Changes in sleep effort mediate insomnia severity in older adults following online cognitive behavioural therapy
Disclosure: the authors have no potential conflicts of interest to disclose.
Abstract
Background
To examine treatment mechanisms of digitally delivered cognitive behavioural therapy for insomnia (CBT-I), this study assessed the mediating effects of dysfunctional beliefs, hyperarousal, locus of control, self-efficacy, sleep effort, and safety behaviours on self-reported insomnia severity in older adults before and following the completion of a self-guided, online CBT-I program.
Methods
The baseline and follow-up measurements were completed by 62 older adults (55 female, 89%). This was a two-condition within-participant design. Mediation analysis using a parallel mediation model was conducted using the MEMORE macro for repeated measure designs.
Results
Out of all the included mediator variables, only a reduction in sleep effort scores (0.88; SE 0.51; 95% CI 0.001–2.00) significantly mediated changes in insomnia severity scores following the intervention. Insomnia severity scores significantly reduced following the intervention (Mpre = 9.84, SD = 5.89, Mpost = 6.87, SD = 4.90); t(61) = 5.19, P = <0.001; d = 0.55 95% CI 0.38–0.93.
Conclusions
Sleep in older adults improved following digitally delivered CBT-I, and these changes were influenced by a reduction in sleep control efforts exerted by participants. These findings highlight possible treatment pathways of CBT-I. Further investigation of CBT-I as a strategy to prevent sleep problems is warranted.
Trial Registration
Australian New Zealand Clinical Trials Registry (ANZCTR), ACTRN 12619001509156; http://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=378451.
INTRODUCTION
There is strong evidence that cognitive behavioural therapy is efficacious for a myriad of conditions ranging from depression and anxiety to post-traumatic stress,1 and it is also heralded as the gold standard treatment approach for insomnia, a common sleep disorder.2 Older adults are disproportionately affected by insomnia and are more likely to develop sleep problems than younger adults, with about 60% of older individuals in Australia complaining of insomnia.3 Cognitive behavioural therapy for insomnia (CBT-I) comprises several treatment components: sleep hygiene, cognitive therapy, sleep restriction, stimulus control, and relaxation training.4 Sleep hygiene focusses on creating an optimal sleep environment, including bedroom temperature and light and noise reduction. Cognitive therapy aims to challenge unrealistic or maladaptive thoughts about sleep. Sleep restriction promotes adherence to a strict sleep – wake schedule to decrease time spent in bed awake, whereas stimulus control addresses associations between the bedroom environment and sleep. Finally, relaxation training lowers anxiety around sleep and decreases cognitive as well as physiological arousal. Meta-analyses of the effectiveness of CBT-I have demonstrated medium to large effect sizes for CBT-I on several sleep outcomes: Insomnia Severity Index (ISI, g = 0.82, 95% CI 0.74, 0.91), sleep efficiency (SE, g = 0.63, 95% CI 0.56, 0.69) and sleep onset latency (SOL, g = 0.47, 95% CI 0.41, 0.53).4
While CBT-I is an effective treatment method, the mechanisms of change that underpin any improvements in sleep outcomes remain uncertain. A review by Schwartz and Carney5 highlighted the need for research to examine mediators of CBT-I to determine the underlying treatment mechanisms. However, it has taken almost 10 years since the publication of the original review for newer studies and reviews to emerge,6 and at the time this research was conceived there were very few studies that conducted mediation analysis. An evaluation of the most effective mechanisms of change would allow clinicians to better understand and personalise CBT-based treatment for insomnia.
In this study, we examined the mediating effects of cognitive, behavioural and hyperarousal mechanisms, including dysfunctional beliefs, hyperarousal, locus of control, self-efficacy, sleep effort, and safety behaviours, on the outcome of self-reported insomnia severity in older adults following a digitally delivered, self-guided CBT-I program. Since these mechanisms have been highlighted by previous research as potential mediators of CBT-I,5, 6 we anticipated that: (a) insomnia severity would reduce following CBT-I treatment; and (b) that a reduction in insomnia severity would be mediated by dysfunctional beliefs, hyperarousal, locus of control, self-efficacy, sleep effort, and safety behaviours. We examined the sleep outcomes of older adults regardless of their insomnia status, to elucidate whether CBT-I has the potential to improve sleep even in individuals without insomnia.
METHODS
Ethics approval
Ethics approval for this study was granted by the university's Human Research Ethics Committee and informed written consent was obtained from all participants prior to their participation. This study was conducted in accordance with the ethical standards outlined in the Declaration of Helsinki and with the National Statement on Ethical Conduct in Human Research (2007, Updated 2018). This manuscript is a secondary analysis of data collected as part of a previous clinical trial.7 The research question and analysis outlined in the current manuscript were not part of the primary study.
Recruitment
Participants were recruited between March 2020 and March 2021, and were eligible to take part if they were aged 60–80 years. Exclusion criteria were a diagnosis with a sleep disorder (other than insomnia), a psychiatric or cognitive disorder, shift worker, epilepsy and a high risk of falling. Sleep restriction therapy is contraindicated in individuals with epilepsy, sleep disordered breathing, bipolar disorder and schizophrenia and individuals who reported any of these conditions were excluded. Decreased cognitive performance can also be a side effect of sleep restriction and can result in an increased risk of falls in older adults. Therefore, older individuals with an existing high fall risk were also excluded. The exclusion criteria were assessed during a 15–30 min screening phone call. Age, presence of sleep disorders or cognitive disorders, shift work status, an existing diagnosis of epilepsy or chronic fatigue syndrome were assessed by self-report. The risk of developing sleep disorder breathing was assessed using the STOP Bang (snoring, tiredness, observed apnoea, high blood pressure, body mass index, age, neck circumference, and male gender) questionnaire, a brief self-report instrument.8 The STOP Bang is effective at detecting obstructive sleep apnoea, and has a reported sensitivity of 84%.8 Older adults with a high risk score (>2) were excluded from taking part. The fall risk assessment was based on the STEADI algorithm.9 Participants were assessed based self-reported unsteadiness when standing or walking, concerns about falling, and occurrence of a fall within the past year.
Participants were not excluded if they took sedatives, as CBT-I also has demonstrated effectiveness in those taking sleep medication;4 however, medication type, dosage and frequency were documented as part of the initial assessment.
Design
Participants completed the questionnaires using a digital software distribution platform (Qualtrics XM Platform™ software, Qualtrics, Provo, UT, USA). The estimated completion time was around 20–25 min.
Sleep-related dysfunctional beliefs were assessed with the Dysfunctional Beliefs and Attitudes about Sleep scale (DBAS-16).10 The DBAS-16 has 16 statements rated on a 10-point Likert scale, which examines beliefs and attitudes about sleep. The DBAS-16 reliably discriminates between self-reported good and poor sleepers in older adult populations and has adequate internal consistency (Cronbach α = 0.79 for research samples).11 A DBAS-16 score of ≥4 suggests a clinically significant level of dysfunctional beliefs.11
The Pre-Sleep Arousal Scale (PSAS) was used to examine cognitive and physiological hyperarousal.12 The PSAS has two subscales each consisting of eight items. The items are rated using a five-point Likert scale, with scores ranging from one to five. The internal consistency of the PSAS has been reported as Cronbach α = 0.67 for the cognitive, and 0.84 for the physiological arousal subscales in normal sleepers, and 0.76 and 0.81 respectively for individuals with insomnia.12
Internal and chance locus of control were evaluated with the Sleep Locus of Control Scale (SLOC).13 The SLOC is an eight-item measure which explores whether an individual believes that sleep outcomes are controlled by themselves or by chance. The measure uses a six-point Likert scale and has an internal consistency of Cronbach α = 0.72 for internal locus of control, and 0.59 for chance SLOC.13
The Self-Efficacy for Sleep Scale (SE-S)14 examines self-efficacy. Self-efficacy relates to an individual's confidence in their ability to demonstrate behaviours required to achieve desired health outcomes.15 The SE-S is a nine-item measure using a five-point scale, with higher scores equalling increased sleep self-efficacy. The SES has fair internal consistency (Cronbach α = 0.71).16
The Glasgow Sleep Effort Scale (GSES)17 assesses sleep effort, an individual's effort to control the process of falling asleep. The GSES is comprised of seven items, which are rated on a three-point scale. In the initial validation study internal consistency of the GSES was reported as fair, with a Cronbach α = 0.77.17
The Sleep-Related Behaviours Questionnaire (SRBQ)18 appraises safety behaviours relating to sleep. It contains 32 items, which are rated on a five-point scale ranging from zero to four. The measure has high internal consistency with a Cronbach α = 0.92.18
The outcome measure, the ISI19, 20 is a brief self-report questionnaire. It assesses not only severity of insomnia, but also impairment of daytime functioning and worry about sleep and is used as an instrument to examine treatment response. The ISI has excellent internal consistency in community samples (Cronbach α = 0.90). Scores below seven indicate clinical insomnia, scores between eight and 14 subthreshold insomnia, scores between 15 and 21 moderate clinical insomnia, and scores ≥22 severe clinical insomnia.19
Procedure
Prior to commencement of the study, the lead author arranged an appointment for a screening call to assess participant eligibility. After the participants had provided written informed consent, they were emailed the baseline questionnaire. Once completed, they then received the instructions for the online CBT-I intervention via email. All participants received the same instructions. After completion of the CBT-I program, participants were emailed the follow-up questionnaire.
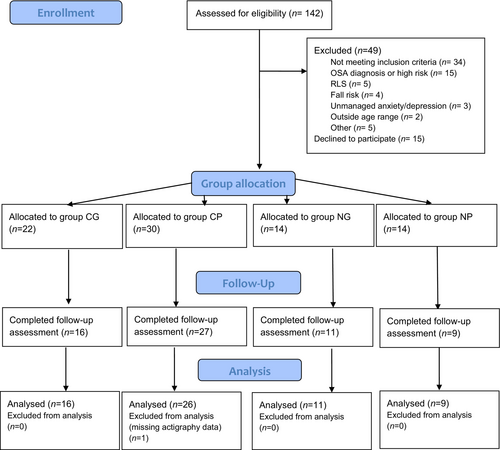
One hundred and seventy-five expressions of interest were received, and 142 participants agreed to be assessed for eligibility. The remaining participants were excluded due to non-response. Thirty-four individuals were not eligible to participate; of those, 15 were excluded due to sleep apnoea risk, five as a result of self-reported restless legs syndrome, four because of a high risk of falls, three due to poorly managed mental health conditions. In addition, two participants were excluded as they were not aged between 60 and 80 years, two reported having bipolar disorder or post-traumatic stress disorder, and one participant was a shift worker. Two additional participants were excluded because of polycythemia vera and chronic fatigue syndrome, which are medical conditions which can be exacerbated by sleep restriction therapy. Twenty-eight participants discontinued following eligibility screening, stating they lacked the time to take part. Eighty participants completed the baseline data collection. Following the intervention, 15 participants were lost to follow-up: five participants withdrew due to health reasons, one due to a lack of time, one had no access to a computer, and the remaining eight did not give a reason for dropping out. Three more participants did not provide sufficient actigraphy data and were excluded from the data analysis. A total of 62 participants (55 female, 89%) completed both baseline and follow-up measurements (Fig. 1).
Description of intervention
Participants completed a digital CBT-I program over 4 weeks. The program has demonstrated efficacy at resolving sleep problems, with earlier research reporting insomnia remission in 65% of users.21 This self-guided program consists of four modules providing information about sleep hygiene, the management of sleep-interfering thoughts and behaviours and relaxation techniques. The program is currently not customisable but covers the most common characteristics of insomnia (e.g., undesired early morning awakenings). Each of the modules takes about 20 min to complete, and in addition, participants are required to fill in a sleep diary.
Data analysis
A power analysis (G*Power version 3.1.9.7) for sample size estimation showed that a minimum of 40 participants would be needed to detect a moderate effect size, using a significance criterion of α = 0.05 and power = 0.80.22 This calculation was based on data from an earlier study (N = 103 participants; mean = 72.81 years; SD = 7.12)23 comparing subjective and objective sleep patterns in older adults. This study reported an effect size of 0.68, a medium effect using Cohen's (1988) criteria. However, due to its complexity, power analysis tools do not typically produce power calculations for mediation analysis, and as a result our calculations were based on a multivariate regression model, as used in the previous study. Nevertheless, Fritz et al.24 recommended several methods of boosting statistical power in mediation models without increasing the sample size. Such methods are for example the inclusion of additional predictors in the mediation model (pre- and post-test scores), as well as additional mediators. They suggested that additional mediators increase power by controlling the shared effects of these mediators.
Subsequently, a paired samples t-test was performed to compare insomnia severity pre- and post-treatment, showing a significant difference in insomnia severity scores pre-test (mean = 9.84, SD = 5.89) compared with post-test (mean = 6.87, SD = 4.90; t(61) = 5.19, P = <0.001). This was a large effect (Cohen's d = 4.51; 95% CI 0.38–0.93), indicating that the average insomnia severity score following intervention was more than four SDs lower than the average score prior to treatment.
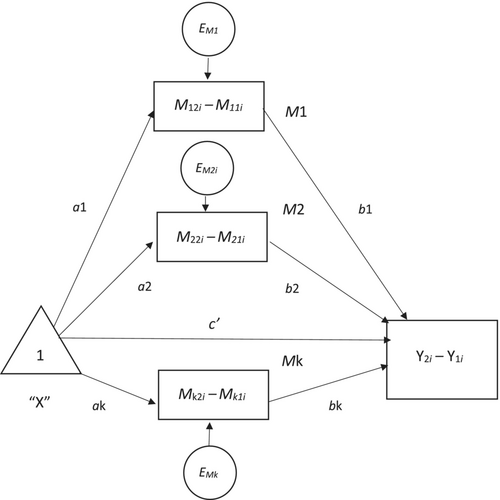
Mediation analyses were conducted using MEMORE for SPSS v2.1 (Montoya 2019).25 MEMORE is a macro for mediation and moderation models in repeated measures designs. It allows for up to 10 parallel mediators to be entered (Montoya and Hayes 2017)26 (see Fig. 2). MEMORE calculates the difference between the two mediator measurements and the difference between the two dependent variable measurements, and these are then modelled using the procedure described by Montoya and Hayes26 and Judd et al.27 Prior to analysis, we tested model assumptions of linearity, homoskedasticity, and normality of estimation error; these were confirmed by examining residuals plots. A parallel mediation model using 10 000 bootstrap samples and bias-corrected bootstrap (Model 1 in MEMORE) was run to estimate the total, direct, and indirect effects of the intervention on insomnia severity, as measured by the ISI before and after the intervention, through changes in the mediators dysfunctional beliefs, hyperarousal, locus of control, self-efficacy, sleep effort, and safety behaviours. As there was no statistically significant difference in the somatic subscale of the PSAS following CBT-I, this mediator was excluded from the analysis. All mediator variables were mean centred prior to analysis.
RESULTS
The completion rate for the online CBT-I program was 77.5%. Treatment adherence is higher than in previous research using this program, which reported a completion rate of 37.2%.21
Table 1 shows the demographic characteristics of the sample, and the changes in the mediator variables before and after the intervention.
| Variable | Pre-intervention mean (SD/%) N = 80 | Post-intervention mean (SD/%) N = 62 |
|---|---|---|
| Age | 66.74 (4.39) | 66.14 (3.90) |
| Alcohol consumption, ≤4 units, (%) | 74 (92.50%) | 60 (95.20%) |
| Gender, female (%) | 71 (88.8%) | 56 (88.9%) |
| Employment status, retired (%) | 52 (65%) | 43 (68.3%) |
| Caffeine consumption, in mg | 228.10 (123.89) | 227.79 (135.87) |
| Dysfunctional beliefs, DBAS-16 | 3.76 (1.54) | 3.22 (1.44) |
| Hyperarousal, PSAS, cognitive subscale | 18.62 (6.23) | 15.32 (4.73) |
| Locus of control, SLOC† | 27.18 (6.03) | 30.62 (6.36) |
| Self-efficacy, SE-S | 28.12 (7.35) | 32.38 (7.18) |
| Sleep effort, GSES | 3.84 (2.73) | 2.56 (2.12) |
| Safety behaviours, SRBQ | 39.75 (13.84) | 36.68 (12.69) |
- GSES, Glasgow Sleep Effort Scale; PSAS, Pre-Sleep Arousal Scale; SE-S, Self-Efficacy for Sleep Scale; SLOC, Sleep Locus of Control Scale; SRBQ, Sleep-Related Behaviours Questionnaire; DBAS-16, Dysfunctional Beliefs and Attitudes about Sleep scale.
- † A higher score signifies greater internal locus of control.
Using a parallel mediation model, analysis showed that changes in dysfunctional beliefs, hyperarousal, locus of control, self-efficacy and safety behaviours did not mediate insomnia severity following a CBT intervention for insomnia. Changes in sleep effort fully mediated the changes in insomnia severity following CBT-I (Table 2).
| Outcome | Mediator | Intervention effect on outcome (direct effect) | Mediated effects (indirect effects) | P-value | Conclusion |
|---|---|---|---|---|---|
| (SE/95% CI) | SE/95% CI | ||||
| Insomnia severity | 0.40 (0.81/−1.22, 2.01) | 0.626 | Full mediation | ||
| Dysfunctional beliefs | −0.10 (0.33/−0.82, 0.49) | ||||
| Hyperarousal† | 0.80 (0.53/−0.21, 1.89) | ||||
| Locus of control | 0.09 (0.42/−0.72, 0.97) | ||||
| Self-efficacy | 0.69 (0.53/−0.27, 1.87) | ||||
| Sleep effort | 0.88 (0.51/0.001, 2.00)‡ | ||||
| Safety behaviours | 0.22 (0.32/−0.37, 0.93) | ||||
| Total | 2.58 (0.80/1.11, 4.24) |
- † Cognitive subscale of Pre-Sleep Arousal Scale.
- ‡ Significant mediation effect.
DISCUSSION
In this study we examined treatment efficacy and potential treatment mechanisms underlying CBT-I. We found that online CBT-I improved insomnia severity in older adults, and that the improvement was mediated by a reduction in sleep effort. We did not find a mediating effect for dysfunctional beliefs, hyperarousal, locus of control, self-efficacy, or safety behaviours as hypothesised.
The role of sleep effort as a mediating variable has previously been examined by Ebert et al.,28 who found that it mediated insomnia severity in teachers with elevated symptoms of insomnia. In addition, Hertenstein et al.29 investigated the association between sleep effort, dysfunctional beliefs and attitudes about sleep, pre-sleep arousal, and insomnia in a sample of 47 individuals with insomnia and 52 good sleepers. In line with the current study, Hertenstein et al. discovered an association between sleep effort and insomnia severity, but also did not find a mediating function of either dysfunctional beliefs or arousal, even though these had been highlighted as potential mediators in earlier literature.30, 31
The model of sleep effort proposed by Broomfield and Espie17, 32 suggested that sleep effort is a complex construct consisting of multiple cognitive and behavioural components, which include dysfunctional beliefs, as well as maladaptive behaviours and arousal: a period of acute insomnia, for example, can trigger dysfunctional beliefs about sleep, which subsequently progress into chronic insomnia. It is possible that there is an overlap between the concept of sleep effort and dysfunctional sleep beliefs and other mediators, or that changes in dysfunctional sleep beliefs, hyperarousal, locus of control, self-efficacy, or safety behaviours are mediated via changes in sleep effort. This may explain why previous research investigating only dysfunctional sleep beliefs found a mediating effect, while other studies that examined sleep effort failed to uncover a mediating effect of dysfunctional beliefs. Indeed, when we ran simple mediation models for each mediator, we found significant results for four mediators – dysfunctional beliefs, sleep effort, self-efficacy, and safety behaviours – but in the parallel mediation model sleep effort fully mediated insomnia severity. Montoya and Hayes26 stipulated that it is possible to estimate a set of single-mediator models, but doing so confounds estimates of the direct effect for each mediator with indirect effects through the mediators that are not present in the model, and therefore a parallel model was considered the most appropriate choice.
Further, Broomfield and Espie17 have emphasised that several diagnostic tools measure single items relevant to sleep effort: The DBAS-16, for example, which examines dysfunctional attitudes and beliefs about sleep, and which has been utilised in this study for this purpose, incorporates items relevant to sleep effort. In a previous study online CBT-I ameliorated dysfunctional sleep beliefs in older adults regardless of whether they complained of poor sleep and regardless of whether they had objectively measured poor sleep,7 but whether sleep effort changed following CBT-I or whether it mediated dysfunctional sleep beliefs was not examined. If sleep effort is the main treatment process underpinning CBT-I, and is closely related to other mediators, such as dysfunctional beliefs, then insomnia treatment components that focus solely on reducing sleep effort, for example relaxation and meditation, should improve insomnia severity in older individuals. Future research is warranted to disentangle sleep effort and dysfunctional sleep beliefs and other mediators in an effort to further pinpoint which components of CBT-I are effective in improving sleep in older individuals.
It has further been suggested that sleep effort is an underlying mechanism that explains discrepancies between subjective and objectively measured sleep.33 Some studies have shown that sleep effort predicts subjective insomnia severity,28, 29 whereas dysfunctional beliefs predict objective insomnia outcomes.29 Hertenstein et al.29 also showed in their study that polysomnography-determined total sleep time was associated with dysfunctional beliefs and attitudes about sleep. The current study has not examined whether sleep effort mediates objective sleep outcomes, such as wake after sleep onset measured using actigraphy or polysomnography. Future investigations should explore whether dysfunctional beliefs and sleep effort affect different treatment pathways.
In this study we examined sleep in older adults over the age of 60. The fact that sleep effort mediated insomnia severity following CBT-I in this sample indicates CBT-I's potential at preventing insomnia in older adults overall, regardless of their sleep status. Affective sleep preoccupation shares similarities with the concept of sleep effort, as they both relate to processes associated with preoccupation with sleep and a desire to control it. Ellis et al.34 compared adults with acute insomnia and normal sleepers to assess which factors increased the likelihood of developing chronic insomnia. They found that none of the examined predisposing, precipitating (apart from depression) or coping factors predicted the transition from acute insomnia to its chronic form. However, they did discover that older age and affective sleep preoccupation, the daytime preoccupation about one's sleep, were predictive of acute insomnia. These findings also suggest that therapy approaches aimed at preventing acute insomnia in older adults might be useful by targeting sleep preoccupation and sleep effort. Again, more prospective research studies are required to examine this notion further.
The use of a convenience sample of individuals, who self-selected as being interested in learning more about their sleep, is a limitation of this study. Furthermore, in our analysis we focused on self-reported insomnia severity but did not examine other sleep outcomes following CBT-I, for example, changes in waking after sleep onset, nor did me measure objective changes in sleep outcomes using actigraphy or polysomnography.
However, to the best of our knowledge, this is the first study to examine CBT-I treatment mechanisms in older individuals irrespective of their insomnia status. We have demonstrated that sleep effort mediates insomnia severity even in a sample containing non-treatment-seeking individuals. Future research is needed to examine the relationship between sleep effort and dysfunctional beliefs and attitudes about sleep and investigate whether these findings signify a possible serial mediation, an overlap in the concepts of sleep effort and dysfunctional sleep beliefs, or whether sleep effort and dysfunctional beliefs indeed target different mechanisms of CBT-I treatment. It is also recommended that the use of online CBT-I used as a preventative measure against insomnia in older people is investigated and whether self-guided therapy focusing on reducing sleep effort can improve sleep in older adults.
CONCLUSION
Only changes in sleep effort mediated the outcome of self-reported insomnia severity in older adults following a digitally delivered, unguided CBT-I program. Since research into the treatment mechanisms and the preventative potential of CBT-I is scarce, we recommend that future research studies incorporate these elements. Unlike previous research, which focused only on individuals with insomnia, we did not find that dysfunctional sleep beliefs and other commonly hypothesised mediators influenced the outcomes of CBT-I. Our findings further suggest that CBT-I could be effective as a preventative measure in older adults.
ACKNOWLEDGMENTS
YK is supported through an Australian Government Research Training Program Scholarship. The authors would like to thank Dr. Saiyidi Mat Roni and Associate Professor Chandra Salgado Kent for their support.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




