Transcription Factor ClTCP4 Regulates Leaf Size and Wavy Margin in Leaf Morphogenesis of Citrullus lanatus
Funding: This work was supported by China Postdoctoral Science Foundation (2022M711064 and 2023M741062), Joint Fund of Henan Province Science and Technology Research and Development Plan (222103810009), Henan Provincial Science and Technology Research Project (242102110176) and National Natural Science Foundation of China (32202514).
ABSTRACT
The formation of leaf morphology is crucial for the normal role of leaves in various growth and development processes of plants. Citrullus lanatus (watermelon), is an important economic crop and its leaf development mechanism is still not well understood. In this study, a CIN-TCP transcription factor, ClTCP4, was identified as participating in the process of watermelon leaf morphogenesis. ClTCP4 knockout lines (CR lines) generated by the CRISPR/Cas9 system show a wavy leaf margin. The levels of auxin, gibberellin, and cytokinin hormones were elevated in CR lines, and transcriptome analysis indicated altered gene expression in the hormone metabolism pathways mentioned above. At the cellular level, the functional loss of ClTCP4 causes smaller cells and an increased cell number in the leaves of CR lines. A negative regulatory relationship between ClTCP4 and ClWOX1 were determined with the tobacco transient overexpression system. Leaf margin regulator “ClWOX1” may inhibit the transcriptional activation effect of ClTCP4 on the leaf margin suppressor “ClNGAL1” in the process of leaf morphology formation. This study revealed the regulatory role of ClTCP4 in leaf morphogenesis in watermelon plants, and provided a theoretical basis and gene resources for improving leaf morphology in watermelon.
1 Introduction
Leaves are one of the most important above-ground organs of plants, and the morphology of plant leaves and their margin can be roughly classified into compound leaves, incised leaves, entire margin leaves, serrated leaves, wavy leaves, etc. (Blein et al. 2010; Duan et al. 2023). Early studies identified two key determinants of leaf margin morphogenesis: the size of the proliferative active zone and the maintenance time of the proliferative active zone (Hagemann and Gleissberg 1996). Plants regulate cell differentiation in time and space through multiple pathways, thereby generating polymorphic changes in leaf margin morphology (Palatnik et al. 2003). For most species, cells in different parts of the leaf divide and elongate in a balanced and orderly manner, thus keeping the leaf surface flat (Hayakawa et al. 2016; Yang, Wang, et al. 2018). Rapid growth of leaf marginal cells can lead to a saddle shape or wavy margin, while excessive division or elongation in the leaf center can form a cup-shaped leaf (Hayakawa et al. 2016; Yang, Wang, et al. 2018).
After the polarity of the leaves is established, the size and shape of the leaves are determined by cell number and cell size (Wang et al. 2021). Plant hormones are pivotal in the cell proliferation and the expansion processes. Cytokinin maintains cell proliferation activity and affects leaf size by restricting the transition from the cell proliferation stage to the cell volume expansion stage during leaf development (Černý et al. 2013; Skalák et al. 2019). The gibberellin and brassinosteroid pathways have been shown to promote both cell proliferation and cell expansion processes (Ueguchi-Tanaka et al. 2007; Oh et al. 2011). Elevated expression of the gibberellin synthesis gene GA20ox can increase leaf area (Ueguchi-Tanaka et al. 2007; Wang, Jiang, et al. 2020). Similarly, increased expression of the brassinosteroid response gene BRI1 enlarges leaf size (Shang et al. 2011; Oh et al. 2011). Auxin modulates leaf morphogenesis processes via key factors in the auxin response pathway (Mizukami and Fischer 2000; Okushima et al. 2005).
In previous studies, some transcription factors play key regulatory roles in leaf margin development. The TCP family was categorized into two subfamilies: the class I (PCF) and the class II (CIN-like and CYC/TB1-like; Cubas et al. 1999; Chen et al. 2019; Wang et al. 2022). The CIN-TCP genes have been extensively studied for their roles in leaf curling and inhibiting cell proliferation, and the functional loss of CIN-TCP genes causes larger leaves with a saddle shape and wavy margin in Antirrhinum, Arabidopsis, tomato, and jujube (Nath et al. 2003; Martín-Trillo and Cubas 2010; Schommer et al. 2014; Zhang et al. 2021; Yang et al. 2022). In Arabidopsis, the quadruple mutant tcp2 tcp3 tcp4 tcp10 shows wavy or serrated leaves (Bresso et al. 2018). High levels of TCP4 gene expression in the distal region of young Arabidopsis leaves will restrict the activity of leaf meristematic tissue, resulting in continuous marginal growth of cotyledons, leaves, and floral organs (Efroni et al. 2013). Furthermore, TCP transcription factors regulate cell proliferation and differentiation in Arabidopsis leaves via the IAA and cytokinin signaling pathways (Das Gupta et al. 2014). CnTCP4 expression is abundant in both the leaf and ligulate florets of Chrysanthemum nankingense, and the autotetraploid plants produced larger leaves than diploid plants (Qi et al. 2019). The WUSCHEL-related homeobox 1 (WOX1) homologs are recognized for their regulatory roles in leaf expansion and margin morphogenesis (Li et al. 2024; Nardmann et al. 2004; Tadege et al. 2011; Vandenbussche et al. 2009; Wang, Zhao, et al. 2020; Wang, Niu, et al. 2020; Zhuang et al. 2012). For instance, the mutants of WOX1 homologs such as narrow sheath (ns) in Zea mays L. (Nardmann et al. 2004), maewest (maw) in Petunia × hybrida (Vandenbussche et al. 2009), lam1 in Nicotiana sylvestris (Tadege et al. 2011), stenofolia (stf) in Medicago truncatula (Tadege et al. 2011; Li et al. 2024), lath in Pisum sativum L. (Zhuang et al. 2012), lam1 in Solanum lycopersicum (Wang, Zhao, et al. 2020), and mf in Cucumis sativus (Wang, Niu, et al. 2020), all display defects in leaf expansion and leaf margin development. It has been shown that the functional mutation of WOX1 has resulted in decreased leaf area and increased cell volume in cucumber plants (Wang, Niu, et al. 2020).
In Empire lettuce, a retrotransposon insertion within the 3′UTR of the LsTCP4 allele results in reduced transcription level of LsTCP4 in leaves, consequently affecting leaf margin morphology (Seki et al. 2020). The LsAP2 has been shown to directly regulate TCP family genes, inducing the formation of curly leaves in lettuce (Luo et al. 2021). While the above studies have reported on the role of CIN-TCP transcription factors in leaf margin morphogenesis in Arabidopsis thaliana and lettuce, the molecular mechanisms by which CIN-TCP members regulate leaf morphogenesis in Cucurbitaceae crops remain unclear. Watermelon, as an important economic crop of the Cucurbitaceae family, has deeply lobed leaf margins and a complex leaf shape. In the yeast two-hybrid test of this study, a watermelon TCP transcription factor (Cla97C07G136640) was screened out to interact with the leaf shape regulatory factor WOX1. Therefore, the ClTCP4 gene edited lines (CR lines) were created. Through the morphological analysis of CR lines, gene expression detection, and transcriptional regulation analysis, the mechanism by which ClTCP4 regulates watermelon leaf development was revealed. By identifying the role of the ClTCP4 gene in regulating the morphogenesis of watermelon leaves, this study is helpful for understanding the role of CIN-TCP members during the morphogenesis process of different leaf types, which is of great significance for revealing the molecular mechanisms of leaf shape development.
2 Materials and Methods
2.1 Experimental Materials
The high-efficiency transformation material YL (a conventional diploid inbred line material) was used for gene editing in watermelon. Watermelon seedlings, including the inbred line YL and CR lines, were grown in controlled light chambers at 28°C for a 16-h light period and 20°C for an 8-h dark period, along with a relative humidity of 70%. Seedlings with two fully expanded true leaves were transplanted to the horticultural greenhouses, and the leaf morphology of watermelon plants was continuously observed during the subsequent growth process through photography and microscopic examination.
2.2 Bioinformatics Analysis
The watermelon genetic information in this study was obtained from the cucurbitaceae genome database (CuGenDB, http://cucurbitgenomics.org/). The TCP family members in Arabidopsis were retrieved from the TAIR (http://www.arabidopsis.org) database. The above sequences of TCP family members are listed in Table S1. The TCPs in Citrullus lanatus and A. thaliana were aligned using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). The cis-acting elements in the 2 kb promoter sequence of ClTCP4 were analyzed using the PlantCARE online analytical tool (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/). A phylogenetic tree was generated by the MEGA 6 software, using the neighbor-joining method with the following parameters: pairwise alignment, 1000 bootstrap replicates, p-distance model, uniform substitution rates, and complete deletion.
2.3 Gene Expression Analysis
The main stem, young leaf petioles, leaves of different developmental stages, male and female flowers, and young fruits from 6-week-old YL seedlings were collected for gene expression analysis. Following RNA extraction, cDNA was synthesized via reverse transcription. Total RNA isolation (Lot#P4825, Tiangen) and the synthesis of first-strand cDNA (Lot#AK3701, TaKaRa) were performed following the manufacturer's instructions. Quantitative real-time PCR (qRT-PCR) primers for the CIN-TCP members were designed (Table S2) by Primer Premier 5. qRT-PCR was performed according to the previous steps in Niu et al. (2022). β-Actin was used as the reference gene (Kong et al. 2014), and the data of qRT-PCR was calculated according to the previous study by Livak and Schmittgen (2001). Three biological replicates were performed for each gene.
2.4 Subcellular Localization Analysis
Gene sequences were amplified with K5 HiFi DNA Polymerase (ZT107, ZOMANBIO). The ClTCP4 cDNA was inserted into the pGreenII-GFP vector using the pEASY-Uni Seamless Cloning and Assembly Kit (CU101-01, TRANSGEN). The recombinant plasmid was transformed into Agrobacterium tumefaciens strain GV3101, and the above bacterial liquid containing recombinant plasmids was injected into the leaves of 4- to 6-week-old Nicotiana benthamiana using sterile syringes. Cells of the infected leaves were observed using a fluorescent microscope (BX63, Olympus) after 48 h. DAPI was used to stain and label the nucleus. Specific gene primers are listed in Table S2.
2.5 Creation of Gene Edited Lines (CR Lines) for ClTCP4
Gene editing primers of ClTCP4 were designed by an online gene editing target design platform (http://crispr.dbcls.jp/), and two editing targets with minimal off-target potential were selected within the TCP domain. The editing target sequences were cloned into the pBSE402 CRISPR vector, and the recombinant plasmid was transformed into A. tumefaciens strain EHA105. The genetic transformation process followed previous description (Wang et al. 2024). Transgenic seedlings were screened using GFP fluorescence detection with a stereoscopic fluorescence microscope (MZ10F, Leica). ClTCP4 fragments of transgenic seedlings were amplified by target detection primers (Table S2) and were sequenced to confirm gene edited forms.
2.6 Leaf Morphology Observation
The leaf morphology of YL and CR lines was photographed, and a ruler was used to indicate the size of the leaves. The ZEISS image analysis software ZEN3.9 was utilized for leaf size analysis. Three leaves were selected from each identical node of YL and CR lines to measure the leaf area and the number of wavy folds. For the preparation of paraffin sections, the mature leaves of the YL and CR lines were fixed, embedded, sectioned, and dewaxed following the method of Bai et al. (2004), and the leaf structure of longitudinal sections, including the morphology and size of palisade tissue cells and spongy tissue cells, was monitored. The morphology and size of the lower epidermal cells of leaves were observed by light microscopy (Axioscope 5, ZEISS). For the different leaf regions in the observation of lower epidermal cells, the top, middle, and base leaf regions were defined following the criteria in Figure S1. The leaf tissue attached to the upper one-third of the main vein is defined as the top region; the leaf tissue attached to the middle one-third of the main vein is defined as the middle region; the leaf tissue attached to the lower one-third of the main vein is defined as the base region. The ZEN3.9 software was utilized for the analysis of cell size and cell number within the 100*100 μm area of the lower epidermal cells of leaves of YL and CR lines. Three views were randomly selected for the statistics of the cell number, and six cells were randomly selected for the measurement of cell size.
2.7 Endogenous Hormone Measurements
A total of 0.3 g young leaves from 6-week-old plants of YL and CR lines were sampled for the measurements of endogenous hormones (auxin, cytokinin, gibberellin). Three biological replicates were performed. The leaf samples are quickly frozen with liquid nitrogen, and the endogenous hormones determination assays were conducted by Nanjing Zhongding Biotechnology Co. Ltd. For the determination of hormones, [2H5] trans-zeatin (D-tZ), [2H5] trans-zeatin riboside (D-tZR), D2-GA3, D5-IAA (Olchemim, Olomouc) were spiked into the extraction solutions as internal standards. All the measurements for hormones were performed with ACQUITY UPLC I-Class coupled to Waters Xevo TQ-XS triple quadrupole mass spectrometer according to the description of Dong et al. (2023).
2.8 Transcriptome Analysis
Young leaves about 3 cm length from YL and CR lines were collected for transcriptome analysis (Beijing Biomaker Biotechnology Co. Ltd). Three biological replicates were collected for each line. The concentration and purity of extracted RNA were assessed using Nanodrop 2000 (Thermo Fisher) and the degradation degree of RNA was observed using agarose electrophoresis (1% agarose gel). The VAHTSTM Universal DNA Library Prep Kit (Vazyme) was employed to construct libraries from qualified samples. Subsequently, the constructed libraries were sequenced using the NovaSeq6000 instrument (Illumina) with a sequencing read length of PE150. The NovaSeq 6000 S4 Reagent Kit (Illumina) was used for sequencing. During the result analysis, genes exhibiting at least a 2-fold change in expression between YL and CR lines, with a false discovery rate (FDR) of less than 0.05, were considered as differentially expressed genes. Differentially expressed genes were identified using the DESeq2 tool. KOBAS (Mao et al. 2005) database and clusterProfiler software were used to test the statistical enrichment of differential expression genes in KEGG pathways. The watermelon “97103” genome (http://cucurbitgenomics.org/v2/organism/16) served as the reference genome.
2.9 Yeast Two-Hybrid and Bimolecular Fluorescence Complementation Assays
The yeast two-hybrid (Y2H) assays were conducted as described previously (Wang, Niu, et al. 2020). The full-length CDS of ClTCP4 and ClWOX1 were cloned into pGADT7 and pGBKT7 vectors, respectively, using the pEASY-Uni Seamless Cloning and Assembly Kit (CU101-01, TRANSGEN). A combination of pGBKT7-53 and pGADT7-T was used as the positive control, with a combination of pGBKT7-lam and pGADT7-T as the negative control (Clontech).
For bimolecular fluorescence complementation (BiFC) assay, the CDS of ClWOX1 was cloned into pSPYNE-35S vector and the CDS of ClTCP4 was cloned into pSPYCE-35S vector. Transient overexpression in N. benthamiana epidermal cells was performed following the study of Wang, Niu, et al. (2020). The recombinant plasmids were transformed into A. tumefaciens strain GV3101, and these two strains with recombinant plasmids were co-transformed into the leaves of 6-week-old N. benthamiana using sterile syringes. Fluorescence was visualized using a microscope (BX63, Olympus) within 48–72 h after infiltration of Agrobacterium carrying those two strains with recombinant plasmids.
2.10 Luciferase Complementation Assay
The CDS of ClTCP4 and ClWOX1 were cloned into the pCAMBIA 1300-CLuc and pCAMBIA 1300-NLuc plasmids, respectively, using the pEASY-Uni Seamless Cloning and Assembly Kit (CU101-01, TRANSGEN). The recombinant plasmids were transformed into the GV3101 strain, and these two strains with recombinant plasmids were co-transformed into the N. benthamiana leaves. After 48 h, the leaves were then sprayed with 5 mM luciferin (CL6928, Coolaber) and kept in the dark for 10 min. A cooled CCD imaging apparatus (Tanon-5200) was used for capturing images.
2.11 GUS Report Assay
The CDS of ClTCP4 and ClWOX1 were fused into the pGreenII vector to construct the effectors, respectively, and the pCambia1381-proClNGAL1-GUS recombinant plasmid was used as a reporter. The recombinant plasmids were then transformed into the GV3101 strain. Four bacterial solutions (Vector, ClTCP4, ClWOX1 and proClNGAL1-GUS) were mixed according to three volume ratios of 4.5:4.5:0:1, 0:4.5:4.5:1 and 9:0:0:1, and these mixed solutions were infiltrated into the N. benthamiana leaves. The assay determining GUS activity was performed as described previously (Niu et al. 2022). Each experimental combination was subjected to five biological replicates.
3 Results
3.1 Protein Characteristic Analysis of ClTCP4
A phylogenetic tree was constructed for Cla97C07G136640 and Arabidopsis TCP family members, revealing that Cla97C07G136640 is situated within the CIN branch of the TCP family and shows the closest evolutionary relationship with AtTCP4 (Figure 1A). Consequently, Cla97C07G136640 was renamed ClTCP4. Protein sequence alignment of ClTCP4 with adjacent AtTCP members showed the presence of a conserved TCP domain, which is a characteristic of TCP family members (Figure 1B). The TCP domain, a basic helix–loop–helix (bHLH) motif comprising 59 amino acids, is capable of binding to DNA or mediating protein–protein interactions (Cubas et al. 1999). The subcellular localization of ClTCP4 indicated that it was predominantly localized to the cell nucleus (Figure 1C).
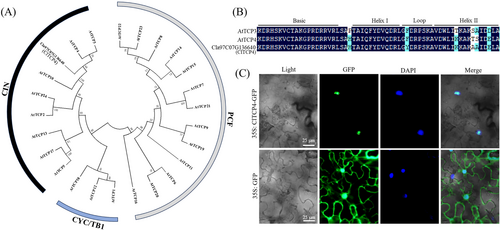
The 2 kb promoter sequence of ClTCP4 was used to determine cis-acting elements by the PlantCARE online tool (Table S3). There are diverse hormone response elements in the ClTCP4 promoter, including auxin, ABA, ethylene, and gibberellins response elements, implying a role for ClTCP4 in hormone regulatory pathways.
3.2 Construction of CR Lines
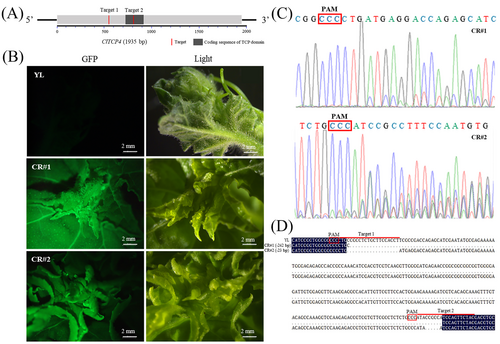
Specific target editing primers were designed in 5′ terminal sequence of ClTCP4 (Figure 2A). Two positive transgenetic lines were obtained by genetic transformation. Fluorescence detection showed that these two independent lines can produce GFP green fluorescence, confirming the successful embedding of T-DNA into the genome (Figure 2B). The detection primers were designed in the peripheral regions of the two target sites, and the corresponding sequence of the two transgenic lines was amplified and sequenced. As shown in Figure 2C, the sequencing results displayed overlapping peaks, indicating the target sequences of ClTCP4 were successfully edited. The sequencing results of CR lines of T2 homozygous generations showed a 242 bp fragment deletion in CR#1 and two short fragment deletions (a total of 23 bp) in CR#2 (Figure 2D). The TCP sequence variations in the two CR lines both caused the shift of the nucleotide coding box. These two CR lines were used to identify the function of ClTCP4.
3.3 Leaf Morphology Analysis of CR Lines
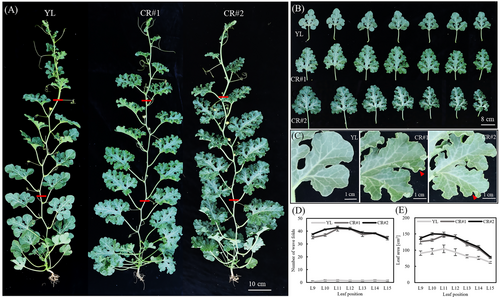
Phenotypic analysis of CR lines revealed that, in contrast to the flat leaf margins of YL plants, the CR lines exhibited numerous wavy folds along their margins (Figure 3A). A comparative assessment of leaves at continuous leaf nodes demonstrated the distinct leaf shape between YL and CR lines (Figure 3B), and the wavy folds were uniformly distributed along the leaf margin in CR lines (Figure 3C). The count of these folds indicated that there is an extreme increase in wavy folds in CR lines compared with YL (Figure 3D). Furthermore, we found that the leaf size of CR lines was significantly increased compared with that of YL (Figure 3E).
3.4 Cell Morphology Analysis of CR Lines
The mutation of leaf morphology frequently correlates with the change of cell morphology (Wang, Niu, et al. 2020). The lower epidermal cells in different leaf regions of YL and CR lines were examined microscopically, and there are obvious differences in terms of cell morphology and cell number (Figure 4A). Additionally, paraffin sections of leaves were made to observe the longitudinal profile of the leaves of YL and CR lines (Figure 4B). The palisade tissue cells in YL plants were elongated, and the spongy tissue cells were loosely packed. Conversely, the CR lines displayed shorter palisade tissue cells, and the spongy tissue exhibited a sparser arrangement. In addition, the upper and lower surfaces of the leaves of YL plants are flatter compared to CR lines (Figure 4B).
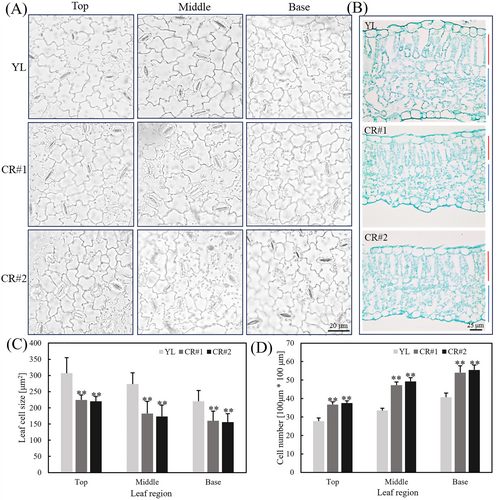
Further quantitative analysis was conducted on the cell morphology and cell number. Across the top, middle, and base leaf regions, the epidermal cells of CR lines exhibited diminished cell size compared to those of YL plants, and the quantitative analysis showed that the decrease in leaf cell size was approximately 25% (Figure 4C). Under the observation field (100*100 μm), quantitative analysis of the number of the lower epidermal cells of the leaves of CR lines was more than that of the YL plants, and the increase is approximately 30% (Figure 4D).
3.5 Analysis of the Regulatory Pathway of ClTCP4
Transcriptome analysis revealed that the functional loss of ClTCP4 resulted in the downregulation of many genes regulating leaf development. KEGG enrichment analysis (Figure S2) showed at the Environmental Information Processing level, the differential expression genes were mainly enriched into the categories of “ABC transporters” and “plant hormone signal transduction.” At the “Metabolism” level, the two main enriched categories of the differential expression genes were “phenylpropanoid biosynthesis” and “cutin, suberine, and wax biosynthesis”; and at the “Organismal Systems” level, the differential expression genes were mainly enriched into the category of “plant-pathogen interactions.”
Differentially expressed genes of hormone metabolism, cell proliferation, and cell expansion were listed in Table 1. For the gibberellin synthesis pathway, the genes of Cla97C09G164590 (Gibberellin 3-beta-dioxygenase 1, GA3ox1), Cla97C05G083220 (Ent-kaurenoic acid oxidase 1, KAO1), and Cla97C09G172140 (Beta-amyrin 11-oxidase) were upregulated in CR lines (Table 1; Figure S3A). Abscisic acid metabolism gene Cla97C10G202910 was downregulated (Table 1). For the cytokinin pathway, the UDP-glycosyltransferase 73C3 gene (Cla97C10G202910) was downregulated (Figure S3B). Auxin polar transport genes (Cla97C09G172568 and Cla97C11G219090) showed upregulation. The BRI1 kinase inhibitor 1 (BKI1), a negative regulator in brassinosteroid signal transduction that can suppress the brassinosteroid receptor BRI1, was downregulated (Table 1; Figure S3C). Conversely, WOX1, controlling leaf margin morphogenesis and cell morphology, was upregulated. Wall-associated receptor kinase 2 (WAK2) and expansion-like B1, both regulating cell expansion, exhibited downregulation. These indicate that the functional mutation of ClTCP4 induces alterations in gene expression in the downstream hormone pathways and genes associated with cell proliferation and expansion.
| ID | FC CR/YLa | FC log2 | FDR | Swiss-Prot_annotationb | Function descriptionc |
|---|---|---|---|---|---|
| Cla97C09G164590 | 3.8571 | 1.9475 | 5.12E-04 | Gibberellin 3-beta-dioxygenase 1 | Involved in gibberellic acid biosynthesis |
| Cla97C05G083220 | 3.1102 | 1.6370 | 5.42E-10 | Ent-kaurenoic acid oxidase 1 | Involved in gibberellic acid biosynthesis |
| Cla97C09G172140 | 2.5796 | 1.3671 | 4.60E-08 | Beta-amyrin 11-oxidase | Involved in gibberellic acid biosynthesis |
| Cla97C10G202910 | 0.4612 | −1.1165 | 1.99E-04 | UDP-glycosyltransferase 73C3 | Involved in trans-zeatin metabolism |
| Cla97C09G172568 | 2.7988 | 1.4848 | 1.00E-05 | Auxin efflux carrier component 6 | Involved in auxin transport and homeostasis |
| Cla97C11G219090 | 2.3864 | 1.2549 | 1.46E-07 | ABC transporter B family member 4 | Auxin efflux transmembrane transporter |
| Cla97C02G029120 | 0.3858 | −1.3742 | 7.11E-04 | Cytochrome b561 | Auxin-responsive family protein |
| Cla97C05G101410 | 0.3971 | −1.3326 | 8.80E-06 | BRI1 kinase inhibitor 1 | Regulate the sensitivity to BR signal |
| Cla97C10G196435 | 0.3859 | −1.3735 | 5.95E-05 | Gibberellin regulated protein GAST1 | Involved in cell expansion. |
| Cla97C08G148950 | 0.4229 | −1.2416 | 1.37E-06 | Wall-associated receptor kinase 2 (WAK2) | Control cell expansion |
| Cla97C09G175230 | 0.2183 | −2.1956 | 3.39E-03 | Expansin-like B1 | Regulate cell morphology |
| Cla97C04G077500 | 2.2148 | 1.1472 | 5.18E-03 | WUSCHEL-related homeobox 1 | Control leaf expansion and morphology |
| Cla97C07G135270 | 0.4565 | −1.1313 | 1.97E-03 | B3 domain-transcription factor NGA3 | Negatively regulate cell proliferation of lateral organs |
| Cla97C03G056100 | 2.8101 | 1.4906 | 3.17E-08 | MERISTEM LAYER 1 (ML1)-like | Control epidermal cell differentiation |
| Cla97C02G036810 | 0.2989 | −1.7421 | 6.56E-05 | NAC transcription factor 29-like | Involved in leaf morphogenesis |
- a FC CR/YL, the fold change of gene expression in CR to YL plants.
- b The function annotation in Swiss-Prot database.
- c The function description of homologous gene in TAIR database.
3.6 Change of Endogenous Hormone Level in CR Lines
The differential expression genes of gibberellin metabolism are highlighted in the KEGG map of diterpenoid biosynthesis (Figure S3A). The gibberellin synthesis gene Cla97C09G164590 was upregulated in CR lines. This enzyme catalyzes the conversion of GA4 to GA3, enhancing the endogenous production of GA3 (Cheng et al. 2023). In the synthesis process of bioactive gibberellin precursors, the metabolic enzymes Cla97C05G083220 and Cla97C09G172140 of gibberellin biosynthesis were also upregulated. Conversely, in the cytokinin pathway, the UDP-glycosyltransferase 73C3 gene (Cla97C10G202910) was downregulated, potentially inhibiting the breakdown of trans-Zeatin-related products (Figure S3B).
Meanwhile, the endogenous hormone contents were detected. In CR lines, there was a significant increase in the content of indole-3-acetic acid (IAA), GA3, and trans-Zeatin (Figure 5). The content of GA3 (Figure 5A) and tZ (Figure 5B) in CR lines was more than double that of YL.

3.7 ClTCP4 Interacts With WOX1 in Watermelon
By yeast two-hybrid screening, it has been found that ClTCP4 can interact with the WOX1 homolog, ClWOX1 (Cla97C04G077500), in watermelon. The yeast test result indicated that all combinations grew normally on SD/−leu/−trp medium. Notably, only the experimental group (AD-ClTCP4, BD-ClWOX1) and the positive control group grew on SD/−leu/−trp/−his/−ade medium. Meanwhile, the experimental group (AD-ClTCP4, BD-ClWOX1) catalyzed the substrate X-α-gal to turn blue (Figure 6A), which indicated that there is a protein–protein interaction between these two transcription factors.
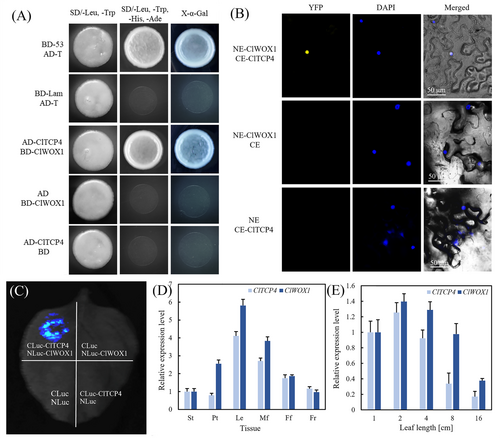
The BiFC assay further confirmed the interaction between ClWOX1 and ClTCP4 (Figure 6B). The interaction signal of the experimental group (NE-ClWOX1, CE-ClTCP4) was observed in the nucleus of lower epidermal leaf cells. In the luciferase complement assay (Figure 6C), yellow fluorescence was also detected in the experimental group (ClWOX1-NLuc, ClTCP4-CLuc). Therefore, these results confirmed the protein–protein interaction between ClWOX1 and ClTCP4.
Tissue-specific expression levels of ClTCP4 and ClWOX1 in YL plants were detected in young stems, petioles, leaves, male and female flowers, and young fruits. These two genes can be expressed in all the above tissues, and both exhibited relatively higher expression in leaves than in other tissues (Figure 6D). During the process of leaf development, the expression patterns and trends of ClTCP4 and ClWOX1 were consistent, and they both showed relatively higher expression levels in young leaves with a leaf length of 1–4 cm (Figure 6E).
3.8 qRT-PCR Analysis of Genes Related to Leaf Morphogenesis
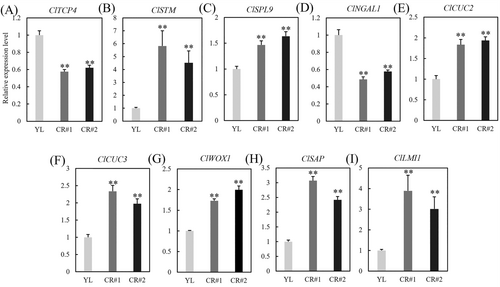
CIN-TCP transcription factors modulate leaf morphogenesis by controlling genes involved in cell proliferation/extension and leaf margin development (Rath et al. 2022; Sarvepalli and Nath 2018). Therefore, the expression patterns of related genes were investigated, including SHOOT MERISTEMLESS (STM), SQUAMOSA PROMOTER BINDING PROTEIN-LIKE 9 (SPL9), NGATHA-LIKE 1 (NGAL1), Cup-shaped cotyledon 2/3 (CUC2/3), LATE MERISTEM IDENTITY1 (LMI1), STERILE APETALA (SAP) and WOX1 (Figure 7). The results revealed that the expression levels of ClSTM (Figure 7B), ClSPL9 (Figure 7C), ClCUC2/3 (Figure 7E,F), ClWOX1 (Figure 7G), ClSAP (Figure 7H) and ClLMI1 (Figure 7I) were significantly upregulated in CR lines, whereas the expression of ClNGAL1 (Figure 7D) in CR lines was significantly downregulated compared to YL plants. Therefore, ClTCP4 may have affected the formation of leaf morphology through transcriptional regulation of these leaf-forming-related genes.
3.9 The Effect of the Interaction Between ClWOX1 and ClTCP4 on the Expression of ClNGAL1
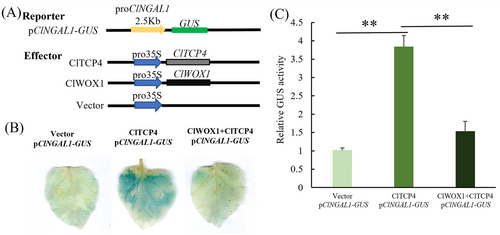
The tobacco transient overexpression system was used to detect the regulatory effect of ClTCP4 on ClNGAL1 (Figure 8A), and the result showed that the GUS staining effect of the test group (ClTCP4, pClNGAL1-GUS) was significantly stronger than that of the control (Figure 8B). The quantitative test result showed that the GUS enzyme activity of the test group (ClTCP4, pClNGAL1-GUS) was four times that of the control (Figure 8C). However, when ClWOX1 and ClTCP4 were co-injected, its GUS staining effect was significantly weakened compared to the other test group (ClTCP4, pClNGAL1-GUS; Figure 8B), and the quantitative detection of GUS enzyme activity also confirmed this (Figure 8C). In conclusion, ClTCP4 can activate the expression of ClNGAL1, while ClWOX1 inhibits the promotion effect of ClTCP4 on the expression of ClNGAL1.
4 Discussion
The knockout of ClTCP4 in watermelon resulted in a significant increase in the levels of auxin, gibberellin, and trans-zeatin (Figure 5). Previous evidence indicates these hormones significantly influence cell proliferation and expansion by moderating the shift from proliferation to expansion, thereby impacting leaf size (Skalák et al. 2019; Wang, Jiang, et al. 2020; Spartz et al. 2012). Therefore, ClTCP4 could affect leaf morphology by regulating the levels of these endogenous hormones.
Similarly, recent research revealed that CIN-TCPs regulated cell development via multiple hormonal pathways in plants. For instance, in Arabidopsis, the diminished activity of TCP3 triggers the activation of the gibberellin signaling inhibitor, GAINSENSITIVE (GAI), which decreases gibberellin levels (Koyama et al. 2010). In this study, the expression of auxin polar transport genes was upregulated in CR lines (Table 1), which indicates ClTCP4 may modulate the auxin homeostasis via changing the expression of these genes. The overexpression of the brassinosteroid receptor gene BRINSENSITIVE 1 (BRI1) is correlated with increased leaf size (Shang et al. 2011; Oh et al. 2011). In this study, the downregulated expression of BR signal suppressor BKI1 (BRI1 kinase inhibitor 1) in CR lines indicates ClTCP4 is a negative regulator in brassinosteroid signaling (Table 1; Figure 6C). Therefore, ClTCP4 may negatively regulate BR signal transduction and subsequently affect the size of watermelon leaves.
CIN-TCPs are pivotal in leaf development and cause phenotypic variations in plants through the control of cell proliferation and cell differentiation processes (Sarvepalli and Nath 2018; Yang et al. 2022). In loss-of-function mutants of CIN-TCPs, the size of epidermal cells was significantly decreased, whereas in gain-of-function mutants of CIN-TCPs, the size of epidermal cells was significantly increased (Sarvepalli and Nath 2011). In this study, elevated cell number and diminished cell size have been observed in CR lines, which is consistent with previous research (Sarvepalli and Nath 2011). Meanwhile, transcriptomic analysis revealed the gene expression changes of cell-proliferation and cell-expansion related genes, potentially implicating their role in ClTCP4-mediated cell development (Table 1).
This study confirms the protein–protein interaction between ClWOX1 and ClTCP4. WOX1 homologs have been recognized for their regulatory roles in leaf expansion and leaf margin morphogenesis (Wang, Zhao, et al. 2020; Wang, Niu, et al. 2020). The ClTCP4 mutation resulted in increased leaf area and reduced cell size. In contrast, the mutations of WOX1 homologs have been associated with decreased leaf area and increased cell size (Wang, Niu, et al. 2020), which implies the potential antagonistic relationship between ClWOX1 and ClTCP4. Furthermore, in cucumber, a transcriptional negative feedback loop between WOX1 and TCP4 is considered to stabilize their expression balance and coordinate them to jointly regulate cell division, cell differentiation, and leaf expansion (Wang, Niu, et al. 2020). In CR lines, ClWOX1 was upregulated and ClTCP4 was downregulated, indicating a negative transcriptional correlation between ClWOX1 and ClTCP4 also exists in watermelon. The negative correlation between ClWOX1 and ClTCP4 may maintain the morphogenesis of watermelon leaves.
In this study, the functional mutation of ClTCP4 in watermelon resulted in significant upregulation of genes associated with serrated/curled leaf margin, including ClSTM, ClSPL9, and ClCUC2/3, while the expression of ClNGAL1 was remarkably downregulated. Previous research has established that CUC2 and CUC3 facilitated leaf serrations during leaf growth, and the upregulated expression of CUC2 induced a serrated and curled margin (Rubio-Somoza et al. 2014). SPL loss-of-function mutants and CIN-TCP gain-of-function mutants exhibited similar phenotypes, which are characterized by diminished leaf size, indicating their antagonism relationship in leaf growth (Wu et al. 2009). Furthermore, STM expression influenced cell proliferation capacity, and its overexpression in Arabidopsis resulted in lobed leaves by activating the expression of CUC genes (Lincoln et al. 1994; Spinelli et al. 2011). In lettuce, overexpression of the STM homolog LsKN1 led to varying degrees of leaf curling (Jia et al. 2022). In our study, the ClTCP4 mutation resulted in the upregulation of ClSPL9 and ClSTM, which could cause the leaf curling by promoting the upregulation of ClCUC2/3 expression (Wu et al. 2009; Spinelli et al. 2011). NGAL1 exerts the negative regulation of serrated leaf margin by repressing CUC2 transcription (Shao et al. 2020). The expression of ClNGAL1 was downregulated in CR lines, which may enhance ClCUC2 expression and increase the number of wavy leaf serrations (Shao et al. 2020). The transient overexpression experiment of tobacco confirmed that ClTCP4 played a positive promoting role in the expression of ClNGAL1, which is consistent with the previous report that CIN-TCP genes in Arabidopsis regulate leaf development by promoting NGA expression (Ballester et al. 2015). Further, the experiment of co-injection of ClWOX1 and ClTCP4 shows that the promotion effect of ClTCP4 on ClNGAL1 is inhibited when ClWOX1 is present. The above results reveal that ClWOX1 may jointly regulate the expression of downstream leaf development genes with ClTCP4 through the protein–protein interaction. In addition, the expression of ClSAP was significantly upregulated in CR lines. In previous studies, the cucumber Little Leaf (LL) gene, an ortholog of Arabidopsis STERILE APETALA (SAP), is associated with a smaller leaf size in the little leaf (ll) mutant (Yang, Liu, et al. 2018). Therefore, ClTCP4 may modulate leaf area via the ClSAP pathway.
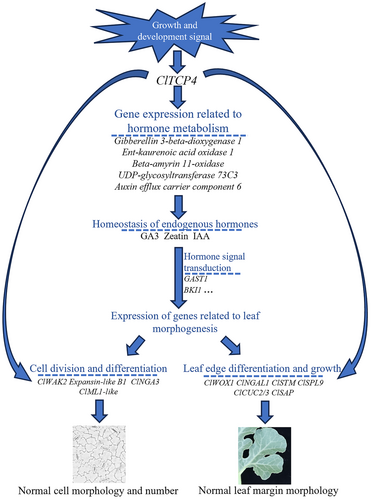
Based on this current research and our previous studies, the possible role of ClTCP4 in leaf morphogenesis is described by a mechanism diagram (Figure 9). In watermelon plants, external environmental cues and leaf developmental signals induce the expression of ClTCP4, and the contents of endogenous hormones (IAA, Zeatin and GA3) related to leaf development remain stable. The downstream gene expression of leaf development is normal, which ensures the process of cell division and cell expansion, as well as margin morphology (Figure 9). In contrast, the gene knockout of ClTCP4 induces expression changes of those genes involved in hormone synthesis and metabolism, causing elevated contents of IAA, Zeatin, and GA3. These changes disrupt the homeostasis of the above hormone pathways and the gene expression associated with leaf development, leading to increased cell proliferation, reduced cell size, and the wavy leaf margin. Overall, the regulatory role of ClTCP4 in watermelon leaf morphogenesis has been confirmed. This study expanded our understanding of the molecular mechanism of leaf morphogenesis and provided gene resources for optimizing leaf morphology.
Author Contributions
H.W., H.N., H.X., and Y.L. designed the experiments. H.W., W.Z., D.Z., X.L., and X.H. conducted the experimental work. H.W., W.Z., X.W., and M.L. performed data analysis. H.W., Q.D., and J.L. wrote the manuscript. J.W., H.N., H.X., and Y.L. reviewed and revised the manuscript.
Acknowledgements
We thank Mr. Liu Yangyang for the suggestions on the preparation of paraffin sections of watermelon leaves.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data supporting the findings in this study are available within the paper and Supporting Information published online.




