Can transcutaneous auricular vagus nerve stimulation mitigate vigilance loss? Examining the effects of stimulation at individualized versus constant current intensity
Abstract
According to the arousal model of vigilance, the locus coeruleus-norepinephrine (LC-NE) system modulates sustained attention over long periods by regulating physiological arousal. Recent research has proposed that transcutaneous auricular vagus nerve stimulation (taVNS) modulates indirect physiological markers of LC-NE activity, although its effects on vigilance have not yet been examined. Aiming to develop a safe and noninvasive procedure to prevent vigilance failures in prolonged tasks, the present study examined whether taVNS can mitigate vigilance loss while modulating indirect markers of LC-NE activity. Following a preregistered protocol (https://osf.io/tu2xy/), 50 participants completed three repeated sessions in a randomized order, in which either active taVNS at individualized intensity set by participant, active taVNS set at 0.5 mA for all participants, or sham taVNS, was delivered while performing an attentional and vigilance task (i.e., ANTI-Vea). Changes in salivary alpha-amylase and cortisol concentrations were measured as markers of LC-NE activity. Self-reports of feelings associated with stimulation and guessing rate of active/sham conditions supported the efficacy of the single-blind procedure. Contrary to our predictions, the observed vigilance decrement was not modulated by active taVNS. Pairwise comparisons showed a mitigation by active taVNS on cortisol reduction across time. Interestingly, Spearman's correlational analyses showed some interindividual effects of taVNS on indirect markers of LC-NE, evidenced by positive associations between changes in salivary alpha-amylase and cortisol in active but not sham taVNS. We highlight the relevance of replicating and extending the present outcomes, investigating further parameters of stimulation and its effects on other indirect markers of LC-NE activity.
1 INTRODUCTION
When performing prolonged tasks without breaks, vigilance (that is, the challenging ability to sustain attention during long periods) usually declines across time, a phenomenon known as “vigilance decrement” (Hancock, 2017; Neigel et al., 2020; Thomson et al., 2016). According to the arousal model of vigilance, physiological arousal is critical to regulate sustained attention, for which the locus coeruleus-norepinephrine (LC-NE) system would play a major contributing role (Esterman & Rothlein, 2019; Maness et al., 2022; Oken et al., 2006; Unsworth & Robison, 2016). In the last years, there has been growing interest in using noninvasive techniques such as transcranial direct or alternating current stimulation to mitigate vigilance loss (Al-Shargie et al., 2019; Pobric & Hulleman, 2023). Transcutaneous auricular vagus nerve stimulation (taVNS), a relatively new noninvasive brain stimulation technique, might be an effective method to this end, as recent research has proposed that it specifically targets the LC-NE system (Butt et al., 2020; Farmer et al., 2021; Redgrave et al., 2018). However, to date, evidence about the effects of taVNS on indirect markers of LC-NE activity is still controversial (Burger, D'Agostini, et al., 2020; Giraudier et al., 2022), and there is considerable variability between studies in some parameters (e.g., current intensity, stimulation onset, and duration) when using taVNS (Farmer et al., 2021; Ridgewell et al., 2021). In the present study, we examined the possibility of alleviating vigilance loss while modulating LC-NE activity by comparing the effects of taVNS at different current intensities.
There has been a long-standing discussion regarding whether vigilance should be considered a single mechanism or if the concept refers to different components of sustained attention that are captured in the lab via different tasks (Esterman & Rothlein, 2019; Fortenbaugh et al., 2017; Oken et al., 2006; van Schie et al., 2021). On the one hand, in signal-detection tasks like the Mackworth Clock Test (Mackworth, 1948), vigilance is assessed as the capacity to detect infrequent critical events (e.g., a double jump in the clock's hand) from other noise events (e.g., a regular jump in the clock's hand). In such tasks, the vigilance decrement is observed as a drop in hits across time-on-task. On the other hand, in simple reaction time (RT) tasks like the Psychomotor Vigilance Test (Basner & Dinges, 2011; Lim & Dinges, 2008), vigilance is rather measured as the capacity to maintain a fast reaction to a single stimulus (e.g., a down counter) without selecting a specific response. Here, vigilance decrement is observed as a progressive increase in the mean and variability of RT.
Different neural mechanisms have been proposed to modulate vigilance across time, further supporting the idea that vigilance might be regulated by different components of sustained attention (Esterman & Rothlein, 2019; Fortenbaugh et al., 2017; Langner & Eickhoff, 2013; Oken et al., 2006; Sarter et al., 2001; Sturm & Willmes, 2001; van Schie et al., 2021). According to the model by Sarter et al. (2001), sustained attention is regulated by the interaction of (a) a top-down mechanism, supported by cholinergic neural activity that modulates behavioral responses of vigilance in detecting infrequent targets, and (b) a bottom-up mechanism, regulated by the LC-NE system, which is not associated with specific behavioral responsiveness but is critical to modulate arousal levels of attention to maintain vigilance. Although Sarter et al. did not link the bottom-up mechanism of sustained attention with a behavioral response, later evidence has associated changes in physiological markers of LC-NE activity with performance in vigilance tasks (Esterman & Rothlein, 2019). In particular, it has been reported that states of hypo-arousal, indicated by a reduction in pupil size, are associated with vigilance loss, as evidenced by slower RT in the Psychomotor Vigilance Test (Unsworth et al., 2018; Unsworth & Robison, 2016; Yamashita et al., 2021).
Noting the diversity in how vigilance is both conceptualized and measured, in the last years we have proposed a theoretical and empirical dissociation of vigilance in two different components (Coll-Martín et al., 2023; Luna et al., 2018; Luna, Roca, et al., 2021). While executive vigilance (EV) might be considered a cognitive component involved in monitoring and detecting infrequent critical signals and supported by top–down neural mechanisms of sustained attention, arousal vigilance (AV) might be rather an automatic component that maintains a fast reaction to stimuli from the environment without implementing much control on the response and likely modulated by bottom–up neural mechanisms of sustained attention. To empirically dissociate vigilance components, we developed a new version of the classic attentional networks test (ANT) that simultaneously assesses EV and AV under the same participant's attentional state (Coll-Martín et al., 2023; Luna et al., 2018). The ANT for Interactions and Vigilance—executive and arousal components (ANTI-Vea) is a task suitable to measure, within a single session: (a) the EV decrement in a signal-detection sub-task similar to the Mackworth Clock Test (Mackworth, 1948), (b) the AV decrement in a RT sub-task similar to the Psychomotor Vigilance Test (Lim & Dinges, 2008), and (c) the main effects and interactions of the classic attentional networks' components (i.e., phasic alertness, orienting, and executive control) as in the ANT for Interactions by Callejas et al. (2004), Coll-Martín et al. (2023), Luna et al. (2018), Luna, Barttfeld, et al. (2021), Luna, Roca, et al. (2021). The ANTI-Vea captures the EV decrement as a drop in hits and false alarms, and the AV decrement as similar increases in mean and variability of RT and lapses. Interestingly, SD of RT seems to represent a proxy measure of AV, as it has been associated with “out of the task” states in previous vigilance research (Esterman et al., 2013; Esterman & Rothlein, 2019).
Dissociable patterns at the behavioral (Luna, Barttfeld, et al., 2022; Luna, Tortajada, et al., 2022; Román-Caballero et al., 2021), physiological (Feltmate et al., 2020; Sanchis et al., 2020; Sanchis-Navarro et al., 2024), and neural (Hemmerich et al., 2023, 2024; Luna et al., 2020, 2023a, 2023b) levels were observed for EV and AV when measuring vigilance components via the ANTI-Vea task. Most importantly, the EV and AV decrements were independently mitigated by modulating different mechanisms of sustained attention. While anodal high-definition transcranial direct current stimulation (tDCS) over the right frontoparietal network mitigated only the EV decrement in hits (Hemmerich et al., 2023; Luna et al., 2020), caffeine intake alleviated the AV decrement in SD of RT but not the EV decrement in hits (Sanchis et al., 2020). Noting the positive outcomes observed in our lab in mitigating the EV decrement through noninvasive stimulation at the cortical level (Hemmerich et al., 2023; Luna et al., 2020) and in reducing AV loss by modulating LC-NE activity via caffeine intake (Sanchis et al., 2020), in the present study, we examined the possibility of specifically alleviating the AV decrement while modulating indirect markers of LC-NE activity via taVNS.
taVNS is a noninvasive technique that stimulates afferent fibers of the auricular branch of the vagus nerve at the cymba conchae in the left auricle, which joins the main bundle of the vagus nerve and projects to nuclei in the brainstem, such as the LC and the nucleus tractus solitarius (Butt et al., 2020; Redgrave et al., 2018; Wang et al., 2022). Different neural systems have been proposed to be modulated by taVNS, mainly the LC-NE and the GABAergic, but also the cholinergic one (Beste et al., 2016; Butt et al., 2020; Farmer et al., 2021; Van Leusden et al., 2015; Wang et al., 2021). Following the hypothesis that taVNS can target the LC-NE system, some positive modulatory effects have been observed on different attentional processes, such as response inhibition (Beste et al., 2016), conflict-triggered adjustment (Fischer et al., 2018), and cognitive control in multitasking (Sommer et al., 2023). Several efforts have been undertaken to validate effects of taVNS on LC-NE activity by analyzing changes in indirect physiological markers as, for instance, pupil size (Burger, Van Der Does, et al., 2020; Capone et al., 2021; D'Agostini et al., 2023; Keute et al., 2019; Villani et al., 2022), alpha oscillations (Lloyd et al., 2023; Sharon et al., 2021), and salivary alpha-amylase (sAA) and cortisol (sCort) (D'Agostini et al., 2021, 2022; Giraudier et al., 2022; Warren et al., 2019). However, evidence collected so far is still inconsistent, showing mixed effects on these markers (for a review, see Burger, D'Agostini, et al., 2020).
Given its small cost and reduced time in sampling collection, saliva markers have received increased interest as markers of LC-NE activity in taVNS studies (Burger, D'Agostini, et al., 2020). sAA and sCort are considered indirect markers of central NE activity mediated by the activation of the sympathetic nervous system or the hypothalamo-pituitary–adrenal axis, respectively (D'Agostini et al., 2021; Engert et al., 2011; Strahler et al., 2017; Warren et al., 2019). If taVNS can modulate LC-NE activity, then it would be expected to increase sAA and mitigate the reduction of sCort across time (D'Agostini et al., 2021, 2022; Warren et al., 2019). Although inconsistent effects on sAA have been reported across studies (Burger, D'Agostini, et al., 2020), a pool-mega analyses of raw data from ten independent experiments showed that there seems to be a positive, albeit small, effect of taVNS in increasing sAA across time (Giraudier et al., 2022). Regarding sCort, only a few studies have reported positive effects of taVNS in mitigating its reduction, either via the planned interaction (Warren et al., 2019) or pairwise comparisons (D'Agostini et al., 2021).
It is worth noting that there is considerable methodological variability between studies regarding saliva sampling collection and taVNS protocols of stimulation. On the one hand, saliva samples have been either collected via chewing a cotton swab or via the spitting method. However, only collecting saliva via the spitting method allows us to analyze salivary flow rate to rule out salivary parasympathetical activity and to correct sAA and sCort concentrations by the volume secretion per min (Burger, D'Agostini, et al., 2020; Giraudier et al., 2022). On the other hand, stimulation parameters are relatively heterogeneous between taVNS studies (Colzato & Beste, 2020; Farmer et al., 2021). There seems to be no consensus on how long the pre-task and on-task stimulation periods should be (for a review, see tab. 2, Farmer et al., 2021). Regarding current intensity, some studies have set a constant level of current at 0.5 mA for all participants (Beste et al., 2016; Burger, Van Der Does, et al., 2020; Maraver et al., 2020; Warren et al., 2019), in some other studies current intensity has been set individually for each participant at the maximum of mA tolerated below a painful/uncomfortable sensation (D'Agostini et al., 2022; Fischer et al., 2018; Sharon et al., 2021; Sommer et al., 2023; Ventura-Bort et al., 2018; Ventura-Bort & Weymar, 2024).
Previous research has shown either positive or null effects by taVNS at individualized or constant current intensity on indirect markers of LC-NE activity (Burger, D'Agostini, et al., 2020). However, note that outcomes from independent studies are difficult to compare with each other, as the study design, methods, and other taVNS parameters vary considerably between studies. For instance, applying taVNS at a constant intensity of 0.5 mA, two recent studies did not find effects of taVNS on pupil size (Burger, Van Der Does, et al., 2020). However, Warren et al. (2019) did observe changes in sAA and sCort, thus showing some positive evidence of taVNS modulating indirect markers of LC-NE activity. Similarly, applying taVNS at individualized intensity, Sharon et al. (2021) observed that short bursts of taVNS increased pupil size and attenuated alpha oscillations, but in a replication study by Lloyd et al. (2023) only the modulation of pupil size was observed. Yet, other studies failed to observe a modulation by taVNS at individualized intensity on pupil size (D'Agostini et al., 2021, 2022), but reported positive effects on sCort reduction (D'Agostini et al., 2021).
To date, there seem to be only two studies in human participants that examined effects of individualized versus constant taVNS intensities in the same participants sample (Borges et al., 2019; D'Agostini et al., 2023). By delivering short bursts of 5 s of stimulation, D'Agostini et al. (2023) found a positive effect on pupil size via the individualized intensity (against constant intensity at 0.2 or 0.5 mA) and the highest pulse width (400 compared to 200 μs) stimulation condition. However, Borges et al. (2019) did not observe an effect of active taVNS at an individualized or constant intensity at 1.0 mA on heart rate variability in a stimulation period of 10 min. Importantly, in the two cited studies, effects were measured in a short period and only at the physiological level, without assessing a potential taVNS modulation on behavioral performance (Borges et al., 2019; D'Agostini et al., 2023). Thus, noting the controversial outcomes observed with taVNS at individualized or constant intensity on indirect markers of LC-NE activity and the scarce evidence reported to date about effects at different current intensities while maintaining other stimulation parameters in the same participants, we decided to conduct the study reported here.
1.1 The present study
The present study aimed to examine whether some of the typical taVNS protocols used in human experimental research are effective to mitigate the AV decrement while modulating indirect markers of LC-NE activity. To this end, two different stimulation protocols were examined, that is, taVNS at individualized versus constant current intensity, on behavioral and self-report scores associated with AV loss. To validate the effects of taVNS at the physiological level, effects of taVNS on saliva markers (i.e., sAA and sCort) associated with LC-NE activity were investigated. In short, participants completed three sessions in randomized order, in which they performed the ANTI-Vea while receiving either active taVNS at individualized intensity, active taVNS at constant intensity, or sham taVNS. Saliva samples and self-report questionnaires were collected before and after the ANTI-Vea. The study followed a preregistered protocol (i.e., methods, procedure, sample size estimated a-priori, hypotheses, and data analyses plan) publicly available in OSF (https://osf.io/tu2xy).
1.2 Hypotheses
Noting that previous research has shown both positive (D'Agostini et al., 2021; Ventura-Bort et al., 2018; Warren et al., 2019) and null (D'Agostini et al., 2021, 2022; Koenig et al., 2021) effects of taVNS at individualized or constant intensity on sAA and sCort, and that, to the best of our knowledge, this is the first study in which taVNS at individualized versus constant intensity are compared in a long period of stimulation, effects of different intensities of taVNS on behavioral, physiological, and self-report scores were examined without a priori hypotheses.
Regarding the behavioral measures, first, if AV is modulated by the LC-NE system, then probably active taVNS should mitigate the AV decrement. Second, if vigilance components are modulated by independent neural mechanisms (Hemmerich et al., 2023; Luna et al., 2020; Sanchis et al., 2020), then probably the EV decrement will not be modulated by taVNS. Lastly, effects of taVNS on phasic alertness, orienting, and executive control were examined without a-priori hypotheses.
Regarding physiological markers, if taVNS is suitable for modulating indirect activity of the LC-NE system, then probably active taVNS would increase sAA (Giraudier et al., 2022) and mitigate the typical reduction of sCort (D'Agostini et al., 2021; Warren et al., 2019) across time. Following previous research (D'Agostini et al., 2021; Giraudier et al., 2022; Warren et al., 2019), we anticipated that taVNS effects on sAA and sCort might be relatively small, or even only observed in pairwise comparisons by stimulation condition. Lastly, if the effects of taVNS on LC-NE are due to increased noradrenergic activity but not mediated by parasympathetical activity in saliva, then a similar decrease in salivary flow rate across time should be observed in active and sham taVNS conditions (Burger, D'Agostini, et al., 2020).
Regarding self-report scores, if active taVNS mitigates the AV decrement, then probably participants will self-report a small change across time in the alertness state in the active taVNS conditions, but a large decrease in the sham one. Given that mental workload might be specifically associated with EV rather than with AV, we expected that self-reported mental workload would not be different among taVNS conditions.
Finally, we anticipated a series of hypotheses concerning the effectiveness of our procedure. First, we expected to observe no differences in vigilance and attentional components in the baseline period. Second, we anticipated that self-report feelings associated with stimulation would be considerably small and similar between active/sham conditions, and that participants would not correctly guess the active/sham stimulation received. Finally, we expected that the short “method of limits” to calculate the individualized intensity level will not affect the self-reported state before and after the method of limits.
2 METHODS
2.1 Participants
Using Superpower (Lakens & Caldwell, 2021), sample size was a-priori estimated taking the modulation of the AV decrement by taVNS as the effect of interest. 10,000 data simulations were run to estimate a high-powered interaction between stimulation condition (active individualized/active constant/sham) and blocks (six levels) as within-participant factors, and SD of RT as a dependent variable. For the sham condition, SD of RT across blocks was computed from a large sample size (N = 589) of a previous study with the ANTI-Vea (Luna, Roca, et al., 2021). For the two active conditions, a similar and stable SD of RT across blocks was simulated (see OSF, https://osf.io/tu2xy/). Considering a significance level of α < .05 and a sample size of N = 50, the interaction between stimulation conditions and blocks would be observed with an effect size of = 0.06 and a power of 1 − β = .82.
Participants were 51 healthy adults between 18 and 40 years old, who were undergraduate students from the University of Greifswald, Germany. One participant voluntarily withdrew after the first session (final N = 50; 38 women; age: M = 22.52; SD = 3.14). All participants had normal or corrected to normal vision and met the following key inclusion criteria: not being diagnosed with an acute and chronic physical disease, a mental or an addiction disorder; not using medication; not having pacemakers, cochlear implants, or metals in the body; and not being pregnant. Prior to data collection, participants were informed about the study and signed a written informed consent. Participation was compensated on a basis of either 10 eu/h or 1 credit course/h. The study was conducted according to the ethical standards of the 1964 Declaration of Helsinki (last update: Fortaleza, 2013) and was approved by the Ethics Committee of the Greifswald University Medicine (protocol code: BB 032/22a).
2.2 Procedure and design
A single-blind, sham-controlled, and repeated-measures design was used. Each participant completed three sessions in randomized order at the lab, separated between 24 hs and 72 hs (average days between sessions: M = 1.41; SD = 0.72). To control variability in sAA and sCort throughout the day, each participant completed the three sessions at the same time (n at 10 am = 15; n at 12 pm = 13; n at 2 pm = 11; n at 4 pm = 11).
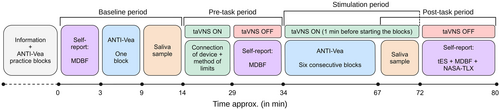
To familiarize participants with the ANTI-Vea task and reduce potential practice effects, in the first session participants completed four practice blocks with and without feedback as in the standard ANTI-Vea (Luna et al., 2018) and then two consecutive experimental blocks, following a rest period of 5 min (total duration of these steps: ~45 min). In the second and the third session, instead, participants received the task's instructions and completed only one practice block without feedback (~5 min). Then, in all sessions, after familiarization with the task, participants followed the same procedure (see Figure 1). The stimulation condition was manipulated between sessions (see taVNS section below). In total, the first session lasted ~2 h and the second and the third one ~1:30 h.
2.3 Instruments
2.3.1 taVNS
Stimulation was delivered via a NEMOS® taVNS device (Cerbomed GmbH, Erlangen, Germany), using two titan electrodes attached to a mount that is wired and connected to the stimulation unit. Electrodes set-up followed first the application of an alcohol wipe (Softa® Swabs, B. Braun, Melsungen, Germany) over the left ear and then electrode pads (tVNS Technologies GmbH, Erlangen, Germany) soaked with highly conductive electrode gel (Signagel®, Parker Laboratories, Inc.) onto the electrodes. In the two active stimulation conditions, electrodes were placed in the left auricle at the cymba concha, a region innervated with afferent vagus nerve's fibers that project to the LC, while in the sham condition electrodes were placed in the left earlobe, a region with no projected fibers of the vagus nerve (Butt et al., 2020; Farmer et al., 2021). Current was delivered with a pulse width of 200–300 μs at 25 Hz and was alternated between on/off periods every 30 s.
The stimulation condition was manipulated between sessions as a function of current intensity and active/sham stimulation, as follows: (a) active at individualized intensity, (b) active at constant intensity, and (c) sham, set in half of the participants at individualized intensity and in the other half at constant intensity. The order of stimulation conditions across sessions was counterbalanced between participants.
Constant intensity was set for all participants at 0.5 mA, as in Warren et al. (2019). Individualized intensity was set following a short “method of limits” (D'Agostini et al., 2021, 2022; Sharon et al., 2021). To this end, participants scored their subjective feelings in a series of short trials of 5 s of stimulation on a scale from 0 to 9, using the following descriptions: 0 = no sensation, 1 = light, 3 = mild, 6 = moderate, 7 = intense, 8 = highly intense but not annoying/uncomfortable, 9 = highly intense and slightly annoying/uncomfortable. Stimulation intensity started at 0.1 mA and increased gradually by 0.1 mA in each trial after the participant's response, until reporting a score of 9 or reaching the maximum intensity (i.e., 5 mA). If the participant reported a score of 9, the intensity was decreased until the participant reported a score of 7. The method of limits was repeated twice and individualized intensity was set as the average of intensities scored as 8 in the two increasing and decreasing series of trials, or at 5 mA if a score of 9 was never reported.
As control of the single-blind design and to follow the same procedure in all sessions, regardless the stimulation condition, the method of limits was performed in all sessions (see Figure 1). After the method of limits, taVNS was turned off for 5 min, and then, before starting the stimulation period, taVNS was turned on for 30 s at the intensity level of that session. After that, participants were asked if they were feeling comfortable with that sensation or if they preferred to reduce the intensity before starting the blocks (see Figure 1). In case the participant asked for a lower intensity, the current was reduced by 0.1 mA and tested again for another series of 30 s, and this procedure was repeated until the participant did not report an uncomfortable sensation. This step aimed to prevent an uncomfortable feeling about stimulation and, therefore, participants' responses were not collected here.1
2.3.2 Behavioral task: ANTI-Vea
The task was designed and run using PsychoPy (Peirce et al., 2019). The ANTI-Vea combines three embedded sub-tasks that are randomly completed across three types of trials: (a) ANTI, a flanker paradigm combined with a warning signal and a visual cueing paradigm, suitable to assess phasic alertness, orienting, and executive control as in the ANTI task (Callejas et al., 2004); (b) EV, a signal-detection task as the Mackworth Clock Test (Mackworth, 1948) that measures the EV decrement; and (c) AV, a simple RT task that mimics the Psychomotor Vigilance Test (Lim & Dinges, 2008) to assess the AV decrement. The procedure of the trials is represented in Figure 2 and can be revised in further detail in previous studies (Luna et al., 2018; Luna, Roca, et al., 2021).
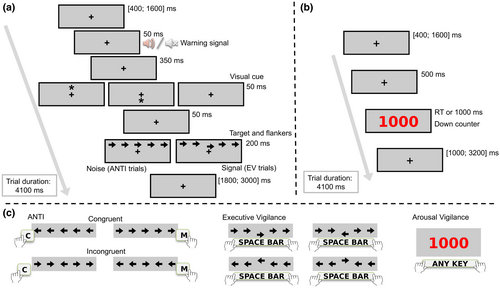
In short, participants have to fix their gaze on the fixation point at all times. In the ANTI trials (60%, see Figure 2a), a string of five horizontal arrows is presented either above or below the fixation point. Participants have to select the direction pointed by the target (i.e., the central arrow), while ignoring the direction pointed by the surrounding arrows (see Figure 2c). To assess executive control, the direction pointed by the target and distractors can be the same (congruent condition, 1/2 of trials) or opposite (incongruent condition, 1/2 of trials). To assess phasic alertness, the target can be anticipated by an auditory warning signal (tone condition, 1/2 of trials) or not (no tone condition, 1/2 of trials). To assess attentional orienting, target's position regarding the fixation point can be predicted by an exogenous visual cue either correctly (valid condition, 1/3 of trials), incorrectly (invalid condition, 1/3 of trials), or not predicted at all (no cue condition, 1/3 of trials).
EV trials (20%) follow the same procedure as the ANTI, except that the target is vertically displaced from its central position either upward or downward (see Figure 2a). Participants have to detect the infrequent displacement of the target by pressing the space bar, ignoring in these cases the direction pointed by the target (see Figure 2c). Importantly, if participants press the space bar in the ANTI trials (i.e., when the target is not vertically displaced), the response is categorized as a false alarm (FA). Lastly, in AV trials (20%), no warning signal nor visual cue is presented and the string of arrows is replaced by a red millisecond-down counter, starting at 1000 (see Figure 2b). Participants have to stop the counter as fast as possible by pressing any available key from the keyboard (see Figure 2c).
Participants completed the same structure of experimental blocks in all sessions (see Figure 1): one block before receiving active/sham stimulation (baseline period) and six consecutive blocks while receiving active/sham stimulation (stimulation period). Each block comprised 80 randomized trials (48 ANTI, 16 EV, and 16 AV) and lasted for 5 min 28 s.
2.3.3 Salivary samples
Saliva samples were collected via the spitting method using the SpeciMAX Saliva Collection Kit (Thermo Fisher Scientific Inc.). Samples were collected at two periods (see also Figure 1): (a) baseline and (b) post-task, while active/sham stimulation was still on, as suggested by Burger, D'Agostini, et al. (2020). Participants were asked to passively accumulate the saliva in their mouth and to spit it into the tube once per min for a 3 min period (D'Agostini et al., 2022; Warren et al., 2019). Samples were stored in a freezer at −21°C.
Aiming to reduce some side effects on LC-NE activity that might influence analyses of saliva samples (Strahler et al., 2017), participants were asked to avoid some activities before coming to the lab: (a) 8 h before the session, drinking alcohol and any drug consumption; and (b) 1 h before the session, any food and drink intake (including caffeine and water), smoking, tooth brushing, and exercise. At the beginning of each session, participants informed if they have avoided these activities through a brief survey.
2.3.4 Self-report questionnaires
Across each session, participants completed a series of questionnaires (see Figure 1). To assess changes in the subjective states of mood, calmness, and alertness, participants completed the Multidimensional Mood Questionnaire (Mehrdimensionale Befindlichkeitsfragebogen, MDBF; Steyer et al., 1997) at three different periods: baseline, pre-task, and post-task. The MDBF is a questionnaire of 24 one-word items that are scored in a five-points scale. The score for mood, calmness, and alertness is calculated by the sum of the responses in the items measuring each state.
In the post-task period, participants completed the transcranial electrical stimulation (tES) survey (Fertonani et al., 2015) to report their subjective perception of feelings associated with stimulation. The tES survey was adapted for measuring other feelings (i.e., neck pain and nausea) related to taVNS, as in D'Agostini et al. (2021, 2022). Importantly, as control of the single-blind procedure, the tES survey also asks the guessing (active/sham/don't know) about the stimulation received. Lastly, participants completed the short version of the NASA-TLX (Nygren, 1991), in which they reported the mental workload perceived about completing the ANTI-Vea through six items that are rated between 0 and 20. A summary score of mental workload is obtained as the sum of the six responses.
2.4 Data analyses
Analyses were conducted with R version 4.1.2 (R Core Team, 2021) in RStudio version 2023.9.1.494 (Posit team, 2023), using the following packages. Data was preprocessed with dplyr (Wickham et al., 2023). Analysis of variance (ANOVA) was conducted with afex (Singmann et al., 2023), and pairwise comparisons were run with emmeans (Lenth, 2023). Effect sizes and their 95% confidence intervals (CI) were computed with effect size (Ben-Shachar et al., 2020). For data figures and tables, a 95% CI of the mean was computed following the Cousineau-Morey method (Morey, 2008) with hausekeep (Lin, 2019). Correlational analyses were conducted with easy stats (Lüdecke et al., 2022). Data figures were plotted in Python 3.9.13 using Matplotlib (Hunter, 2007).
ANOVAs are reported with partial eta-squared () and 95% CI around them as a measure of effect size (Cumming, 2014; Kelley & Preacher, 2012). When the sphericity assumption was not met (Greenhouse-Geiser ε < 1) ANOVAs are reported with the Greenhouse–Geisser correction. The stimulation condition was factorized with the following levels in all analyses: active at individualized intensity, active at constant intensity, and sham.
2.4.1 Behavioral measures: ANTI-Vea
Attentional and vigilance components were analyzed following standard analyses of the ANTI-Vea task (Luna, Barttfeld, et al., 2021), as follows. Analyses were conducted separately for the baseline and the stimulation period. One participant was excluded from all behavioral analyses due to a high rate (33.33%) of no responses in one of the sessions. In addition, another participant was excluded only from analyses on the baseline period because, due to a technical issue, responses were not registered in that block.
For ANTI trials, RT analyses excluded trials with responses <200 ms or >1500 ms (0.44%) and incorrect responses (6.82%). Separated repeated-measures ANOVAs were conducted with mean correct RT or the percentage of errors as a dependent variable. In the baseline period, data was collapsed across tone, visual cue, and congruency factors, and the stimulation condition was included as a within-participant factor. For analyses on the stimulation period, data was collapsed across blocks and ANOVAs included stimulation condition, tone (no tone/tone), visual cue (invalid/no cue/valid), and congruency (congruent/incongruent) as within-participant factors.
Regarding vigilance components, changes across time-on-task were analyzed by computing average scores by block. For EV, hits were computed as the percentage of correct responses in EV trials and FA as the percentage of space bar responses in ANTI trials, as in Luna, Barttfeld, et al. (2021). Nonparametric metrics of signal detection theory were computed as A′ for sensitivity and B″ for response bias (Grier, 1971; Stanislaw & Todorov, 1999). For AV, mean RT, SD of RT, and the percentage of lapses (i.e., responses ≥600 ms) in AV trials were computed. Separated repeated-measures ANOVAs were conducted, with each EV or AV score as a dependent variable. In the baseline period, the stimulation condition was included as within-participant factor. Analyses on the stimulation period included stimulation condition and blocks (6 levels) as within-participant factors. Main effects and interactions were analyzed via pairwise contrasts and analyses of the polynomial linear component.
2.4.2 Saliva measures
Two participants were excluded from saliva analyses due to an insufficient volume in some of their samples. Samples were analyzed at the Institute of Pharmacy, University of Greifswald, Germany. Assay kits from TECAN IBL International GmbH (Hamburg, Germany) were used to analyze concentrations of sAA (alpha-Amylase Saliva Assay RE80111) and sCort (Cortisol Saliva ELISA RE52611). For sAA, the concentration of eight samples exceeded the linear range of the standard curve, prompting the need for dilution to ensure they were within the appropriate range. Flow rate was calculated as the volume of secretion (ml) by min. Concentrations in sAA (U/ml) and sCort (nmol/L) were computed as a product of that value by the flow rate, as in previous taVNS studies (D'Agostini et al., 2021, 2022, 2023; Warren et al., 2019) and considered the “gold standard” in the field (Burger, D'Agostini, et al., 2020; Strahler et al., 2017). Visual inspections of saliva scores showed a high-skewness in their distribution. Although not anticipated in our preregistered protocol, and noting that previous studies transformed saliva scores by the natural logarithm (D'Agostini et al., 2021), we inspected the residuals in separated models of saliva measures. Using the lme4 package (Bates et al., 2015), linear mixed-effects models for raw or natural log transformed scores of salivary flow rate, sAA, and sCort were fitted, with period and stimulation condition as fixed effects and the participant as a random effect. Visual inspection of QQ-plots and test of residuals computed with the DHARMa package (Hartig, 2022) showed an improvement in residuals deviation in all models with transformed scores.
Analyses of transformed values of sAA, sCort, and flow rate were conducted via separated repeated-measured ANOVAs, including stimulation condition and the period (baseline/post-task) as within-participant factors. Note that our preregistered protocol anticipated that modulations by taVNS on sAA and sCort could be relatively small and likely observed in separated analyses by the stimulation condition (D'Agostini et al., 2021; Warren et al., 2019). Thus, pairwise comparisons between periods were conducted even when the interaction between period and stimulation condition was not significant.
2.4.3 Self-report measures
The scores of calmness, alertness, and mood from the MDBF were analyzed via separated repeated-measures ANOVAs, including stimulation condition and period (baseline/pre-task/post-task) as within-participant factors. The summary score computed in the NASA-TLX was analyzed in a repeated-measures ANOVA, with stimulation condition as within-participant factor.
Scores of intensity (i.e., 0 to 4) about feelings associated with stimulation in the tES survey were transformed as dichotomic variables (e.g., perceived/not perceived). Absolute frequencies of each feeling and the guessing response were analyzed by Χ2 tests as a function of the stimulation condition. Lastly, in addition to our preregistered protocol and as further control of the sham-controlled procedure, the individualized intensity level was analyzed via a repeated-measures ANOVA, with stimulation condition as within-participant factor.
2.4.4 Correlational analysis
Our preregistered protocol anticipated the analysis of Pearson's correlations between changes across time in behavioral, self-report, and saliva scores as a function of the stimulation condition. Using the rockchalk package (Johnson, 2019), the size of the linear slope across blocks for AV and EV scores was computed at the participant level. Changes in sAA and sCort and the alertness factor of the MDBF were computed as the difference between post-task and pre-task measurements. Importantly, correlations are reported here with the following deviations from our preregistered protocol. First, given that log-transformed scores can bias Pearson's correlations, we decided to compute changes in sAA and sCort from raw data and to analyze correlations via the Spearman's rank coefficient. Second, to adjust significant values by Holm correction for multiple comparisons, correlations included those variables in which stimulation effects are usually reported, that is: (a) sAA and sCort and (b) behavioral measures, including the most sensitive score of the AV (i.e., SD of RT) and the EV (i.e., hits) decrement, which are the ones used in previous studies with the ANTI-Vea to analyze modulations by other manipulations, as tDCS (Hemmerich et al., 2023; Luna et al., 2020) or cognitive load (Luna, Barttfeld, et al., 2022). Nevertheless, for the sake of transparency, Supplementary Material presents full-matrices of bi-variate Pearson's correlations anticipated in our protocol (see Figures S1–S3).
3 RESULTS
3.1 Effects of taVNS on attentional and vigilance components
3.1.1 Arousal vigilance
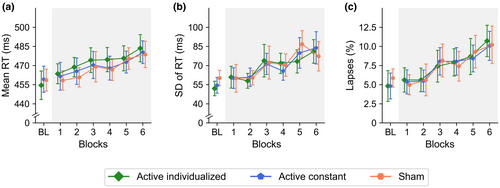
In the baseline period, the main effect of stimulation was not significant for mean RT, F(1.84, 86.66) = 0.26, p = .751, = 0.01, 95% CI [0.00, 0.05], SD of RT, F(1.98, 93.02) = 1.98, p = .144, = 0.04, [0.00, 0.13], and lapses, F(1.53, 72.13) = 0.53, p = .545, = 0.01, [0.00, 0.07] (see Figure 3). In the stimulation period, the main effect of stimulation was also not significant for mean RT, SD of RT, and lapses (see Table 1), showing a similar overall performance in the three stimulation conditions (see Table 2).
| Dependent variable | Effect or interaction | F | df | p | [95% CI] |
|---|---|---|---|---|---|
| Arousal vigilance | |||||
| Mean RT | Stimulation | 0.53 | 1.99, 95.73 | .589 | 0.01 [0.00, 0.07] |
| Blocks | 9.28 | 3.36, 161.46 | <.001 | 0.16 [0.07, 0.24] | |
| Stimulation * Blocks | 0.21 | 7.54, 361.85 | .987 | <0.01 [0.00, <0.01] | |
| SD of RT | Stimulation | 0.13 | 1.80, 86.39 | .861 | <0.01 [0.00, 0.03] |
| Blocks | 10.47 | 4.18, 200.42 | <.001 | 0.18 [0.09, 0.25] | |
| Stimulation * Blocks | 0.61 | 7.19, 345.23 | .753 | 0.01 [0.00, 0.01] | |
| Lapses | Stimulation | 0.01 | 1.56, 74.98 | .982 | <0.01 [0.00, 0.03] |
| Blocks | 11.53 | 3.54, 169.89 | <.001 | 0.18 [0.09, 0.25] | |
| Stimulation * Blocks | 0.20 | 7.81, 374.89 | .990 | 0.01 [0.00, 0.01] | |
| Executive vigilance | |||||
| Hits | Stimulation | 0.77 | 1.89, 90.72 | .460 | 0.02 [0.00, 0.08] |
| Blocks | 19.90 | 4.04, 194.00 | <.001 | 0.29 [0.19, 0.37] | |
| Stimulation * Blocks | 0.24 | 7.27, 349.04 | .976 | <0.01 [0.00, <0.01] | |
| False alarms | Stimulation | 1.81 | 1.89, 90.65 | .172 | 0.04 [0.00, 0.12] |
| Blocks | 5.22 | 3.65, 175.23 | <.001 | 0.10 [0.03, 0.16] | |
| Stimulation * Blocks | 1.13 | 6.75, 323.93 | .345 | 0.02 [0.00, 0.04] | |
| Sensitivity (A′) | Stimulation | 1.21 | 1.54, 73.95 | .295 | 0.02 [0.00, 0.10] |
| Blocks | 6.94 | 4.19, 201.33 | <.001 | 0.13 [0.05, 0.19] | |
| Stimulation * Blocks | 0.52 | 8.46, 406.23 | .849 | 0.01 [0.00, 0.01] | |
| Response bias (B″) | Stimulation | 0.28 | 1.89, 90.70 | .746 | 0.01 [0.00, 0.05] |
| Blocks | 9.62 | 4.04, 194.06 | <.001 | 0.17 [0.08, 0.24] | |
| Stimulation * Blocks | 1.07 | 7.45, 357.54 | .386 | 0.02 [0.00, 0.04] | |
- Note: Significant outcomes are highlighted in bold.
- Abbreviations: df, degrees of freedom; RT, reaction time; SD, standard deviation.
| Dependent variable | Active individualized | Active constant | Sham | |||
|---|---|---|---|---|---|---|
| M | 95% CI | M | 95% CI | M | 95% CI | |
| Arousal vigilance | ||||||
| Mean RT (ms) | 473 | [469, 478] | 470 | [465, 474] | 468 | [464, 473] |
| SD of RT (ms) | 70 | [65, 74] | 70 | [66, 75] | 71 | [66, 76] |
| Lapses (%) | 7.65 | [6.71, 8.59] | 7.57 | [6.73, 8.41] | 7.61 | [6.61, 8.61] |
| Executive vigilance | ||||||
| Hits (%) | 70.49 | [68.42, 72.57] | 68.77 | [66.91, 70.63] | 67.98 | [65.98, 69.99] |
| False alarms (%) | 8.05 | [7.10, 8.99] | 6.51 | [5.54, 7.47] | 7.63 | [6.74, 8.52] |
| Sensitivity (A′) | 0.90 | [0.89, 00.90] | .90 | [00.89, 0.90] | .89 | [0.88, 0.89] |
| Response bias (B″) | 0.47 | [0.40, 0.53] | 0.50 | [0.44, 0.56] | 0.50 | [0.43, 0.56] |
| ANTI | ||||||
| RT (ms) | 589 | [584, 594] | 575 | [570, 580] | 585 | [580, 591] |
| Errors (%) | 6.87 | [6.28, 7.47] | 5.57 | [4.99, 6.15] | 7.10 | [6.82, 8.60] |
- Abbreviations: CI, confidence intervals; M, mean; ms, millisecond; RT, reaction time.
The AV decrement usually observed with the ANTI-Vea was found as a significant main effect of blocks (see Table 1), with a significant linear increase in mean RT, t(48) = 4.50, p < .001, = 0.30, [0.10, 0.48], SD of RT, t(48) = 5.63, p < .001, = 0.40, [0.19, 0.56], and lapses, t(48) = 5.16, p < .001, = 0.36, [0.15, 0.53] (see Figure 3). However, contrary to our predictions, stimulation did not modulate the change across blocks in any of the dependent variables for AV (see Figure 3 and Table 1).
3.1.2 Executive vigilance
In the baseline period, the main effect of stimulation was not significant for hits, F(1.83, 86.03) = 1.28, p = .283, = 0.03, [0.00, 0.11], FA, F(1.95, 91.48) = 0.13, p = .871, < 0.01, [0.00, 0.04], sensitivity, F(1.98, 93.14) = 1.23, p = .297, = 0.03, [0.00, 0.10], and response bias, F(1.85, 87.07) = 1.13, p = .326, = 0.02, [0.00, 0.10] (see Figure 4). In the stimulation period, the main effect of stimulation was also not significant in none of the dependent variables for EV (see Table 1). As observed in Table 2, overall performance in the experimental blocks was similar in the three stimulation conditions.
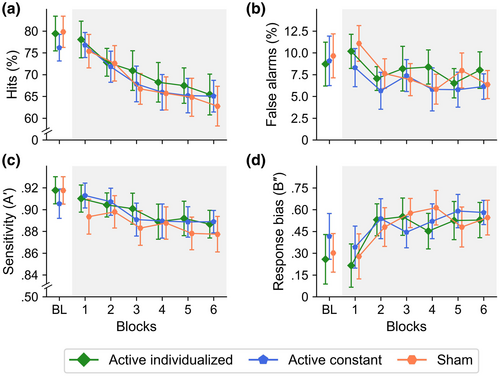
The EV decrement usually observed with the ANTI-Vea was found as a significant main effect of blocks (see Table 1), with a significant linear decrease in hits, t(48) = −7.17, p < .001, = 0.52, [0.32, 0.66], FA, t(48) = −3.37, p = .002, = 0.19, [0.03, 0.38], and sensitivity, t(48) = −4.77, p < .001, = 0.32, [0.12, 0.50], and a significant linear increase in response bias, t(48) = 4.13, p < .001, = 0.26, [0.08, 0.45] (see Figure 4). Similarly to the AV decrement, however, stimulation did not modulate the change across blocks in any of the dependent variables for EV (see Figure 4 and Table 1).
3.1.3 Phasic alertness, orienting, and executive control
In the baseline period, the main effect of stimulation was not significant, neither for RT, F(1.79, 84.10) = 0.33, p = .694, < 0.01, [0.00, 0.06], nor for errors, F(1.43, 67.23) = 1.97, p = .159, = 0.04, [0.00, 0.13]. Overall performance was similar in the active individualized (RT: M = 584 ms, 95% CI [568, 600]; errors: M = 5.86%, [4.33, 7.04]), active constant (RT: M = 577 ms, [565, 589]; errors: M = 6.29%, [4.81, 7.77]), and sham (RT: M = 577 ms, [562, 592]; errors: M = 7.99%, [5.81, 10.17]) stimulation conditions.
In the stimulation period, the main effects usually observed with the ANTI-Vea were replicated in the present study (see Figure 5). The main effect of warning signal was significant for both RT, F(1, 48) = 48.42, p < .001, = 0.50, [0.30, 0.64], and errors, F(1, 48) = 18.35, p < .001, = 0.28, [0.09, 0.46], with faster and more precise responses in the tone than no-tone trials. The main effect of visual cue was significant for RT, F(1.65, 79.34) = 140.99, p < .001, = 0.75, [0.66, 0.80], but not for errors, F(1.51, 72.44) = 2.57, p = .097, = 0.05, [0.00, 0.15], showing the typical validity (valid < invalid), t(48) = −14.46, p < .001, d = 2.09, 95% CI [1.58, 2.59], benefits (valid < no cue), t(48) = −14.57, p < .001, d = 2.10, [1.59, 2.61], and costs (no cue < invalid), t(48) = −5.34, p < .001, d = 0.77, [0.45, 1.09], effects for RT. Lastly, the main effect of congruency was significant for RT, F(1, 48) = 163.83, p < .001, = 0.77, [0.66, 0.84], but not for errors, F(1, 48) = 0.69, p = .412, = 0.01, [0.00, 0.14], with faster responses in congruent than incongruent trials.
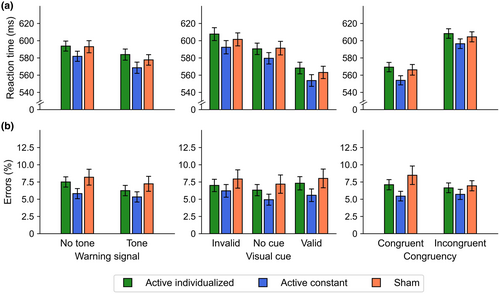
The main effect of stimulation was not significant neither for RT, F(1.88, 90.03) = 1.92, p = .156, = 0.04, [0.00, 0.13] nor for errors, F(1.53, 73.29) = 2.55, p = .098, = 0.05, [0.00, 0.15] (see Table 2). Moreover, as depicted in Figure 5, stimulation did not modulate the effect of warning signal (RT: F(1.66, 79.62) = 1.35, p = .264, = 0.03, [0.00, 0.11]; errors: F(1.86, 89.15) = 0.76, p = .461, = 0.02, [0.00, 0.08]), visual cue (RT: F(3.29, 158.08) = 0.79, p = .509, = 0.02, [0.00, 0.05]; errors: F(3.46, 166.29) = 0.61, p = .631, = 0.01, [0.00, 0.04]), or congruency (RT: F(1.83, 87.70) = 0.57, p = .552, = 0.01, [0.00, 0.07], errors: F(1.24, 59.44) = 0.80, p = 0.400, = 0.02, [0.00, 0.08]). For the sake of conciseness, the remaining interactions between attentional components and stimulation conditions and the descriptive statistics for each orthogonal condition are reported in Supplementary Material (see Tables S1 and S2).
3.2 Effects of taVNS on sAA, sCort, and flow rate
The main effect of period was significant for sCort, F(1, 47) = 11.65, p = .001, = 0.20, [0.04, 0.39], showing a decrease from baseline to post-task period (see Figure 6). In contrast, the main effect of period was not significant for sAA, F(1, 47) < 0.01, p = .975, < 0.01, [0.00, <0.01], and flow rate, F(1, 47) = 0.45, p = .507, = 0.01, [0.00, 0.13] (see Figure 6).
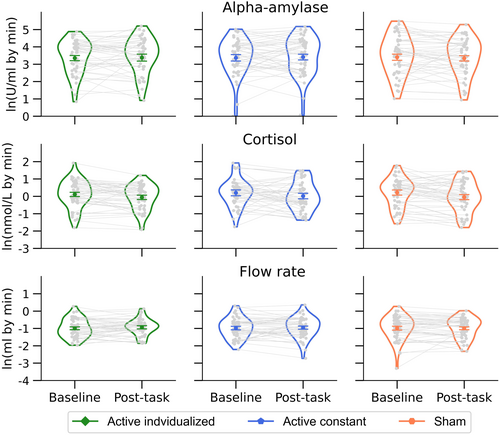
The main effect of stimulation condition was not significant for sAA, F(1.97, 92.41) = 0.02, p = .982, < 0.01, [0.00, <0.01], sCort, F(1.88, 88.58) = 0.61, p = .538, = 0.01, [0.00, 0.08], and flow rate, F(1.94, 91.26) = 0.15, p = .853, < 0.01, [0.00, 0.04]. Importantly, the stimulation condition neither modulate the effect of period for sAA, F(1.98, 93.09) = 0.94, p = .395, = 0.02, [0.00, 0.09], sCort, F(1.84, 86.44) = 0.29, p = .734, < 0.01, [0.00, 0.05], or flow rate, F(1.99, 93.37) = 0.32, p = .722, = 0.01, [0.00, 0.06] (see Figure 6).
Following planned analyses anticipated in our preregistered protocol, pairwise comparisons between periods were separately conducted by the stimulation condition. For sAA, there were no significant differences between periods for any of the stimulation conditions (all ps < .330). Interestingly, for sCort, the change between baseline and post-task periods was significant for sham, t(47) = 2.89, p = .006, d = 0.42, [0.12, 0.72], and active individualized conditions, t(47) = 2.11, p = .040, d = 0.31, [0.01, 0.60], but not for the active constant one, t(47) = 1.60, p = .117, d = 0.23, [−0.06, 0.52]. However, it should be noted that the change in sCort in the active constant condition was not significantly different from that change in the sham condition, t(47) = −0.56, p = .595, d = −0.08, [−0.36, 0.21].
3.3 Self-report measures
3.3.1 Feelings associated with taVNS
In the tES survey, participants did not guess differently if they received real or sham stimulation between stimulation conditions, Χ2(4) = 9.13, p = .058. Correct guessing was not above chance in the active constant (18.37%) and active individualized (42.55%) conditions, and only slightly above chance in the sham one (51.02%). The perception that stimulation could have influenced performance was not different between stimulation conditions, Χ2(2) = 4.85, p = .088. The perception of feelings, such as itching, Χ2(2) = 4.64, p = .098; pain, Χ2(2) = 0.22, p = .896; burning, Χ2(2) = 2.33, p = .313; warmth, Χ2(2) = 2.87, p = .238; pinching, Χ2(2) = 1.14, p = .566; fatigue, Χ2(2) = 1.50, p = .472; and neck pain, Χ2(2) = 1.08, p = .582, was not different between stimulation conditions. Feelings such as metallic/iron taste (2.03%), nausea (4.05%), and others (8.11%), were reported at a very low rate (see Table S3 in Supplementary Material). The individualized intensity in the method of limits was not significantly different between stimulation conditions (see Tables 3 and 4). Altogether, this set of outcomes further supports the validity of the single-blind design in the present study.
| Dependent variable | Active individualized | Active constant | Sham | |||
|---|---|---|---|---|---|---|
| M | 95% CI | M | 95% CI | M | 95% CI | |
| Individualized intensity (mA) | 4.16 | [3.87, 4.45] | 4.04 | [3.77, 4.32] | 4.35 | [4.09, 4.61] |
| NASA-TLX score | 11.06 | [10.61, 11.51] | 11.02 | [10.49, 11.56] | 11.10 | [10.62, 11.59] |
| MDBF | ||||||
| Alertness—baseline | 28.32 | [26.92, 29.72] | 27.26 | [25.45, 29.07] | 28.62 | [27.27, 29.97] |
| Alertness—pre-task | 26.90 | [25.49, 28.31] | 26.38 | [25.22, 27.54] | 26.96 | [25.69, 28.23] |
| Alertness—post-task | 23.44 | [22.34, 25.54] | 23.40 | [22.11, 24.69] | 23.34 | [21.86, 24.82] |
| Mood—baseline | 34.00 | [33.17, 34.83] | 33.34 | [32.42, 34.26] | 34.12 | [33.19, 35.05] |
| Mood—pre-task | 33.30 | [32.58, 34.02] | 33.02 | [32.31, 33.73] | 32.98 | [32.01, 33.95] |
| Mood—post-task | 33.18 | [32.43, 33.93] | 32.90 | [32.29, 33.51] | 33.10 | [32.10, 34.10] |
| Calmness—baseline | 33.20 | [32.35, 34.05] | 33.44 | [32.53, 34.35] | 32.42 | [31.33, 33.51] |
| Calmness—pre-task | 32.54 | [31.79, 33.29] | 32.88 | [32.14, 33.62] | 31.56 | [30.51, 32.61] |
| Calmness—post-task | 32.54 | [31.80, 33.28] | 32.34 | [31.48, 33.20] | 32.66 | [31.73, 33.59] |
- Abbreviations: CI, confidence intervals; M, mean.
| Dependent variable | Effect of interaction | F | df | p | [95% CI] |
|---|---|---|---|---|---|
| Individualized intensity | Stimulation | 1.27 | 1.96, 96.13 | .285 | 0.03 [0.00, 0.10] |
| NASA-TLX score | Stimulation | 0.03 | 1.93, 94.33 | .970 | <0.01 [0.00, <0.01] |
| MDBF—Alertness | Stimulation | 0.39 | 2.00, 97.99 | .680 | <0.01 [0.00, 0.06] |
| Period | 45.38 | 2.00, 97.89 | <.001 | 0.48 [0.34, 0.59] | |
| Stimulation * Period | 0.45 | 3.25, 159.09 | .734 | <0.01 [0.00, 0.03] | |
| MDBF—Mood | Stimulation | 0.35 | 1.67, 82.02 | .670 | 0.01 [0.00, 0.06] |
| Period | 5.30 | 1.92, 93.96 | .007 | 0.10 [0.01, 0.21] | |
| Stimulation * Period | 0.47 | 3.17, 155.29 | .717 | 0.01 [0.00, 0.03] | |
| MDBF—Calmness | Stimulation | 1.13 | 1.79, 87.70 | .322 | 0.02 [0.00, 0.10] |
| Period | 2.07 | 1.92, 93.95 | .134 | 0.04 [0.00, 0.13] | |
| Stimulation * Period | 1.53 | 3.30, 161.87 | .205 | 0.03 [0.00, 0.07] |
- Note: Significant outcomes are highlighted in bold.
- Abbreviation: df, degrees of freedom.
3.3.2 MDBF and NASA-TLX scores
In the MDBF, the main effect of stimulation condition was not significant for alertness, mood, and calmness (see Table 4). Alertness and mood, but not calmness, significantly change across periods (see Table 4). Participants reported a higher alertness state at baseline than at the pre-task period, t(49) = 2.61, p = .032, d = 0.37, [0.08, 0.66], at baseline than at the post-task period, t(49) = 9.11, p < .001, d = 1.30, [0.92, 1.68], and at the pre-task than at the post-task period, t(49) = 6.73, p < .001, d = 0.96, [0.62, 1.30] (see Table 3). The state of mood was more positive at baseline than at the pre-task period, t(49) = 2.61, p = .032, d = 0.37, [0.08, 0.66], at baseline than at the post-task period, t(49) = 3.25, p = .006, d = 0.46, [0.17, 0.76], but not significantly different between the pre-task and post-task periods, t(49) = 0.15, p = .989, d = 0.02, [−0.26, 0.30] (see Table 3).
Importantly, the stimulation condition did not modulate the state of alertness, mood, and calmness across periods (see Table 4). In addition, the mental workload perceived about the ANTI-Vea task reported in the NASA-TLX was not significantly different between stimulation conditions (see Table 4).
3.4 Correlational analyses
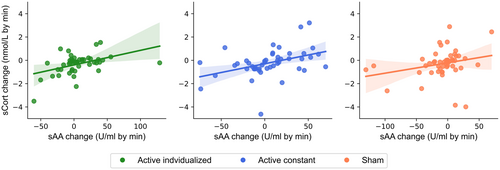
Spearman's rank correlations showed positive and significant associations between changes in sAA and sCort in both active individualized and active constant conditions, but not in the sham one (see Table 5 and Figure 7), thus showing some interindividual effects of active taVNS on salivary markers of LC-NE activity. However, the change in sAA or sCort was not associated with the change in hits for EV or the change in SD of RT for AV in any of the stimulation conditions (see Table 5).
| Stimulation | Parameter 1 | Parameter 2 | n | Spearman's ρ | 95% CI | p |
|---|---|---|---|---|---|---|
| Active individualized | sAA change | sCort change | 47 | 0.41 | [0.13, 0.63] | .013 |
| Hits slope | 47 | 0.09 | [−0.21, 0.37] | .645 | ||
| SD of RT slope | 47 | −0.15 | [−0.42, 0.15] | .645 | ||
| sCort change | Hits slope | 47 | −0.07 | [−0.36, 0.23] | > .999 | |
| SD of RT slope | 47 | <0.01 | [−0.29, 0.30] | > .999 | ||
| Active constant | sAA change | sCort change | 48 | 0.47 | [0.20, 0.67] | .002 |
| Hits slope | 48 | −0.06 | [−0.34, 0.24] | .697 | ||
| SD of RT slope | 48 | −0.23 | [−0.49, 0.07] | .244 | ||
| sCort change | Hits slope | 48 | 0.02 | [−0.28, 0.31] | .912 | |
| SD of RT slope | 48 | −0.14 | [−0.42, 0.16] | .683 | ||
| Sham | sAA change | sCort change | 49 | 0.31 | [0.03, 0.55] | .083 |
| Hits slope | 49 | 0.15 | [−0.15, 0.42] | .628 | ||
| SD of RT slope | 49 | 0.06 | [−0.23, 0.35] | .670 | ||
| sCort change | Hits slope | 49 | −0.17 | [−0.44, 0.12] | .466 | |
| SD of RT slope | 49 | 0.12 | [−0.17, 0.40] | .466 |
- Note: p Value are reported with Holm correction for multiple comparisons. Change in salivary alpha-amylase (sAA) and cortisol (sCort) denotes the difference between post-task and baseline periods. Slope in hits and SD of RT denote the change across blocks. Significant outcomes are highlighted in bold.
- Abbreviations: CI, confidence intervals; RT, reaction time; SD, standard deviation.
4 DISCUSSION
The aim of the present study was to investigate whether taVNS can mitigate vigilance loss while modulating indirect markers of LC-NE activity. Noting the relative variability between studies in some parameters of taVNS (Farmer et al., 2021; Yap et al., 2020), we decided to compare two of the most used taVNS protocols in human experimental research by manipulating current intensity in a randomized order across sessions at a constant level set at 0.5 mA for all participants versus an individualized level set by participants below an uncomfortable sensation. Importantly, to validate the effects of taVNS at the physiological level, changes in sAA and sCort were measured as indirect markers of LC-NE activity. The study was conducted via a single-blind, sham-controlled, and within-participants design and followed a preregistered protocol publicly available in the OSF (https://osf.io/tu2xy/). To the best of our knowledge, this is the first study investigating the effects of taVNS on (a) vigilance performance across time-on-task, and (b) behavioral, physiological, and self-report measures at constant versus individualized current intensities in a long stimulation period. Contrary to our hypotheses, the AV decrement was not mitigated by active taVNS, neither at the constant nor at the individualized intensity level. At the physiological level, while only mitigation on sCort reduction by active taVNS at the constant intensity level was observed via planned comparisons, correlational analyses between changes in sAA and sCort seem to emphasize some interindividual effects of taVNS on LC-NE activity.
Vigilance was measured via the ANTI-Vea, a task that has proved to be effective in simultaneously measuring the decrement in two vigilance components (i.e., EV and AV) along with other attentional functions in several lab and online behavioral studies (for a review, see Coll-Martín et al., 2023), as well as in psychophysiological research (Hemmerich et al., 2023; Huertas et al., 2019; Luna et al., 2023a, 2023b; Sanchis et al., 2020). Previous studies showed acceptable reliability (i.e., Spearman-Brown split-half correlations >.80) in the EV and AV scores of the ANTI-Vea, and at least a similar reliability in phasic alertness, orienting, and executive control scores as other versions of the ANT (Cásedas et al., 2022; Coll-Martín et al., 2021; Luna, Roca, et al., 2021). Most importantly, previous research conducted in our lab has shown positive modulatory effects in mitigating the EV decrement by high-definition anodal tDCS over the right parietal cortex (Hemmerich et al., 2023; Luna et al., 2020) and the AV decrement by caffeine intake (Sanchis et al., 2020). In the present study, the main effects usually observed with the ANTI-Vea were clearly replicated, supporting the validity of the task to measure vigilance and attentional components of interest. In short: (a) the AV decrement was observed as an increase in mean and SD of RT, and lapses; (b) the EV decrement was observed as a drop in hits and FA; and (c) attentional components showed the classic effects for phasic alertness (tone effect), attentional orienting (cueing validity effect), and executive control (congruency effect). Nonetheless, no modulatory effects by active taVNS over AV, EV, and attentional components were observed.
Our preregistered hypotheses anticipated that taVNS might specifically modulate the AV decrement. We developed this prediction based on: (a) the critical role of the LC-NE system in regulating sustained attention by the arousal model of vigilance (Aston-Jones & Cohen, 2005; Esterman & Rothlein, 2019; Sarter et al., 2001); (b) previous research supporting that taVNS can target LC-NE activity (D'Agostini et al., 2023; Farmer et al., 2021; Lloyd et al., 2023; Sharon et al., 2021; Warren et al., 2019); and (c) previous studies from our lab with the ANTI-Vea supporting that the EV decrement can be mitigated via tDCS, while the AV decrement can be mitigated by caffeine intake (Hemmerich et al., 2023; Luna et al., 2020; Sanchis et al., 2020). The arousal model of vigilance predicts that physiological arousal fluctuates through different states represented as an inverted-U shape, with the optimal state in the middle and vigilance impairments observed in the extremes (Aston-Jones & Cohen, 2005; Esterman & Rothlein, 2019; Nieuwenhuis, 2024). While at one extreme of the inverted-U shape there are hyper-arousal states causing attentional lapses due to high distractibility, at the opposite extreme there are hypo-arousal states associated with low-tonic LC-NE activity leading to low task engagement and inattentive states. There is extensive research supporting the critical role of the LC-NE system in modulating tonic arousal, usually associated with vigilance performance in long periods, but also phasic arousal associated with goal-relevant stimuli response (Langner & Eickhoff, 2013; Maness et al., 2022; Oken et al., 2006; Sarter et al., 2001; Sturm & Willmes, 2001). In the same vein, the attentional networks model has also been linked to the LC-NE system through the alerting network, which regulates both phasic alertness and vigilance (Petersen & Posner, 2012; Posner, 2008).
The set of outcomes of the present study can be partially explained by the arousal model of vigilance. It is important to note, however, that the present study was not designed to examine behavioral performance and indirect markers of LC-NE activity across all the states represented in the inverted-U shape function of arousal, that is, hypo-arousal, optimal arousal, and hyper-arousal (Nieuwenhuis, 2024). Nevertheless, the decrement in performance, observed as a change in AV and EV across blocks, was accompanied by a significant reduction in sCort from baseline to post-task periods, which can be both interpreted as a change towards a hypo-arousal state after ~33 min of continuous vigilance period. Note that, while sCort is expected to decrease under non-stimulation conditions such as ~40 min of rest, daytime, or even sham taVNS, sAA is instead expected to increase in the same conditions (Burger, D'Agostini, et al., 2020; Giraudier et al., 2022; Nater et al., 2006, 2007). Regarding sAA, however, no significant change was observed from baseline to post-task period, an outcome that seems to not represent a decline in arousal after a continuous vigilance period in this marker. Importantly, to further support the validity of sAA as an indirect marker of noradrenergic activity, saliva samples were collected here by the spitting method, which allowed us to calculate sAA secretion as the product of sAA concentration by fluid output per min (Burger, D'Agostini, et al., 2020; Giraudier et al., 2022). Although we predicted that salivary flow rate should decrease with time-on-task, no significant change in flow rate was observed between baseline and post-task periods, thus relatively ruling out the influence of parasympathetical activity on sAA (Burger, D'Agostini, et al., 2020).
However, contrary to our predictions, the present outcomes do not clearly support a modulatory effect by active taVNS on changes in sAA and sCort across time. While no effect was observed on sAA, only planned comparisons showed a mitigation on sCort decrease by active taVNS at the constant intensity level which, nevertheless, was not significantly different from the change observed in the sham condition. Note that evidence collected so far about effects of taVNS on sAA is considerably controversial, showing either null or positive effects (Burger, D'Agostini, et al., 2020; Giraudier et al., 2022). Although a pool-mega analysis on ten independent experiments by Giraudier et al. (2022) showed that active taVNS has a positive but small effect in increasing sAA across time, such effect has not been consistently observed across independent studies (D'Agostini et al., 2022, 2023; Sommer et al., 2023). Regarding sCort, evidence is substantially scarce, as it has been examined only in three taVNS studies—to the best of our knowledge—as an indirect marker of LC-NE activity (D'Agostini et al., 2021, 2022; Warren et al., 2019). The mitigation of sCort decrease by taVNS was only observed by Warren et al. (2019), and later by D'Agostini et al. (2021) via a post hoc comparison as in the present study, but not by D'Agostini et al. (2022).
Although the present outcomes do not support the assumption that taVNS can mitigate vigilance loss while modulating indirect markers of LC-NE activity, we nevertheless highlight the need to still collect more empirical evidence on our research questions. While the decrement in vigilance components and the reduction in sCort seem to represent a hypo-arousal state after the continuous vigilance period, it could also be possible that such low-arousal state was not sensitive enough to be modulated by taVNS. Previous studies using tDCS as noninvasive brain stimulation have shown some beneficial effects on vigilance when performance is measured across repeated-sessions in a fatigue state along the day (i.e., at the end of the day, between 8 pm and 12 am; McIntire et al., 2017) or during a night of sleep deprivation (McIntire et al., 2014). Regarding sCort and sAA, it has been shown that these markers reveal larger changes than those observed in the present study when they are measured across several hours across the day (Nater et al., 2007). Therefore, it could be possible that taVNS can be more sensitive to modulate vigilance performance, as well as changes in sAA and sCort, in states of lower arousal than that after ~33 min of a vigilance task as in the present study, as for instance, after a longer task period, fatigue states at late times of the day, or even during sleep deprivation conditions. Future research should explore this path.
Further efforts are also necessary to determine the effects of taVNS on indirect markers of LC-NE activity (Burger, D'Agostini, et al., 2020). While in the present study a beneficial effect by active taVNS at the group level was only observed via pairwise comparisons on sCort, interestingly, correlational analyses seem to show some interindividual effects of active taVNS at constant and individualized current intensities on salivary markers. In particular, the positive correlations observed between changes across time in sAA and sCort showed that the positive effect by active taVNS in both salivary markers seems not to be present in all participants. Moreover, visual inspection of violin plots in Figure 6 and scatter plots in Figure 7 highlight that effects of active taVNS on sAA and sCort were mixed between participants. While in some participants the expected effects (i.e., an increase in sAA and a mitigation in sCort reduction across time) were observed, in some others those effects were rather null or even the opposite. A similar pattern was observed and discussed in the pooled mega-analysis of sAA conducted by Giraudier et al. (2022). While the authors highlight that the small but positive effect of taVNS on sAA seems to be generalizable and not driven only by some participants, there is nevertheless a high variability between participants in the direction and size of the change in sAA from pre- to post-stimulation periods, both in the active and sham stimulation conditions. To disentangle the validity of the effects of taVNS on indirect markers of LC-NE activity at the group and inter-individual levels, further empirical evidence is critically necessary from large sample sizes (Giraudier et al., 2022), replication studies (Lloyd et al., 2023), and from studies simultaneously measuring several markers of LC-NE activity (D'Agostini et al., 2021, 2022; Lloyd et al., 2023; Sharon et al., 2021; Sommer et al., 2023; Warren et al., 2019).
Future research should also consider extending the examination on the effects of taVNS at different stimulation parameters (Farmer et al., 2021; Yap et al., 2020). In the present study, no clear differences in the behavioral, physiological, and self-report measured scores were observed between active taVNS at the current intensities delivered, that is, a constant level set at 0.5 mA for all participants versus an individualized level set by participant, which was overall quite higher than the constant level (average across participants = 4.16 mA, see Table 3). It is also worth noting that the average of individualized intensity observed in the present sample seems to be higher than that average observed in some previous studies, i.e., at or below 3.0 mA (see, for instance, D'Agostini et al., 2022; Fischer et al., 2018; Lloyd et al., 2023; Sharon et al., 2021; Sommer et al., 2023; Ventura-Bort et al., 2018; Ventura-Bort & Weymar, 2024). Currently, we have no explanation for the higher levels of individualized intensity observed. However, these levels do not seem uncommon as they are within the range of intensities observed in the sample of some previous studies (Fischer et al., 2018; Sommer et al., 2023; Ventura-Bort et al., 2018). More importantly, in the present study, the measurement of individualized intensity showed no difference across the three sessions, a difference with some previous studies in which individualized intensities differed between active and sham conditions (Lloyd et al., 2023; Sharon et al., 2021).
Other studies have set current at different constant levels, as 1.0 mA (Keute et al., 2021; Tona et al., 2020) or 3.0 mA (Keute et al., 2019, 2020). To the best of our knowledge, only three studies have examined the effects of taVNS at different constant levels of current intensity. While Tona et al. (2020) only observed some beneficial effects of taVNS at 1.0 mA versus 0.5 mA via post hoc exploratory analyses on speed-accuracy trade-off in task-switching performance, Capone et al. (2021) found that taVNS at 2.0 mA but not at 0.5, 1.0, or 3.0 mA increases pupil diameter under some specific luminosity conditions, and Borges et al. (2019) found no effect of taVNS at 0.5, 1.0, or 1.5 mA on heart rate variability in their Experiment 1.
Regarding the setting of individualized intensity by participant, there seems to be some variability in the “method of limits” across studies. While some studies measure subjective feelings on different intensities between 0.1 and 5.0 mA (Sharon et al., 2021; Sommer et al., 2023), as in the present study, others instead examine those feelings until a maximum of either 4.0 mA (D'Agostini et al., 2022, 2023) or 0.5 mA (Borgmann et al., 2021; D'Agostini et al., 2021). Moreover, the subjective scale used in the “method of limits” also varies across studies: while in some studies the intensity is averaged taking the scores immediately below a “painful” sensation (D'Agostini et al., 2021; De Smet et al., 2021; Giraudier et al., 2020), others as Sommer et al. (2023) use the score on sensations as “strong tingling” (i.e., scored as 6), that is quite below “pain” (represented as 10 in that scale). To avoid a negative experience of taVNS in naive participants, it may be advisable to prevent participants from experiencing a “painful” sensation, even when testing different current intensities before the experimental procedure. To this end, it is recommended that researchers do not include “pain” as a label associated with the maximum score of the subjective scale used in the method of limits, as in the present study (see also Sommer et al., 2023).
According to Yap et al. (2020), a slight tingling or pulsating sensation on the stimulation site might indicate activation of Aβ fibers of the auricular branch of the vagus nerve, a sub-group of thick myelinated fibers that have been associated with therapeutic effects via invasive vagus nerve stimulation (Butt et al., 2020). However, the distribution of Aβ class fibers in the auricular branch of the vagus nerve in humans: (a) has not been yet determined with detail, (b) varies greatly between participants, and (c) seems to be almost six times less than in the cervical branch of the vagus nerve (Butt et al., 2020; Yap et al., 2020). While future research on the anatomical composition of the auricular branch of the vagus nerve might help to account for the observed positive or null effects of taVNS, it seems also necessary to further explore how current intensity but also other stimulation parameters of taVNS might modulate its effects (Butt et al., 2020; Yap et al., 2020). In this vein, a recent registered report by D'Agostini et al. (2023) examined an orthogonal combination of different pulse widths (200 and 400 μs) and current intensities (constant at 0.2 mA, constant at 0.5 mA, individualized intensity) in short bursts of 5 s of stimulation and found that pupil size was increased by the largest pulse width and current intensity combination.
Taking all the above into consideration, our study might present some limitations that motivate us to continue this line of research. Although only current intensity and no other parameters could be manipulated in the taVNS device used here, it is possible that taVNS effects may also depend on different pulse widths and/or of shorter bursts of stimulation between 3 and 5 s (D'Agostini et al., 2023; Lloyd et al., 2023; Sharon et al., 2021; Villani et al., 2022). In addition, although there seems to be no consensus on parameters such as the stimulation onset and duration (Farmer et al., 2021), it is possible that some positive effects of taVNS on behavioral performance or salivary markers might be observed in a longer stimulation period with the current parameters. Although Giraudier et al. (2022) did not observe an effect of taVNS duration on sAA across time in their pooled mega-analysis of ten independent studies, some positive effects have been reported in longer periods of stimulation than in the present study on behavioral performance and sCort. For instance, while Sommer et al. (2023) observed a beneficial effect by taVNS at individualized intensity on cognitive control in a dual-task paradigm but not on changes in sAA across 60 min of stimulation, Warren et al. (2019) found that taVNS at 0.5 mA increased sAA and mitigated sCort reduction across 80 min of stimulation. Note that, unlike the present study, in the two cited studies, stimulation started a few minutes before experimental trials, i.e., around 10 min (Sommer et al., 2023) and 20 min (Warren et al., 2019).
To conclude, the present study showed no clear positive effects of taVNS, either at a constant intensity of 0.5 mA for all participants or at an individualized intensity set by participant, on mitigating vigilance loss. Moreover, while no effects of taVNS were observed on changes in sAA across time, a mitigatory effect on sCort reduction by taVNS at the constant intensity level was observed only via planned pairwise comparisons. Interestingly, correlation analyses between changes in sAA and sCort seem to show some interindividual effects of active taVNS, independently of the current intensity level. No effects of taVNS were observed on other attentional components measured by the ANTI-Vea task. We highlight the need to collect further evidence on the current research questions, replicating and extending the methodological design of the present study, and investigating other stimulation parameters of taVNS and its effects on more indirect markers of LC-NE activity.
AUTHOR CONTRIBUTIONS
Fernando G. Luna: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; validation; visualization; writing – original draft. Juan Lupiáñez: Conceptualization; data curation; methodology; resources; software; validation; writing – review and editing. Stefanie König: Data curation; investigation; resources; validation; writing – review and editing. Ulrike Garscha: Data curation; investigation; resources; validation; writing – review and editing. Rico Fischer: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; writing – review and editing.
ACKNOWLEDGMENTS
We would like to give special thanks to Elsa Motley, Lorenz Neumann, Nano Pirtskhalaishvili, and Michelle Schmidt, undergraduate students at the University of Greifswald, for their collaboration in data collection as student assistants/internship. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
This study was part of a larger research project supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) via a Walter Benjamin Position granted to FGL (project code: 513247174). JL was funded by the Spanish Ministry of Economy, Industry and Competitiveness (research project PID2020.114790 GB.I00).
CONFLICT OF INTEREST STATEMENT
All authors disclose any potential sources of conflict of interest in this submission.
ETHICS APPROVAL
This study was approved by the Ethics Committee of the Greifswald University Medicine (protocol code: BB 032/22a).
Open Research
DATA AVAILABILITY STATEMENT
The raw data (https://osf.io/8khr9/), R scripts for data analyses (https://osf.io/2bg5p/), and PsychoPy scripts (https://osf.io/9bn4h/) used in the present study are openly available in the OSF.
REFERENCES
- 1 Please, note that only a very small number of participants (presumably less than five) asked for a reduction in intensity for a few mA in the active taVNS at individualized intensity condition. The exact number of participants was unfortunately not recorded and cannot be reconstructed.