2 Methods
2.1 Participants
The study sample was composed of 63 male adolescents (Mage = 17.37 years, SDage = 0.41, rangeage = 16.52–18.48) who participated in the study from birth. Recruitment for this prospective longitudinal study was carried out at a local hospital. The inclusion criteria for families were as follows: only families with male newborns were included due to the higher prevalence of ADHD among males compared to females (American Psychiatric Association 2000), and all infants included in the study were born healthy, with normal birth weight (M = 3296.07 g, SD = 419.75) and gestational age (M = 39.19 weeks, SD = 1.59). The study encompassed two-parent families, consisting of native-born or immigrant parents who had received education in the country and were fluent in the local language. At the beginning of the longitudinal study, the mean age of mothers was 29.95 years (SD = 4.90), and the mean age of fathers was 33.65 years (SD = 5.44). The mean number of years of education for mothers was 12.80 (SD = 1.72), and for fathers, it was 12.32 (SD = 1.77). Another inclusion criterion was based on paternal ADHD symptoms, which were assessed using an 18-item yes/no format questionnaire adapted from the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DMS-IV; American Psychiatric Association 2000). During the initial stages of the study, children were categorized into a risk group (i.e., the child's father had seven or more positive responses) or a comparison group (i.e., three or fewer positive responses) based on this symptom count assessment. Between the ages of 2 and 6 months, a more comprehensive assessment of familial risk for ADHD was conducted for both parents, involving self and spousal reports of ADHD symptomatology using the Conners' Adults ADHD Rating Scale (Conners, Erhardt, and Sparrow 1998). These continuous measures of parental symptoms were employed in our study as an indicator of familial risk. The current sample consisted of participants who were assessed at three time points during the study (at ages 54 months, 7 years, and 17 years of age). The children of families that agreed to have their child re-assessed at 17 years of age did not differ from the remaining participants in the original sample in any of the background variables (i.e., birth weight, gestational age, parents' education, parental ADHD symptoms; see Einziger et al. 2021).
2.2 Measures
2.2.1 ADHD Symptoms Throughout Development
The ADHD Rating Scale-IV (DuPaul et al. 1998) was used to assess ADHD symptoms at 54 months of age. This standardized questionnaire was employed to evaluate the frequency of ADHD symptoms displayed by the child over the preceding 6-month period. Each item, comprised of a total of 18 items, required the mother's assessment of her child's behavior using a Likert scale ranging from 0 (never or rarely) to 3 (very often). Cronbach's alpha coefficient was 0.86 for the total ADHD subscale, indicating satisfactory internal consistency.
The Conners' Rating Scales-Revised—CRS-R (Conners 1997) was used to evaluate ADHD symptoms at 7 and 17 years of age. The CRS-R was administered to mothers, who provided ratings for their child/adolescent. Mothers were requested to rate specific symptoms, such as “difficulty doing or completing homework,” exhibited by their child/adolescent within the past month. Ratings were given on a scale ranging from 0 (the behavior rarely or never occurs) to 3 (the behavior occurs very often) for each symptom. Cronbach's alpha coefficient was 0.86 for the total ADHD subscale at 7 years of age and 0.90 at 17 years of age, indicating satisfactory internal consistency at both time points.
Significant correlations were observed between early childhood ADHD symptoms at the two different time points: 54 months and 7 years (r = 0.39, p < 0.01). To derive a more reliable measure that captured the consistency of ADHD symptoms across childhood, a combined childhood ADHD symptomatology measure was calculated (Einziger et al. 2021); standardized scores from the ADHD assessments conducted at 54 months and 7 years of age were averaged. For subjects who participated only in one of the childhood timepoint assessments (i.e., either at age 54 months or age 7 years), the standardized score of that assessment alone was used.
2.2.2 The Stop-Signal Task
This task is based on the stop-signal paradigm (Logan 1994). The fixation sign and stimuli were presented in the center of the screen, in white and on a black background. The task consisted of two phases: a short practice block of 40 trials and three test blocks of 80 trials each, with a short break after each block. The task involved a primary simple discrimination task. In 70% of the trials, the task stimulus (either a “2” or a “Z” inside of a square frame) was presented, and the participants were instructed to respond with a numbered key on an S-R box (specifically, to press “1” when the stimulus was “Z” and to press “4” when the stimulus was “2”). Participants were asked to respond as quickly and as accurately as possible. The go stimulus in each trial remained on the screen until the participant responded, up to a maximum time of 1000 ms. Thirty percent of the trials were stop-signal trials, in which a visual stop-signal (a red square frame) followed the primary go stimulus. In the stop-trials, participants were instructed to inhibit their responses. The stop signal was presented occasionally with variable delays after the primary stimulus. The stop-signal delay (SSD) was initially set to be 500 ms and was adjusted during the tasks using a staircase dynamic-tracking procedure: after a successful inhibition trial, the SSD increased by 50 ms, and after an unsuccessful inhibition trial, the SSD decreased by 50 ms. A blank screen appeared between trials, with randomly generated inter-trial intervals ranging from 1100 to 1900 ms.
We followed Verbruggen et al. (2019) guidelines for the calculation of the stop-signal reaction time (SSRT); we used the integration method with the replacement of go omissions, which has been found to be less biased and more reliable compared to the traditional “mean method.” A full description of this calculation can be found in Einziger et al. (2021). It should be noted that the SSRT could not be estimated for nine participants due to violations of the horse-race model assumptions. For example, the mean RT for unsuccessful stop trials was higher than the mean RT for go trials, or the probability of responding to a stop signal was below 0.25 or above 0.70.
For the calculation of the mean RT of successful go (SG) trials, we first excluded RTs slower than 1500 ms or faster than 150 ms. The average number of SG trials was 133.21 (SD = 28.53, range = 54–163). We also calculated the RTs of two types of errors: (1) failed inhibition (FI) trials, in which participants responded to the stop signal and did not successfully inhibit their response. The average number of FI trials in the behavioral analysis was 28.27 (SD = 7.55, range = 9–40). (2) Failed go (FG) trials, which referred to choice errors in the primary discrimination task. However, it should be noted that the number of these errors was low (M = 10.02, SD = 9.25, range = 1–43). See Table 1 for descriptive statistics of the behavioral performance in this task (for a fuller description of the behavioral analysis of this task, see also Einziger et al. 2021).
| Variable | Mean | SD | Range |
|---|---|---|---|
| Neural measures | |||
| Theta ITC in FI trials | 0.36 | 0.11 | 0.12–0.60 |
| ERN | −1.53 | 3.00 | −9.56 to 5.23 |
| Pe | 9.03 | 4.74 | 1.51–25.19 |
| Theta ITC in SI trials | 0.30 | 0.11 | 0.10–0.63 |
| Behavioral measures | |||
| RT in SG trials | 689.52 | 178.63 | 431.74–1153.65 |
| SD of RT in SG trials | 182.66 | 93.07 | 67.27–355.44 |
| Probability of choice errors in FG trials | 0.07 | 0.07 | 0.00–0.33 |
| RT in FG trials | 582.39 | 238.69 | 255.00–1330.00 |
| SSRT | 169.71 | 51.14 | 57.26–298.61 |
| RT in FI trials | 610.22 | 163.29 | 386.98–1051.73 |
| ADHD symptoms | |||
| Age 17 years | 55.94 | 11.07 | 40–80 |
| Age 7 years | 50.36 | 7.45 | 40–80 |
| Age 54 months | 11.89 | 5.89 | 1–28 |
- Abbreviations: ADHD, attention deficit hyperactivity disorder; ERN, error-related negativity; FG, failed go; FI, failed inhibition; ITC, inter-trial coherence; Pe, positivity error; RT, reaction time; SD, standard deviation; SG, successful go; SI, successful inhibition; SSRT, stop-signal reaction time.
2.3 EEG Analyses
2.3.1 EEG Data Acquisition
EEG data were recorded during the SST from 128 scalp sites using the EGI HydrocCel Geodesic Sensor Net (HCGSN) and system (Electrical Geodesics Inc. 2003). The electrode impedance level was kept below 40 kΩ, an acceptable level for this system (Ferree et al. 2001). During recording, all channels were referenced to the Cz electrode, the recording frequency band was constant at 0.01 to 100 Hz, and the sampling rate was 250 Hz. The data of two participants were damaged due to technical problems and were therefore excluded from all further analyses.
2.3.2 Wavelet Analysis Pre-Processing
EEG-data pre-processing was carried out using the EEGLAB toolbox (version 14; Delorme and Makeig 2004), operating in the MATLAB environment (version 2017b). Continuous EEG data were first high-pass filtered at 0.5 Hz and notched for 50 Hz (local electricity frequency). Data were re-referenced to the average reference and then segmented from 400 ms before stimulus onset to 800 ms after both Go and Stop stimuli onsets. Buffer zones of 2000 ms were added to each epoch to avoid edge artifacts due to the use of complex wavelet convolution. Baseline correction was applied on the segmented data by subtracting the mean amplitude based on a 400-ms-long period before stimulus onset. The used epochs were visually inspected for any large artifacts and bad channels that were not usable for further analysis. Moreover, any trial in which the participant blinked during stimuli presentation was omitted. Then, independent component analysis (ICA) was conducted using EEGLAB's runica function. Components that were visibly recognizable as either blinks, muscle twitches, or heartbeats were subtracted from the segmented data. Next, we used EEGLAB's TBT plugin (Ben-Shachar 2020b) to automatically detect additional artifacts and bad channels. Any trial that contained more than 15 faulted channels was completely removed, and any channel that was faulted in more than 30% of the trials was completely removed from the participant's data. All removed electrodes were interpolated based on activity from neighboring channels. Finally, another visual inspection was conducted to exclude any remaining trials and channels that still contained significant artifacts that might have interfered with data interpretation. For nine of the study participants, more than 10% of the channels (M = 21.55, SD = 12.44, range = 14–52) were removed during pre-processing, and therefore they were excluded from any further analysis. Additionally, two participants were excluded from the final time-frequency analysis due to extremely low behavioral performance on the SST (i.e., the success rate in the stop task tended toward zero or the probability of response to the go signal was approximately 0.5). Eventually, 50 participants had high-quality EEG data that were used in the time-frequency analysis. The primary analysis included trials in which participants failed to inhibit their response after the presentation of the stop stimuli. For the theta ITC calculation in FI trials, the mean number of FI trials after pre-processing was 28.42 (SD = 7.04, range = 9–40).
2.3.3 Wavelet Analysis Time-Window and Electrode Selection
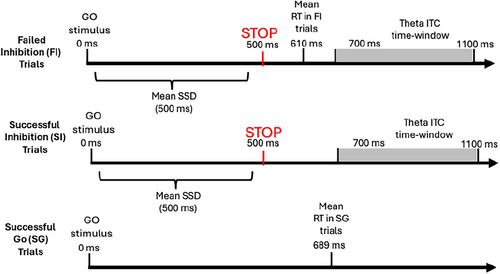
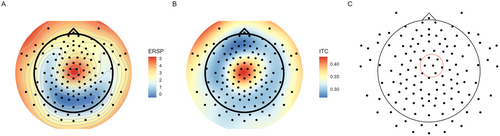
In order to identify the suitable electrodes and time window for this analysis, event-related spectral perturbations (ERSP) power was calculated over all subjects for FI trials from a group of six central electrodes using the epp_getTF function from the EEGLAB's EPP-TB plugin (Ben-Shachar 2020a). In line with McLoughlin et al.'s (2014) procedure for theta-activity inspection, the time window chosen for the analysis was 200–600 ms after stimulus presentation. This time window theoretically starts at the second theta oscillation of the lowest inspected frequency (i.e., 4 Hz) and is long enough to contain approximately two oscillations of this frequency. Therefore, in all further wavelet analyses, the time window used for wavelet-based variable calculation was between 200 and 600 ms after the presentation of the stop stimulus. Figure 1 presents a timeline of the theta ITC time window in relation to stimulus presentation and behavioral response across different trial types.
For the selection of suitable electrodes for analysis, ERSP power in the theta frequency range (i.e., 4–8 Hz) at the chosen time window (i.e., 200–600 ms) was averaged across subjects and across frequency and time domains for each electrode separately. Then, an automatic script identified the six electrodes in which the maximal ERSP power in the chosen time window and frequency range was recorded. The selected electrodes, according to the EGI numbering system, were 106, 80, 7, 105, 112, and 6. These electrodes approximately correspond to FC2, CP2, C1, C2, and FCz in the international 10–10 electrode positioning system. The localization of the six electrodes is consistent with previous literature regarding cognitive control activity (Cavanagh and Frank 2014; see Figure 4 panel C). Theta ERSP was not further used in any of our analyses, since Groom et al.'s (2010) and Aydin et al.'s (2023) studies indicated that theta ERSP is less associated with ADHD compared to theta ITC.
2.3.4 Wavelet ITC Calculation
The Morlet wavelet for FI trials was calculated for each participant using the epp_getTF function from the EPP-TB plugin (Ben-Shachar 2020a). From the resulting complex signal, theta ITC was calculated for each subject, as was previously carried out by Ben-Shachar et al. (2019). Because temporal smoothing from time-frequency decomposition can potentially include FI-related activity in the immediate pre-stimulus time period, the baseline period was set to range from 400 to 200 ms before stop stimulus presentation. Eventually, for each subject, theta ITC was averaged across the chosen time window (i.e., 200–600 ms), the chosen frequency range (i.e., 4–8 Hz), and the six chosen electrodes. For an additional analysis, the exact same process for theta ITC calculation was carried out for successful inhibition (SI) trials (the mean number of SI trials was 42.75, SD = 7.23, range = 27–63).
2.3.5 ERP Analysis Pre-Processing
Due to different filtering requirements, pre-processing was done independently to extract the wavelet-based theta ITC and the ERP variables ERN and Pe. ERP data pre-processing steps were identical to those detailed in Einziger et al. (2021). We used response-locked segments that were re-epoched from the clean FI stimulus-locked segments from 200 ms before motor response and up to 350 ms after motor response.1 For the calculation of the ERP components, the mean number of FI trials after pre-processing was 23.89 (SD = 7.61, range = 9–35).
2.3.6 ERP Analysis Time-Window and Electrode Selection
The six electrodes for the ERP analysis were chosen based on the literature and visual inspection of the topographic map of voltages on the scalp that confirmed the ERN and Pe topography (Falkenstein et al. 2000; Groom et al. 2010; see Figure 2 panel A). Time windows for the ERN and Pe were chosen based on previous research (Aydin et al. 2023; Falkenstein et al. 2000; Overbeek, Nieuwenhuis, and Ridderinkhof 2005) and were confirmed by a visual inspection of the FI grand average ERP waveform across subjects and the chosen electrodes (see Figure 2 panel B). The selected electrodes, according to the EGI numbering system, were 106, 80, 7, 112, 55, and 31. These electrodes approximately correspond to C1, C2, CP1, CP2, CPz, and FC2 in the international 10–10 electrode positioning system. The ERN time window was set between 0 and 120 ms after motor response, and the Pe time window was set between 120 and 350 ms after motor response.
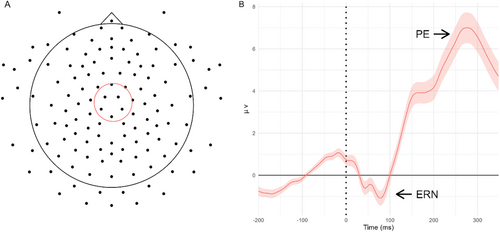
2.3.7 ERN and Pe Amplitude Calculation
For the calculation of both ERP components, ERP data were averaged across FI trials and the six chosen electrodes, separately for each subject. In line with previous studies (Aydin et al. 2023; Groom et al. 2010), the ERN amplitude was calculated as the negative peak amplitude found in the chosen time window, and the Pe amplitude was calculated as the positive peak amplitude found in the chosen time window.
2.4 Data Analysis Plan
Initially, we examined the intercorrelations between the background variables, neural measures, behavioral measures, and ADHD symptomatology. Our two hypotheses regarding the association of neural markers of error processing with concurrent ADHD symptomatology and the superiority of theta ITC compared to traditional ERP components (i.e., ERN and Pe) were examined by conducting a linear regression model constructed hierarchically. In this model, the control variable (i.e., the number of FI trials) was entered in the first step, the traditional ERP measures were entered in the second step, and the theta ITC measure was entered in the third step. Additional regression analyses were conducted to assess the robustness of the results across different measurement calculation methods and to account for potential correlations with behavioral assessments. The third hypothesis of this study considered the association of theta ITC with ADHD symptomatology throughout childhood. This was done by another linear regression model conducted hierarchically. The control variable was entered in the first step, and ADHD symptomatology from both childhood and adolescence was entered in the second step.
3 Results
3.1 Preliminary Analyses
Adolescents' background variables (i.e., age at the adolescence assessment and intelligence scores measured at the age of 13 years of age) and parental background variables (i.e., parents' education and age at the time of the child's birth) did not correlate with any of the dependent variables (i.e., ADHD symptomatology at 17 years of age and theta ITC; rs < |0.21|, ps > 0.19). There were no outliers beyond the range of 2.5 SD above or below the average of each variable of interest. We examined whether the assumptions of linear regression models were met. In general, the required assumptions were met; in one model, the homoscedasticity assumption was violated; however, a bootstrap test confirmed a high resemblance between the parametric model and non-parametric model results, and therefore, further use of the parametric model was valid.
Descriptive statistics are presented in Table 1. Symptomatology throughout childhood is presented separately for ages 54 months and 7 years. As can be seen, our sample presents the full range of symptomatology degree for all three timepoints of measurement.
3.1.1 Preliminary Electrophysiological Analysis
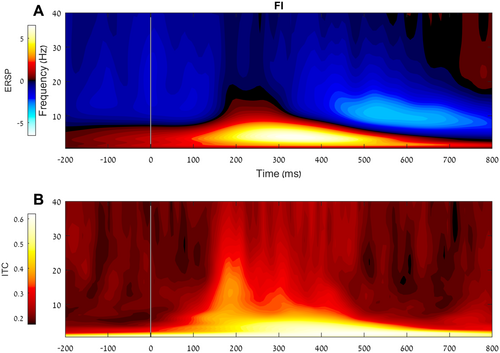
As can be seen in Figure 3 panel A, a positive ERSP is evident in lower frequencies at approximately 150 to 600 ms after the stop stimulus presentation. Moreover, as expected, at approximately the same period, an increase in phase coherence (i.e., less variability) within the similar frequency range can be observed (see Figure 3 panel B). Figure 4 presents the topographic maps of the ERSP (panel A) and the theta ITC (panel B). As expected, both measures peak in a central localization.
3.1.2 Preliminary Behavioral Analyses
Descriptive statistics of the behavioral SST measures are presented in Table 1. In general, none of the behavioral RT measures correlated with ADHD symptoms, except for SSRT, which was positively associated with childhood ADHD symptoms, r = 0.30, p < 0.05 (as previously reported in Einziger et al. 2021). SSRT was also correlated with theta ITC, r = −0.52, p < 0.01, suggesting that lower synchronization in the theta phase during FI trials is associated with longer SSRT and, thus, a less efficient inhibition process.
3.1.3 Correlation Analyses
Intercorrelations among the study variables of interest are presented in Table 2. Theta ITC in FI trials significantly correlated with both concurrent and childhood ADHD symptoms, Pe amplitude, SSRT, and number of FI trials. Pe amplitude significantly correlated with concurrent ADHD symptoms and the number of FI trials; ERN amplitude correlated significantly only with SSRT. Therefore, the number of FI trials was controlled in all the analyses; the SSRT was used for additional analyses.
| Variable | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Number of FI trials | ||||||
| 2. SSRT | 0.85** | |||||
| [0.75, 0.91] | ||||||
| 3. Theta ITC in FI trials | −0.52** | −0.52** | ||||
| [−0.69, −0.28] | [−0.72, −0.26] | |||||
| 4. ERN | 0.11 | 0.33* | −0.18 | |||
| [−0.16, 0.37] | [0.04, 0.57] | [−0.44, 0.11] | ||||
| 5. Pe | −0.38** | −0.27 | 0.57** | 0.22 | ||
| [−0.59, −0.13] | [−0.52, 0.03] | [0.34, 0.73] | [−0.04, 0.46] | |||
| 6. ADHD symptoms during adolescence | −0.07 | 0.21 | −0.40** | −0.02 | −0.31* | |
| [−0.32, 0.18] | [−0.08, 0.47] | [−0.61, −0.13] | [−0.28, 0.25] | [−0.53, −0.05] | ||
| 7. ADHD symptoms throughout childhood | 0.13 | 0.30* | −0.41** | 0.10 | −0.18 | 0.47** |
| [−0.13, 0.38] | [0.01, 0.54] | [−0.62, −0.14] | [−0.17, 0.36] | [−0.43, 0.09] | [0.25, 0.65] |
- Note: Values in square brackets indicate the 95% confidence interval for each correlation.
- Abbreviations: ADHD, attention deficit hyperactivity disorder; ERN, error-related negativity; FI, failed inhibition; ITC, inter-trial coherence; Pe, positivity error; SSRT, stop-signal reaction time.
- * p < 0.05.
- ** p < 0.01.
3.2 Predicting Concurrent ADHD Symptomatology from Error Processing Neural Measures
Table 3 presents the linear regression model in which the error processing neural measures were used to predict ADHD symptoms at 17 years of age. In the first step, the number of FI trials was entered and did not make a significant contribution to the prediction of ADHD symptomatology. In the second step, both ERN and Pe were entered, and only Pe had a unique contribution to the prediction of ADHD symptomatology. In the third step, theta ITC was entered. As can be seen in the table, theta ITC had a unique contribution to the prediction of concurrent ADHD symptomatology above and beyond the traditional ERP measures, with reduced coherence in theta phase being associated with higher ADHD symptomatology. Furthermore, the contribution of Pe to the prediction of ADHD symptomatology was no longer significant, probably due to the significant intercorrelation between Pe and theta ITC (see Table 2). Additionally, the number of FI trials did reach significance in the last step of the regression, with fewer FI trials associated with higher ADHD symptomatology. The reversed directionality of this effect was due to the suppressing effect caused by the multicollinearity between the number of FI trials and theta ITC (Akinwande, Dikko, and Samson 2015). The final model was significant, F(4, 44) = 3.75, p < 0.01. This model's effect size measures (i.e., R2 and adjusted R2) estimate a small-to-medium effect. Bayes factor comparison of the models presented in steps 2 and 3 yielded strong support for the final model, which included theta ITC, BF = 2.59 × 1011; therefore, it supports the second hypothesis by which theta ITC would have a unique contribution to ADHD symptomatology beyond the traditional ERP measures.
| Model | Predictor | ADHD symptoms at 17 years of age | R2 (adjusted R2) | |
|---|---|---|---|---|
| β | ΔR2 | |||
| 1 | Number of FI trials | −0.03 | 0.00 (0.00) | |
| 2 | Number of FI trials | −0.19 | 0.13* | 0.13 (0.08)+ |
| ERN | 0.18 | |||
| Pe | −0.40* | |||
| 3 | Number of FI trials | −0.34* | 0.12* | 0.25 (0.19)* |
| ERN | 0.05 | |||
| Pe | −0.14 | |||
| Theta ITC | −0.49* | |||
- Note: In this linear regression model, the homoscedasticity assumption was slightly violated, but bootstrap analysis with 1000 iterations yielded the same pattern of results as in the parametric test, thus the original model was considered valid. A similar pattern of results was found when we used the mean amplitude method to calculate the ERN and Pe amplitudes (see Appendix A). We also conducted comparable regression analyses using error-related RT measures (i.e., RT on FG trials and RT on FI trials) instead of error-related ERP measures (i.e., ERN and Pe). These error-related RT measures did not significantly predict ADHD symptoms, while theta ITC remained significant after controlling for these RT measures.
- Abbreviations: ADHD, attention deficit hyperactivity disorder; ERN, error-related negativity; FI, failed inhibition; ITC, inter-trial coherence; Pe, positivity error.
- + p < 0.10.
- * p < 0.05.
This model indicates that theta ITC not only has a predictive value over the traditional ERP measures but that the entire association of these measures with ADHD symptomatology can be explained by theta ITC. This finding suggests that theta ITC is a more sensitive measure for neural deficits during error processing.
Figure 5 demonstrates the difference in theta ITC between adolescents with low ADHD symptomatology and adolescents with high ADHD symptomatology. For illustration purposes, the figure presents the averaged theta ITC of adolescents rated in the first quartile of ADHD symptoms at 17 years of age (i.e., low symptomatology) versus the averaged theta ITC of those who were rated at the fourth quartile of ADHD symptoms (i.e., high symptomatology). In line with the linear regression model presented in Table 3, it is apparent that the adolescents with high ADHD symptomatology show significantly reduced theta ITC shortly after the commission of an error.
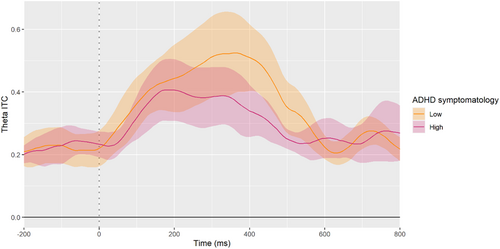
Due to the suppression effect caused by including the number of FI trials in the model, we also conducted this analysis with a different approach to ensure that this suppression effect did not interfere with our results. Instead of controlling for the number of FI trials, we conducted the analysis on a subsample with an equal number of trials per subject. Specifically, we limited our sample to participants who had at least 15 eligible FI trials after pre-processing (n = 41). For each participant in this subsample, we randomly selected 15 FI epochs and recalculated the theta ITC based on these trials. We then performed the same regression analysis without controlling the number of FI trials. This analysis produced the same pattern of results as the main regression presented in Table 3 (see Appendix B for the full results).
3.3 Additional Exploratory Analysis
In the preliminary behavioral analyses, SSRT correlated with theta ITC. Both theta ITC and the SSRT are considered cognitive processes that require the implementation of cognitive control (Gratton et al. 2018). However, theta ITC during FI trials is associated with error processing, whereas SSRT reflects the efficiency of inhibitory control (Lipszyc and Schachar 2010). This raises two additional questions. First, whether theta ITC contributes to the prediction of ADHD beyond the contribution of inhibitory control, as measured by SSRT. Second, whether the deficit in phase synchronization is specific to error processing or indicative of a more general cognitive control process, which would be evident not only in failed inhibition but also in successful inhibition trials.
3.3.1 Predicting Concurrent ADHD Symptomatology from SSRT and Theta ITC in FI Trials
To address the first additional question, we conducted a linear regression model in which SSRT and theta ITC were used to predict ADHD symptoms at 17 years of age. The results are presented in Table 4. In the first step, the number of FI trials was entered and did not make a significant contribution to the prediction of ADHD symptomatology. In the second step, SSRT was entered and made a marginal contribution to the prediction of ADHD symptomatology. In the third step, theta ITC was entered and made a unique contribution to the prediction of concurrent ADHD symptomatology above and beyond SSRT. Furthermore, the contribution of SSRT to the prediction of ADHD symptomatology did not reach statistical significance (which could be due to the significant intercorrelation between SSRT and theta ITC). The final model was significant, F(3, 37) = 4.08, p < 0.05.
| Model | Predictor | ADHD symptoms at 17 years of age | ΔR2 | R2 (adjusted R2) |
|---|---|---|---|---|
| β | ||||
| 1 | Number of FI trials | −0.15 | 0.00 (0.00) | |
| 2 | Number of FI trials | −0.53+ | 0.11+ | 0.10 (0.05) |
| SSRT | 0.54+ | |||
| 3 | Number of FI trials | −0.45+ | 0.15* | 0.25 (0.19)* |
| SSRT | 0.24 | |||
| Theta ITC | −0.46** |
- Note: It should be noted that controlling for the number of FI trials is equivalent to controlling for the number of SI trials, as they are complementary variables. In this analysis, we used the number of FI trials to maintain consistency with the other analyses.
- Abbreviations: ADHD, attention deficit hyperactivity disorder; FI, failed inhibition; ITC, inter-trial coherence; SSRT, stop-signal reaction time.
- + p < 0.10.
- * p < 0.05.
- ** p < 0.01.
3.3.2 Predicting Concurrent ADHD Symptomatology From Theta ITC in Both FI Trials and SI Trials
To determine whether the effect of theta ITC indicates a broader deficit in cognitive control processes rather than being specific to error processing, we also calculated theta ITC in SI trials (which reflects a successful inhibitory control process). We then conducted a linear regression model to predict ADHD symptoms at 17 years of age using theta ITC from both SI and FI trials. This analysis is presented in Table 5.
| Model | Predictor | ADHD symptoms at 17 years of age | R2 (adjusted R2) | |
|---|---|---|---|---|
| β | ΔR2 | |||
| 1 | Number of FI trials | −0.02 | 0.00 (0.00) | |
| 2 | Number of FI trials | −0.15 | 0.05 | 0.05 (0.01) |
| Theta ITC in SI trials | −0.26 | |||
| 3 | Number of FI trials | −0.30+ | 0.17** | 0.23** (0.18) |
| Theta ITC in SI trials | 0.04 | |||
| Theta ITC in FI trials | −0.58** | |||
- Note: In this linear regression model, the homoscedasticity assumption was slightly violated, but bootstrap analysis with 1000 iterations yielded almost identical results to the parametric test, thus the original model was considered valid.
- Abbreviations: ADHD, attention deficit hyperactivity disorder; FI, failed inhibition; ITC, inter-trial coherence; SI, successful inhibition.
- + p < 0.10.
- ** p < 0.01.
In the first step, the number of FI trials was entered and did not make a significant contribution to the prediction of ADHD symptomatology. In the second step, theta ITC in SI trials was entered and did not make a significant contribution to the prediction of ADHD symptomatology. In the third step, theta ITC in FI trials was entered and made a significant contribution to the prediction of concurrent ADHD symptomatology above and beyond theta ITC in SI trials. It should be noted that this effect was significant even though theta ITC in SI trials and theta ITC in FI trials were highly correlated with each other, r = 0.65, p < 0.001. The final model was significant, F(3, 46) = 4.52, p < 0.01.
Due to a significant difference between the number of trials eligible for analysis in the FI condition compared to the SI condition, and in order to ensure the reliability of our results, we conducted an additional version of this regression analysis. Similar to the regression analysis presented in Section 3.2, instead of controlling for the number of FI trials, we conducted the analysis on a subsample with an equal number of SI and FI trials per subject. Specifically, we limited our sample to participants who had at least 15 eligible trials in both conditions (i.e., FI and SI) after pre-processing (n = 40). For each participant in this subsample, we randomly selected 15 epochs in each condition and recalculated the theta ITC measures based on these trials. We then performed a multiple regression analysis without controlling the number of FI trials. This analysis produced the same pattern of results as the main regression presented in Table 5 (see Appendix C for the full results).
3.4 Predicting Error Processing Related Theta ITC from ADHD Symptoms Throughout Development
Our third hypothesis was that childhood ADHD symptomatology would have a unique association with theta ITC, beyond the expected relation with concurrent ADHD symptomatology. As seen in Table 2, ADHD symptoms throughout childhood correlated with theta ITC, which indicated the existence of a first-order association between early childhood symptomatology and theta ITC as measured at 17 years of age. Table 6 presents the linear regression model that was used to examine whether ADHD symptoms throughout childhood had a predictive value beyond concurrent symptomatology during adolescence. In the first step, the control variable (i.e., number of FI trials) was entered and had a significant contribution to the prediction of theta ITC. In the second step, both ADHD symptoms throughout childhood and concurrent ADHD symptoms were entered. Both ADHD measures had a significant unique contribution to the prediction of the theta ITC beyond the control variable. For both measures, higher ADHD symptomatology predicted reduced coherence in theta phase during error processing. The entire model was significant, F(3, 45) = 13.93, p < 0.001.
| Model | Predictor | Theta ITC | R2 (adjusted R2) | |
|---|---|---|---|---|
| β | ΔR2 | |||
| 1 | Number of FI trials | −0.52*** | 0.27*** (0.25) | |
| 2 | Number of FI trials | −0.51*** | 0.22*** | 0.48*** (0.45) |
| ADHD symptoms throughout childhood | −0.25* | |||
| ADHD symptoms during adolescence | −0.30* | |||
- Note: ADHD symptoms in adolescence refers to ADHD symptomatology as assessed at 17 years of age.
- Abbreviations: ADHD, attention deficit hyperactivity disorder; FI, failed inhibition; ITC, inter-trial coherence.
- * p < 0.05.
- *** p < 0.001.
4 Discussion
This study aimed to examine the relation between neurophysiological markers of error processing and ADHD symptomatology during adolescence, specifically theta ITC, which was previously suggested as a marker of a core neural dysfunction in ADHD. Our results suggest that reduced theta ITC during error processing was uniquely associated with ADHD symptoms, above and beyond the traditional ERP measures of error processing (i.e., ERN and Pe). In addition, high ADHD symptoms throughout childhood uniquely predicted reduced theta ITC during adolescence, even beyond concurrent ADHD symptoms. These findings and their interpretations are discussed in detail.
We investigated the relation of three neural markers of error processing (i.e., ERN, Pe, and theta ITC) with ADHD symptomatology. Based on previous research (e.g., Aydin et al. 2023; Czobor et al. 2017; Groom et al. 2010; McLoughlin, Gyurkovics, and Aydin 2022), we expected that all measures would show a reduction in amplitude or coherence in adolescents with high ADHD symptomatology. Indeed, Pe and theta ITC yielded the expected pattern. Importantly, theta ITC had the strongest association with ADHD symptomatology, even beyond the contribution of Pe and ERN. Theta ITC accounted for the entire portion of the variance of ADHD symptoms that was explained by these traditional ERP measures. This finding aligns with Groom et al.'s (2010) suggestion that the abnormalities observed in the ERN and Pe during error processing in ADHD stem from less efficient coordination of theta-evoked brain activity. Moreover, McLoughlin et al. (2014) suggested that ADHD is accompanied by an integral deficit in the ability to recruit cognitive control resources to successfully evaluate ongoing failed performance and adjust behavior accordingly. Our results are in line with these claims and are the first to examine the overlap between these neural error processing measures.
This suggests that the inter-subject difference in the averaged Pe amplitude might be affected by the consistency of elicited theta power during the implementation of cognitive control. Therefore, when the theta power is inconsistent across trials, the averaged Pe amplitude will show a reduction in amplitude, and when the theta power is consistent across trials, the averaged Pe amplitude will show an increase in amplitude. In other words, theta phase synchronization might be the neural mechanism that accounts for the inter-subject variation in Pe amplitude. Thus, it might explain why the Pe had no unique contribution to the prediction of ADHD symptomatology above theta ITC, despite previous evidence demonstrating a diminished Pe amplitude in ADHD (Kaiser et al. 2020; McLoughlin, Gyurkovics, and Aydin 2022). To further explore this possibility, we re-analyzed the regression analysis presented in Table 3 in a manner that considers the onset of neurophysiological markers (see Appendix D). This analysis yielded the same pattern of results and thus does not contradict this possibility; further research is needed to examine this thoroughly. While our study design cannot imply causality from a specific neural dysfunction to ADHD symptomatology severity, it does shed light on a potential abnormal neural mechanism that could be closely linked to the disorder's core neural deficits. Specifically, our findings suggest that the inability to consistently elicit the neural processes involving theta activity explains the impaired implementation of cognitive control during error processing.
It is possible that the effect of theta ITC in FI trials in our analyses could reflect a specific deficit in processing the failure to inhibit a response, or it might be related to processing of the stop stimulus itself. To explore this further, we conducted an additional regression analysis as an exploratory step beyond our original hypotheses. This analysis included theta ITC during both inhibition errors (FI trials) and successful inhibition (SI trials). The results showed that theta ITC during FI trials, but not during SI trials, uniquely predicted ADHD symptoms. This suggests that the deficit in theta phase synchronization may be more pronounced in error processing than in other cognitive control processes. Still, these findings are exploratory, and further research is needed to examine this question thoroughly.
Furthermore, we found that both ADHD symptomatology throughout childhood and ADHD symptomatology during adolescence had unique associations with theta ITC. Aydin et al. (2023) found that approximately 68% of theta ITC shared genetic overlap with ADHD diagnosis, thereby substantiating a genetic link. Our study further supports this by demonstrating an association between theta ITC and ADHD symptomatology across different developmental periods. Throughout development, the manifestation of ADHD characteristics changes. This change is evident both in symptomatology appearance and in the development of neural structures over the years (Franke et al. 2018; Halperin and Healey 2011). For example, it was previously suggested that later cortical development may, in part, diminish some of the disorder's symptoms (Shaw et al. 2006, 2013). Therefore, from a neural perspective, the unique association with adolescent symptomatology may reflect characteristics of persistent ADHD deficits and later cortical maturation. Conversely, the unique association of childhood symptomatology with theta ITC may indicate characteristics that pose potential risks for neural abnormalities later in life. Further research assessing both theta ITC and ADHD symptomatology throughout development is needed to examine this idea.
Our results regarding theta ITC and its association with ADHD symptomatology add to the growing interest in the scientific literature regarding increased neural variability in ADHD (e.g., Einziger et al. 2023; Gonen-Yaacovi et al. 2016; Kuntsi and Klein 2012; McLoughlin et al. 2014; Saville et al. 2015). The cumulative evidence suggests that increased neural variability in ADHD might reflect a global abnormality rather than occurring within a specific cognitive process. While in the current study we found increased neural variability in cognitive control-related neural features, similar abnormalities were also found in primary visual processing (Einziger et al. 2023; Gonen-Yaacovi et al. 2016), primary auditory processing (Gonen-Yaacovi et al. 2016), and response execution (Saville et al. 2015). In addition, increased neural variability in ADHD is apparent in different aspects of the neural response; the current study demonstrated increased neural variability in phase coherence, but different studies also exhibited increased variability in amplitude voltage (Einziger et al. 2023; Gonen-Yaacovi et al. 2016) and amplitude latency (Saville et al. 2015). Therefore, it is plausible that increased neural variability is an inherent neural deficit that affects multiple cognitive processes that underlie the manifestation of different symptoms.
It should be noted that in our study, the ERN was not associated with ADHD symptomatology. While previous evidence has suggested a reduction in ERN amplitude among individuals with ADHD (e.g., Herrmann et al. 2010; Liotti et al. 2005), this relation was found to be less consistent across studies (Kaiser et al. 2020; McLoughlin, Gyurkovics, and Aydin 2022). Our finding that adolescents with high ADHD symptomatology displayed reduced amplitude in the Pe but not in the ERN suggests a deficiency in error awareness rather than conflict monitoring processes (Larson, Clayson, and Clawson 2014; LoTemplio et al. 2023; O'Connell et al. 2007; Overbeek, Nieuwenhuis, and Ridderinkhof 2005). However, it is also plausible that abnormalities in the ERN components are more challenging to detect due to their relatively small magnitude compared to the Pe component. Moreover, it should be noted that the ERN morphology in our study was slightly atypical, displaying two distinctive peaks instead of a single peak (see Figure 2 panel B). This morphology resulted from a bimodal distribution of ERN latency. However, this ERN latency did not correlate with any of the study variables or potential covariates (i.e., participants' age, IQ, ADHD symptomatology, theta ITC, mean RT, SSRT, and number of FI trials; r < |0.21|, p > 0.15). This minor anomaly in ERN morphology might be due to the limited sample size. Identifying a typical distribution of the ERN latency might require larger sample sizes and greater statistical power.
Previous studies have also demonstrated an association between ADHD symptomatology and behavioral measures of inhibitory control, evidenced by both increased SSRT (Crosbie et al. 2013; Lipszyc and Schachar 2010) and a higher rate of commission errors (i.e., incorrect responses to a stop stimulus in tasks involving response inhibition, such as the go/no-go task; Kuntsi et al. 2010; Metin et al. 2012). In our study, individuals with higher childhood symptomatology exhibited longer SSRT in adolescence, with a similar trend observed for concurrent ADHD symptoms. However, SSRT did not make a unique contribution above and beyond theta ITC in FI trials: it could be that it is a less sensitive measure for detecting subtle individual differences in ADHD symptomatology in a non-clinical sample. Moreover, we did not find a higher rate of commission errors, possibly because of the implementation of the staircase dynamic-tracking procedure. This procedure adjusted task difficulty to maintain a 50% success rate, which could compromise the ability to detect subtle individual differences in this measure among adolescents with varying levels of ADHD symptomatology. Indeed, a recent meta-analysis of SST studies in ADHD demonstrated that this association was inconsistent across studies (Senkowski et al. 2024).
It should also be noted that we did not find evidence for a relationship between behavioral error-related RTs and ADHD symptomatology; specifically, RT on FI trials and RT on FG trials. However, theta ITC on FI trials was related to ADHD symptomatology, above and beyond these behavioral measures. This suggests that this electrophysiological marker might be more sensitive than behavioral measures in detecting cognitive control deficits among adolescents with varying levels of ADHD symptoms. However, the design of the SST in the current study limited our ability to thoroughly examine FG errors, as the go task was a simple discrimination task with high accuracy rates. Examining different types of errors, both behaviorally and electrophysiologically, could enhance our understanding of ADHD-related dysfunction, particularly during error processing. Future research could increase the difficulty of the go task to better assess different types of errors within the same task.
5 Limitations
The current study has several limitations that should be taken into consideration when interpreting the results. First, our sample was part of a prospective longitudinal study that began 20 years ago and included only male participants (e.g., Auerbach et al. 2004, 2008; Einziger et al. 2018), limiting the possibility to generalize our results to females with ADHD. The selection of a male-only sample was done at the beginning of the recruitment for the prospective longitudinal study to ensure a higher prevalence of participants who would eventually develop ADHD, due to the higher rate of ADHD in males (American Psychiatric Association 2000). Second, our final sample size was relatively small. Third, the ADHD symptomatology assessment at all time points was based on maternal reports. In future research, it might be advisable to use a teacher or professional neutral observer assessment to strengthen the validity of the symptomatology assessment.
6 Conclusions
To conclude, our study strengthens the view of theta ITC as a neurophysiological marker of a core neural dysfunction in ADHD. This dysfunction is manifested in more varied temporal dynamics of phase synchronization in theta frequencies, which might underlie the deficient implementation of cognitive control during error processing in ADHD. Our findings suggest that theta ITC serves as a more sensitive neural marker for deficits in error processing in ADHD compared to the traditional ERP measurement and behavioral measures related to error processing and inhibitory control. Moreover, ADHD symptomatology throughout childhood was uniquely associated with theta ITC even beyond the ADHD symptomatology during adolescence, thus implying theta ITC's potential significance to the understanding of the neural characteristics that underlie the disorder's symptomatology.
Author Contributions
Tali Devor: conceptualization, formal analysis, investigation, methodology, software, validation, writing – original draft. Tzlil Einziger: conceptualization, investigation, methodology, supervision, writing – review and editing. Mattan S. Ben-Shachar: data curation, software. Christoph Klein: writing – review and editing. Judith G. Auerbach: data curation, writing – review and editing. Andrea Berger: conceptualization, funding acquisition, methodology, resources, supervision, writing – review and editing.
Conflicts of Interest
The authors declare no conflicts of interest.
Endnotes
Open Research
Data Availability Statement
The data that support the findings of this study will be openly available after publication of the manuscript.