Patterns of adaptation to stress cardiovascular responses in smokers during ad libitum smoking and withdrawal
Abstract
There is considerable evidence documenting associations between tobacco smoking, including initiation, maintenance, and relapse of addiction, with diminished cardiovascular responses to acute psychological stress. However, less is known about how smokers respond to repeated stress across time. The current study examined patterns of cardiovascular reactivity and adaptation to recurrent stress among 24-h abstinence smokers, smokers who continued to smoke at their normal rate, and non-smokers. Smokers were randomly assigned to one of two groups; ad libitum (n = 42), or 24 h abstinence (n = 61); non-smokers (n = 43) provided comparative referencing. Across the two laboratory sessions, participants (n = 149) were asked to complete a modified version of the trier social stress test, while monitoring systolic and diastolic blood pressure, and heart rate activity. Results showed that while non-smokers had elevated cardiovascular reactivity to begin with, they showed a greater capacity to habituate to recurrent stress across sessions. The data also suggest that smokers displayed lower cardiovascular reactivity to acute psychological stress and showed little habituation to repeated stress. In adjusted models, smokers exhibited less systolic blood pressure habituation to stress. This response profile in smokers may be a potential mechanism that leads to further cardiotoxic effects on health.
1 INTRODUCTION
Cardiovascular diseases (CVDs) are the leading cause of disease and mortality worldwide. An estimated 50% of the risk of developing CVD is attributed to conventional risk factors such as diabetes, hypertension, high cholesterol, and smoking (Dimsdale, 2008; Schneiderman, 2022). Tobacco smoking reportedly accounts for 8 million deaths globally each year (World Health Organisation, 2024). While racial and ethnic disparities exist in the prevalence and symptom expression of CVD (Hackler III et al., 2019), smoking has been found to be a strong predictor of these cardiac-related differences across racial and ethnic groups (Agarwala et al., 2021; Mazimba & Peterson, 2021; Pool et al., 2017; Zheng et al., 2021). Epidemiological studies show that active smoking, as well as smoking low-tar cigarettes and passive smoking (i.e., environmental), enhance susceptibility to adverse cardiovascular outcomes (Ambrose & Barua, 2004; Gallucci et al., 2020). Smokers have a two-to-four-fold increased risk of developing coronary heart disease compared to non-smokers; the risk of stroke linked to hypertension is amplified in smokers (Aldoori & Rahman, 1998), and 75% of myocardial infarction cases are attributed to smoking (Mähönen et al., 2004; Tonstad & Johnston, 2006). While smoking predisposes individuals to atherosclerotic syndromes, the exact mechanisms linking smoking and CVD are not fully understood. This may in part relate to chemicals found in tobacco (e.g., nicotine, carbon monoxide) known to directly influence the cardiovascular system (Ambrose & Barua, 2004).
It is well established that smoking leads to alterations in the sympathetic nervous system (SNS), which is vital in coordinating the body's stress response (al'Absi, 2020; Chrousos, 2009; Heim et al., 2000; Lovallo et al., 2012). This chronic activation of the SNS, evidenced by increases in blood pressure and heart rate (HR), is one mechanism whereby smoking is considered to increase the development and progression of CVD (Kotlyar et al., 2017). The reactivity hypothesis posits that the magnitude of the stress response can lead to a proliferation in disease with prolonged and exaggerated responses related to indicators of CVDs including atherosclerosis (Barnett et al., 1997; Obrist, 1981), hypertension (Brindle et al., 2016; Carroll et al., 2003; Matthews et al., 1993) and cardiac mortality (Carroll et al., 2012). Further, while controlling for standard risk factors of CVD such as smoking, heightened SBP responses have been linked to carotid intima-media thickness (Jennings et al., 2004), hypertension at a five-year follow-up (Carroll et al., 2012), subclinical CVD in adolescents (Roemmich et al., 2011) and left ventricular mass (Taylor et al., 2003). Lower, or blunted, cardiovascular reactivity (CVR), once considered protective, is related to obesity (Carroll et al., 2008; Phillips et al., 2012), depression (de Rooij et al., 2010), poorer self-rated health (de Rooij & Roseboom, 2010; Phillips et al., 2009), increased cardiac hospitalizations (Sherwood et al., 2017), enhanced risk of CVD mediated by smoking (Ginty et al., 2016), cardiac mortality (Kupper et al., 2015), and a proposed marker for addictive behaviors, including alcohol (Lovallo et al., 2000) and smoking (al'Absi et al., 2003, 2005; Dube et al., 2010).
Smoking and stress independently enhance cardiac activity and in combination, nicotine and stress have an additive effect, which stimulates the cardiovascular system and increases allostatic load (Ashare et al., 2012; Kotlyar et al., 2017; Pomerleau & Pomerleau, 1991). Withdrawal from nicotine may mimic the effects of stress, and negative symptoms associated with abstinence from smoking, including depression, anxiety, and irritability, may render ex-smokers more susceptible to relapse (al'Absi, 2006; al'Absi et al., 2005, 2022; Shiffman, 1982; Stitzer & Gross, 1988). Research examining associations between smoking and CVR have produced mixed results; with reports that smokers who abstain from smoking overnight exhibit larger diastolic blood pressure (DBP) (Tsuda et al., 1996), and systolic blood pressure (SBP) reactivity compared to non-smokers and smokers who continue to smoke at their normal rate (al'Absi et al., 2002). More recently, cigarette smoking has been associated with lower cardiovascular responses to stress, (Ginty et al., 2014; Phillips et al., 2009), with blunted SBP responses observed in ad libitum (i.e., those who continue to smoke their preferred brand at their normal rate) and 24-h abstinence smokers compared to non-smokers (al'Absi et al., 2003). Similarities are also reported for blunted SBP (Dobkin & Pihl, 1992) and HR reactivity (Evans et al., 2012) in adolescent samples.
Physiological responses to stress also aid in our survival and have an adaptive purpose in maintaining homeostasis (Benini et al., 2020), but if sustained overtime can place a burden on the cardiovascular system and lead to a greater risk of disease. As such, assessment of a single stress paradigm is proposed to be less informative when examining the moderators and mediators of the cardiovascular response to stress (Hughes et al., 2018). Recent work suggests that evaluating the magnitude of change to the same stressor over time, be it increases (i.e., sensitization) or decreases (i.e., habituation) in physiological responses to recurrent stress, provides a more detailed profile of not only the initial response to stress, but also the individual's capacity to adapt to recurrent stress (Frankish & Linden, 1991). The term habituation refers to greater decreases in physiological reactions on repeated exposures, is considered a more optimal response to stress (Hughes et al., 2018), an important component of adaptation (Glaser, 1966; Groves & Thompson, 1970), and differs from recovery which is the ability to return to baseline levels after stress (Hughes et al., 2018). Successful habituation is linked to resilience (Lü, Wang, & You, 2016) and higher engagement in social activities (Keogh & Howard, 2024). Less habituation is associated with childhood adversity (Tyra et al., 2021), rumination (Johnson et al., 2012), neuroticism (Hughes et al., 2011), cynical hostility (Tyra et al., 2020), and adiposity (Feda et al., 2016), though prospective studies are required to elucidate the links between habituation and future disease outcomes.
One biological factor that has been shown to disrupt patterns of adaptation is smoking, and to date, has received little attention in research. Hughes and Higgins (2010), in an anthropometrically matched trial, identified that while smokers who abstained from smoking 1 h prior to the task had higher blood pressure response to stress than non-smokers, non-smokers demonstrated blood pressure response sensitization. This study examined within-session exposure to the same stressor but was limited by a small sample size, visuospatial stress task, and lack of biochemical testing to validate smoking status. Given the health implications associated with addiction, investigating how smoking influences adaptation to recurrent stress may be particularly useful in profiling the vulnerabilities of this population. Investigating patterns of habituation to recurrent stress in smokers and identifying the synergistic effects of smoking and stress, may help elucidate the pathological impact on health outcomes, extending our current understanding of the psychophysiological mechanisms of smoking behaviors.
With this in mind, the aim of the present study was to extend on previous research reported by Hughes and Higgins (2010), who found smokers failed to habituate to repeated stress within the same session, by examining the association between smoking and cardiovascular stress-response adaptation across two independent laboratory testing sessions. Further, we aimed to examine if different smoking conditions (i.e., ad libitum, 24-h abstinence) were associated with negative patterns of habituation; that is, would these individuals show less habituation to recurrent stress on exposure to the second stress task (i.e., evidenced by minimal depreciable differences in cardiovascular responses from the first to second exposure). Given the impact of smoking on cardiac health, it was hypothesized that smoking, irrespective of the condition, would be related to less habituation.
2 METHOD
2.1 Study overview
The current article is based on detailed analyses of data obtained from an existing dataset, and design details of this study have been described previously (al'Absi et al., 2021). In brief, using a double-blind design, the primary aim of the overall study from which the data was drawn was to expose participants to an opioid blockade challenge one time in the laboratory, to evaluate the role of endogenous opioid activity in stress response dysregulation. A novel feature of the study design was the inclusion of CVR assessment across two separate standardized stress testing sessions approximately 10 days apart, while also obtaining behavioral, psychological, and health measurements.
Participants responded to flyers posted around the campus community and to online advertisements. Those who met any of the following criteria were excluded from the study; history of hypertension, those with renal, hepatic, cardiovascular, or chronic diseases (e.g., coronary heart disease), history of major psychiatric disorder (e.g., depression; alcohol and drug abuse), currently pregnant, weight not within ±30% of Metropolitan Life Insurance norms, those taking prescription or over-the-counter medications known to effect cardiovascular/endocrine activity (e.g., beta-blockers), or those with an opiate dependency or use of any narcotics within 3 days prior to the study.
Written informed consent was obtained during the medical screening prior to the laboratory sessions. Participants who completed all sessions of the study received $360 for their participation, with an additional $10 bonus added to the payment; an incentive method to increase performance on the speech task. The study was approved by the University of Minnesota Institutional Review Board (IRB).
2.1.1 Participants
After the initial medical screening, a total of 209 participants from the Duluth, MN area enrolled in the study. One-hundred and forty-nine of these individuals completed both laboratory sessions. From this, three outliers were removed from deviating ±3.00 SD from the mean on physiological reactivity scores. The final sample consisted of 146 participants, (mean age = 35.18; SD = 12.57 years; female = 43.2%, White/Caucasian = 69.9%; non-Hispanics = 93.2%). Current smokers were assigned to one of the following smoking experimental groups (n = 42; ad libitum; n = 61; 24 h abstinence) and a control group (n = 43) of non-smokers. Analyses confirmed no differences in demographics (ps > .05) between those who completed the laboratory sessions and those who failed to complete the scheduled sessions. Based on power calculations, a minimum total sample size of 66 participants was needed to detect a significant effect (p = .05, f = 0.25) at 95% power.
2.2 Measures
2.2.1 Smoking groups
Smokers who were uninterested in quitting were considered eligible for the study if they smoked at least 10 cigarettes per day for the past 2 years. Non-smokers were required to have not smoked in the previous 5 years and less than a total of 100 cigarettes in their lifetime (Centers for Disease Control and Prevention, 2024). During the medical screening, smokers were asked about patterns of tobacco use (e.g., cigs/day) and randomly assigned to one of the smoking experimental groups (ad libitum; 24 h abstinence). The smokers in the ad libitum group were asked to smoke the cigarettes of their preferred brand at their own pace for 24 h before each session. They were also allocated a smoking break during the laboratory sessions so that the last cigarette they smoked was <1 h before the task. Those in the 24-h abstinence group were instructed to abstain from all tobacco and nicotine products for 24 h before attending each session until the session was over. Non-smokers completed the same protocol.
2.2.2 Physiological assessment
Indices of cardiovascular activity, SBP, DBP, and HR, were measured non-invasively during lab sessions using a Dinamap oscillometric monitor (Critikon, Tampa, Florida; or Critikon Dinamap® Vital Signs Monitor 1846SX, GE Healthcare, Chicago, Illinois, USA). Baseline activity was defined as the average of four readings taken during the resting baseline period. Task cardiovascular activity was the average of the 10 readings taken across the stress tasks (speech preparation, speech delivery, and mental arithmetic), as previous work suggests this may be a more reliable method (e.g., Kamarck et al., 1992).
Reactivity values were determined as the differences between baseline and stress task values and were calculated separately for each laboratory session (Stress Task 1—Baseline 1; Stress Task 2—Baseline 2). There was a total of six reactivity values calculated: SBP reactivity session 1, SBP reactivity session 2, DBP reactivity session 1, DBP reactivity session 2, HR reactivity session 1, and HR reactivity session 2.
2.2.3 Self-report measures
Mood ratings of distress and positive affect were assessed across both sessions using a subjective questionnaire used in previous studies (al'Absi et al., 2003, 2021). Positive affect consisted of the sum of items on connectedness, cheerfulness, calmness, interest, and controllability. While distress consisted of summed items on irritability, anxiety, restlessness, and impatience. Items were rated on a response scale ranging from 0 (not at all) to 7 (very strong) and participants marked the point on each scale that best represented how they felt in the preceding 30 min. Higher scores indicated greater levels of positive affect and distress. Participants completed the scale during baseline, drug administration absorption period, and again at pre- and post-task stress (total of 7 times). Previous research has reported good psychometric properties across the two scales (al'Absi et al., 1994, 1998, 2003).
2.3 Acute psychological stress task
The laboratory protocol comprised public speaking and mental arithmetic stress tasks, presented in fixed order; speech preparation (4 min), speech delivery (4 min), and a mental arithmetic (8 min) task. During the speech task participants were required to defend themselves against a suspected transgression (shoplifting or hit and run violation), which was counterbalanced across the two laboratory sessions. Participants were told that their speech was being recorded and evaluated and that they could earn an extra $10 bonus based on the evaluation of their speech performance. In reality, no such evaluations took place, and all participants received the additional $10 on study completion. Following the speech task, participants completed a mental arithmetic task where they were given a three-digit starting number and instructed to calculate the sum of the number together and then add this sum to the three-digit number (e.g., 111, the sum is 3; add to 111, equals 114 and follow on to add the sum of 114, etc.). This protocol has been used in previous studies and shown to reliably induce cardiovascular changes (al'Absi et al., 1997, 2003, 2013). Participants were invited back to the laboratory approximately 10 days later and were exposed to the same stress tasks.
2.4 Procedure
Smokers were randomly assigned to one of the two smoking groups (ad libitum; 24 h abstinence), with non-smokers as a comparative group. Participants were scheduled to attend the two laboratory sessions, each lasting approximately 4 h and 10 days apart, with instructions given to refrain from alcohol and analgesic medications for 24 h prior to attending the lab. All lab sessions started early afternoon (i.e., 12 noon) to control for diurnal variations in hormones.
On arrival to the laboratory, a urine analysis was conducted to screen for narcotics and to test for pregnancy in female participants. To confirm smoking status (and abstinence), expired carbon monoxide (CO) was measured using a Bedfont Micro + monitor (coVita, Haddonfield, NJ). Smokers in the 24-h abstinence group with a CO ≥9 ppm, were rescheduled for testing. Participants were then brought to a testing room and provided with a standard lunch. After lunch, the blood pressure cuff was attached and after the baseline period, participants were administered a capsule containing either a placebo or 50 mg of naltrexone (one at each lab, in random order) under double-blind conditions. Given the reported effects of naltrexone on hypothalamic opioid tone (e.g., Chong et al., 2006; Wand & Schumann, 1998), this was controlled as a covariate in the current study.
2.5 Data analyses
Data were screened for outliers prior to analyses. Mean levels of SBP, DBP, and HR were computed for baseline and stress task phases for each laboratory session. To begin with, cardiovascular responses were averaged across tasks (i.e., speech preparation, speech delivery, math). However, separate analyses were then undertaken to determine if obtained results differed when accounting for reactivity and patterns of habituation to each task individually. Reactivity scores were calculated by the differences in change scores (i.e., task–baseline) on all physiological parameters.
A series of repeated measures analyses of variance (ANOVAs) (baseline, task) were used to determine if the stress tasks (separately for session 1 and session 2) successfully perturbed cardiovascular activity. Repeated measures ANOVAs (baseline, task) were also conducted to determine whether the stress tasks (separately for session 1 and session 2) elicited a change in subjective mood ratings. Chi-square (χ2), independent t tests, and one-way ANOVAs were used to examine between-group differences in general study parameters for continuous and categorical variables respectively. Unadjusted associations between study variables were examined using correlational analyses.
To examine the association of smoking withdrawal in responses (SBP, DBP, & HR) to both stressors separately, a series of 2 × 3 mixed factorial ANOVAs were performed, with phase (baseline, task) as the within-subjects factor for each stress task session (session 1, session 2), and smoking (ad libitum, abstinence, non-smokers) as the between-subjects factors.
Then a series of 2 × 3 mixed factorial ANOVAs were undertaken to examine the impact of smoking on physiological adaptation to stress, with phase (reactivity 1, reactivity 2) as the within-subjects factor and smoking (ad libitum, abstinence, non-smokers) as the between-subjects factor.
Effects on cardiovascular outcomes were replicated using a series of mixed-factor analyses of covariance (ANCOVAs) and included baseline values, sex, age, body mass index (BMI), and drug administration as confounding variables. For repeated measures effects, Greenhouse–Geisser corrections (ε) are presented. Bonferroni corrections were used for post hoc comparisons. Post hoc simple effects using one-way ANOVAs were used to examine group differences in habituation, using change scores (reactivity 1–reactivity 2). All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 28.0.1.1 (IBM Corp, Armonk, New York, USA).
3 RESULTS
3.1 Descriptive statistics
Descriptive statistics of study variables are noted in Table 1. Given the nature of the dataset, a series of independent t tests were conducted to examine potential differences in CVR and habituation (SBP, DBP, & HR) due to the order of administration of drug use (placebo vs. naltrexone). While there were no significant differences in CVR due to drug administration at session 2 (all ps > .05), significant group differences in DBP and HR habituation from session 1 to session 2 were observed (ps < .05). As such, drug administration was included as a covariate in adjusted models. No between-group differences were observed in age, sex, race, ethnicity, or BMI (all ps > .05).
| Non-smokers (N = 43) | Ad libitum (N = 42) | 24-h abstinence (N = 61) | |
|---|---|---|---|
| Age | 36.47 (12.84) | 34.43 (12.28) | 34.80 (12.71) |
| Sex (% female) | 51.2 | 38.1 | 41 |
| BMI (kg/m2) | 26.35 (4.43) | 27.88 (6.59) | 26.46 (6.08) |
| Race (% white) | 74.4 | 69 | 67.2 |
| Ethnicity (% not Hispanic) | 90.7 | 92.9 | 95.1 |
| Cigarettes/day | N/A | 13.04 (5.16) | 13.91 (7.02) |
3.2 Manipulation check
A series of repeated measures ANOVAs confirmed that the stress task successfully perturbed physiological activity for SBP, F (1, 145) = 331.62, p < .001, ηp2 = 0.696, DBP, F(1, 145) = 436.32, p < .001, ηp2 = 0.751, and HR, F(1, 145) = 150.86, p < .001, ηp2 = 0.510, at session 1; and SBP, F (1, 144) = 202.23, p < .001, ηp2 = 0.584, DBP, F(1, 144) = 340.06, p < .001, ηp2 = 0.703, and HR, F(1, 144) = 103.36, p < .001, ηp2 = 0.418, at session 2. For subjective mood ratings, repeated measures ANOVAs revealed that positive affect significantly decreased F (1, 137) = 50.37, p < .001, ηp2 = 0.269, at session 1; and F(1,140) = 62.79, p < .001, ηp2 = 0.310, at session 2. Distress scores significantly increased F (1, 139) = 35.85, p < .001, ηp2 = 0.205, at session 1, and F(1, 139) = 36.46, p < .001, ηp2 = 0.208, at session 2. Effects were in the expected direction, with a statistically significant increase from baseline to stress task across both laboratory sessions on physiological parameters (see Table 2) and similar directions on mood ratings of those reported elsewhere (e.g., O'Riordan et al., 2023).
| SBP (mmHg) | DBP (mmHg) | HR (bpm) | Positive affect | Distress | |
|---|---|---|---|---|---|
| Baseline—session 1 | 113.82 (10.85) | 66.45 (8.50) | 68.72 (10.04) | 16.39 (7.04) | 4.64 (4.86) |
| Task—session 1a | 128.82 (14.56) | 76.68 (9.44) | 76.77 (11.52) | 12.08 (6.96) | 7.10 (5.05) |
| Baseline—session 2 | 114.01 (10.46) | 66.29 (8.55) | 69.42 (9.34) | 16.22 (7.09) | 4.18 (4.66) |
| Task—session 2a | 123.64 (23.11) | 73.24 (17.37) | 74.11 (18.86) | 13.16 (7.03) | 6.38 (5.22) |
- Abbreviations: DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
- a Stress significantly differed from the respective baseline at p < .001.
3.3 Smoking and physiological reactivity
3.3.1 Stress task 1
As outlined above, mixed factorial ANOVAs were conducted to assess the impact of smoking on SBP, DBP, and HR throughout the study. There was a significant main effect of smoking on HR, F(2, 143) = 13.00, p < .001, ηp2 = 0.154. The ad libitum group experienced significantly higher HR responses to stress during session 1 compared to the non-smoker (p = .018) and 24-h abstinence (p < .001) groups.
A significant phase × smoking interaction was observed for SBP, F(2, 143) = 5.42, p = .005, ηp2 = 0.070, and HR, F(2, 143) = 8.90, p < .001, ηp2 = 0.111. Post hoc analyses on change scores (Task 1 – Baseline 1 = Reactivity 1) revealed that those in the 24-h abstinence group had significantly lower SBP responses to stress task 1 compared to non-smokers (p = .004); and lower HR responses compared to those in the ad libitum (p < .001) and non-smoking (p = .003) groups. Cardiovascular reactivity (i.e., change scores) for the non-smokers, ad libitum, and 24-h abstinence groups are displayed in Table 3.
| Non-smoker (n = 43) | Ad libitum (n = 42) | 24-h abstinence (n = 61) | |
|---|---|---|---|
| SBP reactivity 1 (mmHg) | 18.89 (10.43) | 14.48 (9.72) | 12.61 (9.04) |
| SBP reactivity 2 (mmHg) | 12.06 (8.38) | 11.29 (10.31) | 10.25 (9.64) |
| DBP reactivity 1 (mmHg) | 10.87 (5.33) | 10.59 (6.18) | 9.07 (5.81) |
| DBP reactivity 2 (mmHg) | 7.64 (4.90) | 9.20 (6.14) | 7.71 (5.07) |
| HR reactivity 1 (bpm) | 9.97 (8.49) | 10.57 (8.77) | 4.96 (5.63) |
| HR reactivity 2 (bpm) | 8.39 (8.08) | 10.22 (8.98) | 3.42 (6.17) |
- Abbreviations: DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
After adjusting for age, sex, BMI, and drug administration, the main effect of smoking on HR, F(2, 138) = 13.24, p < .001, ηp2 = 0.161 and interaction effects of phase × smoking on SBP, F(2, 138) = 6.33, p = .002, ηp2 = 0.084; and HR, F(2, 138) = 9.60, p < .001, ηp2 = 0.122, remained statistically significant. Post hoc Bonferroni correction tests showed that those in the ad libitum group (M = 83.67; SD = 10.39) had significantly higher HR responses to task 1 compared to non-smokers (M = 77.72; SD = 11.66; p = .013) and the 24-h abstinence (M = 71.08; SD = 9.20; p < .001) group. No smoking or interaction effects were observed for DBP on stress task 1.
3.3.2 Stress task 2
A significant main effect of smoking on HR, F(2, 142) = 9.37, p < .001, ηp2 = 0.117, and phase × smoking interaction effect on HR, F(2, 142) = 11.37, p < .001, ηp2 = 0.138 were observed. Similar to stress task 1, those in the ad libitum group (M = 82.81; SD = 11.25) had significantly higher HR responses to stress task 2, compared to those in the 24-h abstinence group (M = 71.19; SD = 9.29; p < .001) on stress task 2.
After controlling for confounds (baseline, drug administration, sex, age, and BMI), the interaction effect of phase × smoking on HR, F(2, 136) = 10.11, p < .001, ηp2 = 0.129, remained statistically significant. Post hoc analyses on change scores revealed that those in the 24-h abstinence group (M = 3.42; SD = 6.17) had significantly lower HR responses to stress task 2 compared to non-smokers (M = 8.39; SD = 8.08; p = .004) and the ad libitum group (M = 10.22; SD = 8.98; p < .001). No smoking or interaction effects were observed for SBP or DBP on stress task 2.
3.4 Physiological habituation
As outlined, mixed factorial ANOVAs were conducted to assess the impact of smoking on changes in physiological responses to recurrent stress. There was a significant main effect of session for SBP, F(1, 143) = 26.87, p < .001, ηp2 = 0.158; and for DBP, F(1, 143) = 13.46, p < .001, ηp2 = 0.086; with reactivity scores decreasing from session 1 to session 2, demonstrating that across sessions, participants blood pressure habituated to the repeated stress session.
A main effect of smoking for HR, F(2, 143) = 13.17, p < .001, ηp2 = 0.156 was also observed, which remained statistically significant when controlling for confounds (drug administration, sex, age, and BMI), HR, F(2, 138) = 13.38, p < .001, ηp2 = 0.162. As can be seen in Table 3, those in the 24-h abstinence group had significantly lower HR reactivity at both sessions compared to the non-smokers and ad libitum groups (all ps < .001).
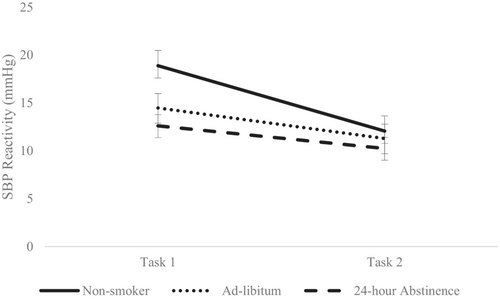
There was no significant session × smoking interaction for DBP, F(2, 143) = 1.25, p = .288, and HR, F(2, 143) = 0.388, p = .679, indicating similar levels of habituation between the non-smokers, ad libitum and the 24-h abstinence groups. However, ANCOVAs revealed a main effect of smoking for SBP, F(2, 138) = 3.18 p = .044, ηp2 = 0.044 and session × smoking interaction effect for SBP, F(2, 138) = 4.12, p = .018, ηp2 = 0.056. Bonferroni correction tests showed that SBP reactivity significantly declined from stress task 1 to stress task 2 (p < .001), with significant between-group differences observed between non-smokers (M = 19.00; SD = 10.53) and the 24-h abstinence group (M = 13.13; SD = 8.59; p = .038) to the stress task during session 1 (please see Figure 1).
3.5 Task-specific effects
Given responses to stress can vary by type of task, exploratory 2 × 3 mixed factorial ANOVAs were conducted for each task (i.e., speech preparation, speech delivery, math) separately. For brevity, a synopsis of effects of task specificity are provided and a more detailed summary of the findings can be found in the supplementary material. For DBP, interaction effects were observed on the math task at session one, and on speech preparation at session two. However, no statistically significant effects were observed for DBP habituation. For HR, the main and interaction effects observed in aggregated analyses remained on individual tasks, with no effects were observed for HR habituation. Lastly, for SBP, the effects observed were similar to those previously reported, and SBP habituation remained statistically significant on the speech delivery task.
4 DISCUSSION
The current study examined associations between smoking and patterns of CVR and habituation to recurrent stress over time. Here, we extend on previous research by examining cardiovascular habituation profiles in smokers (ad libitum, 24-h abstinence) and non-smokers, across two separate laboratory sessions, each 10 days apart. As expected, smoking (i.e., 24-h abstinence) was associated with blunted cardiovascular (SBP & HR) reactivity to acute psychological stress. Further, although the non-smokers showed elevated SBP reactivity compared to the two smokers' groups (ad libitum, 24-h abstinence), non-smokers also showed significant SBP habituation on exposure to recurrent stress. These findings identify that while it appears that smokers who abstain from smoking for 24 h may evidence a blunted cardiovascular response when compared to non-smokers, it may be the case that it is the non-smokers that are showing elevated initial reactivity, that habituates significantly and healthily on repeated exposure to stress.
The ad libitum group had higher HR responses on both tasks, compared to non-smokers and smokers who abstained from smoking overnight. Given that several chemicals found in tobacco smoke (e.g., nicotine, carbon monoxide) have a direct effect on the heart, this is not surprising. Acute doses of nicotine (i.e., comparable to those consumed by smokers) are shown to cause constriction in the heart, increase HR and peripheral vascular resistance, with smoking-related physiological effects observed after smoking one cigarette (al'Absi et al., 2022). Nicotine also remains active in the body for approximately 6-8 h and the ad libitum group in the present study smoked at their regular pace <1 h prior to laboratory testing. In addition, similar to findings reported elsewhere (al'Absi et al., 2003, 2013; Girdler et al., 1997; Phillips et al., 2009; Tsuda et al., 1996), the ad libitum group also had higher resting HR at both testing sessions compared to the other two groups, suggesting chronic smoking alters the activity of the cardiovascular system during rest.
Those who abstained from smoking overnight initially exhibited higher SBP baseline levels compared to non-smokers and the ad libitum group, but then showed low SBP responses to stress during session 1 and lower HR reactivity across both sessions. This aligns with evidence from prospective studies examining the effects of cessation on blood pressure; reporting significant decreases in blood pressure (SBP, DBP) in those whose respective baselines were initially elevated (Farsalinos et al., 2016; Tsai et al., 2021). While the 24-h abstinence group in the current study was uninterested in quitting, the findings may be useful for targeted interventions, indicating the early benefits associated with the cessation of smoking.
In contrast to the Hughes and Higgins (2010) findings, the non-smokers in the current study showed a greater capacity to habituate (SBP) to repeated stress, indicating better adjustment across time. Smokers, regardless of condition, showed little habituation. Given stress and smoking co-occur, the findings may reflect the additive burden of stress and addiction (i.e., smoking), which impacted patterns of adaptation; smokers exhibited less habituation to stress, compared to non-smokers who displayed greater levels of habituation across the two tasks. The findings may also reflect the damage that is directly caused to the cardiovascular system by tobacco smoking, indicating deficits in multiple stress systems regulation, whereby it would not be possible for smokers to mount an adaptative response to stress (al'Absi et al., 2005; Hughes & Higgins, 2010; Koob & Moal, 1997). This failure to adapt to repeated stress in smokers is proposed to increase the risk of poorer physical health, and the findings may reflect the enduring effects of smoking on multiple self-regulatory systems (e.g., autonomic, adrenocortical; al'Absi, 2006; al'Absi, 2020), with increased risk of subsequent disease (al'Absi et al., 2022; Burns, 2003). It also draws into the question of whether smokers who initially exhibit blunted responses to stress are capable of adapting physiologically to stress. Given both smoking groups had varied responses to stress at both sessions, but similar patterns of habituation, it would appear not.
The present study extends the work of Hughes and Higgins (2010) by showing that while smoking (i.e., ad libitum) is associated with increased CVR (i.e., HR) to stress, this is followed by diminished habituation to repeated stress, evidenced by little adjustment of the cardiovascular stress response. The findings align with extensive reports on the adverse impact that chemicals related to tobacco smoking (e.g., nicotine) have on the cardiovascular system. In contrast, those in the 24-h abstinence group initially exhibited higher SBP baseline levels compared to the other groups and displayed lower SBP responses to stress. While it is possible that these blunted responses to the first task created a floor effect, preventing habituation to the second task, this seems unlikely given the notable differences in CVR between smoking groups, but similar patterns of habituation.
Considering person-level shifts in CVR are stable across time, and the well-established links between high and low responses to disease outcomes, it is argued that having less but constant responses to stress is neither protective nor benign (Allen et al., 2014; Phillips et al., 2012). However, heightened responses that are short-lived with notable decreases in subsequent exposure to stress (i.e., habituation), as seen in non-smokers in the current study, may have an adaptive function and reflect an ability to cope with recurrent stress (al'Absi et al., 1997; Frankish & Linden, 1991; Hughes et al., 2018). In contrast, the failure of both smoking groups to habituate successfully may represent an inability to adapt and consequently carry adverse health outcomes (Howard & Hughes, 2013; Hughes et al., 2018; Kelsey, 1993; McEwen & Stellar, 1993). However, the interpretation of these findings is limited by the absence of prospective research looking at the habituation of the cardiovascular stress response and prediction of future health outcomes. Is it the case that a lower but constantly lower reaction to stress is less damaging than a heightened response followed by significant habituation? Can a low response truly habituate? Future research needs to disentangle the precise mechanisms at play in how individuals adapt to recurrent stress and their future health-related outcomes.
In the present study, it was SBP and not DBP or HR that habituated, further highlighting the complexity of patterns of cardiovascular stress-response adaptation and adding to the inconsistencies in research findings to date (i.e., blood pressure and/or heart rate). A possible explanation is that effects on SBP are less likely masked by adjustments in total peripheral resistance compared to DBP (Phillips et al., 2012), and effects on HR are often masked as a result of two opposing forces (i.e., sympathetic, parasympathetic) of the autonomic nervous system (Wright & Kirby, 2001). A possible solution for future research is to consider the inclusion of hemodynamic profile (Howard, 2022) to determine differences between myocardial and vascular profiles in responses to stress. Certainly, in a young healthy sample, smoking status has previously been associated with a mixed, rather than a myocardial, hemodynamic profile to active stress (Howard et al., 2022).
In addition, SBP is considered most sensitive to effort-related processes and particularly responsive to task demands (Brinkmann, 2008; Gendolla et al., 2008; Schwerdtfeger & Gerteis, 2013). The tasks in the current study were aggregated apriori to optimize the reliability of data as this is the most conventional approach to time series analysis (Kamarck et al., 1992). Examining tasks separately can dilute study findings with differences in variability, as seen in the supplementary material. While the different tasks evoked varying responses to task 1 and task 2, findings on habituation remained similar to those on aggregated analyses. Systolic blood pressure habituation was observed but for speech delivery only. While the active tasks utilized are known to reliably perturb cardiovascular activity, components of social evaluation and incentivization were included which may have increased levels of motivation, inducing sympathetic activity.
The present study is not without limitations. First, there might be variation in how individuals inhale cigarettes there is heterogeneity in the level of exposure to tobacco leading to potential variation in the impact of nicotine exposure. In addition, the content of nicotine in each brand was not accounted for, which could have influenced the current results. However, the classification of smoking was obtained using CO measures, and smokers were randomly assigned to one of two conditions prior to attending the first testing session. Second, given the sex differences in smoking reported in the Hughes and Higgins (2010) study and patterns of habituation (Schmaus et al., 2008), examining the interaction of sex in this context may have provided a more comprehensive analysis. Nonetheless, sex was controlled for in all main analyses, with no significant sex differences observed for CVR, smoking, or habituation (all ps > .05). The current findings extend previous research, by providing a more comprehensive assessment of adaptation to repeated stress over time, utilizing a task that reliably induces stress (Allen et al., 2014) and recruiting a sample considerably larger (n = 146) than the modest sample (n = 56) recruited in the Hughes and Higgins (2010) study. Third, the sample in the current study was predominantly Caucasian, and given the notable racial and ethnic disparities in smoking (Agarwala et al., 2021) and CVD (Hackler III et al., 2019; Mazimba & Peterson, 2021), recruiting more racially diverse samples would be beneficial to explore these health-related differences further. Lastly, the associations reported withstood adjustment for several confounding variables; indicating that the observed effects were due to the negative effects of smoking.
To conclude, to the best of our knowledge this is the first study that examines patterns of habituation in smokers (i.e., ad libitum, 24-h abstinence) and non-smokers to repeated stress across time (i.e., 10 days apart). Our findings extend those previously reported on smokers and non-smokers that used a within-session stress exposure, by including a 24-h abstinence group and exposure to the same stressor across time. Contrary to findings reported by Hughes and Higgins (2010), the non-smokers in the current study showed greater SBP habituation to repeated stress, but like the aforementioned study, the smoking groups showed less habituation to stress, evidence of the cardiotoxic impact of smoking on sympathetic activity. Lastly, we suggest that research on cardiovascular reactivity should include multiple exposures to the same stressor, (within-session; across time) to provide a more comprehensive understanding of factors that moderate the cardiovascular stress response.
AUTHOR CONTRIBUTIONS
Tracey M Keogh: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; validation; visualization; writing – original draft; writing – review and editing. Siobhán Howard: Conceptualization; supervision; visualization; writing – original draft; writing – review and editing. Motohiro Nakajima: Data curation; project administration; resources; writing – review and editing. Mustafa al'Absi: Data curation; funding acquisition; project administration; resources; software; supervision; visualization; writing – review and editing.
FUNDING INFORMATION
This study was funded by the National Institutes of Health (RO1DA016351 and RO1DA027232).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.