Amendment of Microbial Metabolites to Develop Next-Generation Formulations for Enhancing Plant Growth and Resilience
Funding: This work was supported by Anusandhan National Research Foundation, CRG/2023/000193; Indian Institute of Technology Delhi.
ABSTRACT
The environmental concerns linked with the overuse of chemical fertilizers necessitate eco-friendly alternatives for sustainable agriculture. Plant growth-promoting (PGP) bioinoculants offer a viable solution; however, their inconsistent performance and short shelf life limit their widespread application. Microbial metabolites, known for boosting plant growth and stress resilience, present a promising alternative. This study evaluated the effectiveness of cell-based and metabolite-based formulations derived from the PGP strain Bacillus haynessi (SD2) on pigeon pea growth under saline conditions. The experiment involved metabolic profiling of SD2 cell-free supernatant (CFS), followed by the development of cell- (SD2 cells) and metabolite-based (exopolysaccharides and CFS) formulations and their application under controlled and natural conditions. Metabolic profiling of CFS revealed the presence of key metabolites linked to plant growth and stress management. Under salt stress, plant growth, total chlorophyll (31.14%), and potassium content declined, while proline (77.52%), malondialdehyde (44.80%), and sodium uptake increased. Both cell- and metabolite-based formulations mitigated the impact of stress by improving plant growth, chlorophyll content, and antioxidant enzyme activities (catalase and ascorbate peroxidase) while reducing the levels of stress markers and sodium-potassium ion ratio. CFS-based formulations were effective under controlled conditions, but exhibited limited performance in natural environment. In contrast, other formulations demonstrated consistent effectiveness. This study highlights the potential of EPS-based formulations as a sustainable and eco-friendly alternative to traditional cell-based formulations, significantly enhancing crop resilience in saline environments.
1 Introduction
Salinity is a critical environmental stressor and a significant constraint to plant growth, productivity, and yield quality worldwide. Over 20% of the cultivable lands around the globe are seriously affected by salt stress, with various natural and anthropogenic activities expanding the salt-affected areas (Hasanuzzaman and Fujita 2022). Salinity stress leads to ion toxicity, oxidative stress, impaired photosynthesis, and alters plant nutritional and hormonal balance (Seleiman et al. 2023). Legumes offer numerous health benefits and have an enormous influence on soil carbon and nutritional (phosphorus and nitrogen) status (Vaz Patto et al. 2015). Among them, pigeon pea (Cajanus cajan) is rich in protein, minerals, vitamins, and carbohydrates, making it vital for nutritional security. Pigeon pea plants are well adapted to diverse environmental conditions such as drought and high temperatures. However, they exhibit low tolerance toward salinity stress and waterlogging conditions (Awana et al. 2019). The susceptibility of pigeon pea to salinity adversely affects their productivity (Jain et al. 2021).
Plant growth-promoting rhizobacteria (PGPR) are widely recognized for their immense role in mitigating salinity stress in plants (Kumawat et al. 2022; Srivastava and Srivastava 2020). PGPR induce salinity stress tolerance in plants through multiple mechanisms, including maintaining water and ionic balance, enhancing nutrient availability, and facilitating biofilm formation. They also modify root system architecture and regulate antioxidant system, osmoprotectants, and hormonal levels in plants (Bhat et al. 2020; Giannelli et al. 2023). Microorganisms produce diverse metabolites that contribute to plant resilience under biotic and abiotic stress conditions (Salazar et al. 2023). These secondary metabolites comprise different osmoprotectants, volatile organic compounds, exopolysaccharides (EPS), and bioactive compounds known to alleviate the salinity stress in plants (Sunita et al. 2020; Chevrette et al. 2022). EPS, produced by many PGPR, help in biofilm formation, root colonization, and protection against environmental stress (Srivastava and Sharma 2023; Srivastava et al. 2024).
Despite their significant potential, bioinoculants share a small segment of the global agrochemical market due to inconsistent and ineffective field performance and limited shelf life (Timmusk et al. 2017; Basu et al. 2021). To overcome the challenges associated with the application and commercialization of PGPR, bioactive compounds derived from microbes can serve as a promising alternative. These compounds can function alone or in combination with microbial cells, offering a potential strategy to enhance crop productivity and stress resilience, while minimizing the dependency on chemical fertilizers (Naamala and Smith 2021; Morcillo et al. 2022). The incorporation of EPS and cell-free supernatants (CFS) in formulation has emerged as a promising strategy to enhance the efficacy of formulations. A deeper understanding of how microbes and their metabolites affect pigeon pea plants under salt stress conditions could lead to the development of efficient formulations for improving plant growth.
Hence, in the present study, we aimed to develop cell- and metabolite-based formulations using salt-tolerant bacterial strain Bacillus haynessi and its metabolites, viz. CFS and EPS, respectively, for managing salt stress in plants. The individual effects of these formulations on pigeon pea growth under salt stress were evaluated under controlled conditions to identify the most effective strategy for enhancing plant growth in saline environment.
2 Materials and Methods
2.1 Growth Conditions of the Bacterial Strain
Salt-tolerant bacterial strain Bacillus haynessi (SD2; Accession no. OM864284) used in this study was procured from the Environmental Genomics Laboratory repository at the Indian Institute of Technology Delhi. SD2 was initially cultured on nutrient agar (peptone 5.0 g/L, sodium chloride 5.0 g/L, beef extract 1.5 g/L, yeast extract 1.5 g/L, and agar 15 g/L with final pH 7.4 ± 0.2) at 30°C for 24 h. A single bacterial colony from nutrient agar medium was subsequently inoculated into the nutrient broth of the same composition (excluding agar), followed by incubation at 30°C for 48 h under continuous shaking conditions at 160 rpm. For long-term preservation, glycerol stock (25%) of SD2 was prepared and stored at −80°C.
2.2 Metabolite Extraction and Analysis
For metabolite extraction, the CFS of 48 h-grown culture of SD2 was collected and sterilized through a 0.22-μm filter. The metabolites were extracted by adding an equal volume of ethyl acetate and collecting the organic phase. This extraction process was repeated twice, after which the organic phase was evaporated using a rotary evaporator at 30°C. The dried metabolites were then redissolved in ethyl acetate. HPLC analysis of samples was performed on a Dionex Ultimate 3000 system (Thermo Scientific) with separation achieved on a Hypersil Gold C18 column maintained at 30°C. The mobile phases were composed of buffer A (0.1% formic acid in water) and buffer B (0.1% formic acid in acetonitrile), with a constant flow rate of 250 μL/min and a total sample run time of 35 min. Mass spectrometric analysis was conducted on a Q Exactive instrument (Thermo Scientific), operating in positive ionization mode with full MS scan. The scan range was set from 100 to 1500 m/z, with a resolution of 70,000 and an AGC target of 1e6. Sheath gas flow was set to 30 arbitrary units, with the auxiliary gas at 10 arbitrary units. The capillary voltage was maintained at +4 kV, and the capillary temperature at 275°C. The S-Lens RF level was set at 50, and the probe heater temperature was 320°C. Raw data were processed using ThermoFisher Scientific Compound Discoverer 2.1 software.
2.3 Development of Formulations
For cell-based formulation, bacterial strain SD2 was cultured in nutrient broth at 30°C with continuous shaking at 160 rpm. After 48 h of incubation, 1.5 mL of bacterial culture (~9 log10 CFU/mL) was added to talc powder pre-mixed with 1% carboxy methyl cellulose (CMC) and 0.1% glycerol. To prepare EPS-based formulation, SD2 was incubated at 30°C for 7 days at 160 rpm in a rotatory shaker, using the growth medium described by Chambi et al. (2021). After incubation, EPS in the supernatant was precipitated by adding chilled acetone (1:2 v/v) and incubating for 24 h at 4°C. The collected EPS was dried at 40°C and mixed with talc, along with 1% CMC and 0.1% glycerol. The final formulations contained EPS at concentrations of 1% and 2% (w/w). The CFS-based formulation was prepared by adding CFS collected by passing the 48 h-grown SD2 supernatant through a filter (0.22 μm) in the formulation mixture. All formulations were mixed properly and dried under aseptic conditions with ~40% moisture content.
2.4 Plant Growth Conditions
Seeds of Cajanus cajan cv. Maruti were obtained from the International Crops Research Institute for the Semi-Arid Tropics, Hyderabad, India. Seeds were surface sterilized with 0.1% sodium hypochlorite for 15 min and washed thoroughly with distilled water. After sterilization, the seeds were coated with formulations and dried overnight under sterile conditions.
2.4.1 Under Controlled Conditions
The seeds were sown in sterilized soil (~300 g/pot) and maintained in a temperature-controlled plant growth chamber under a 16/8 h light/dark cycle at 30°C. For the first 20 days, plants were irrigated with water every 2 days. Salinity stress was induced on the 21st day by irrigating the plants with 15 mL of 200 mM NaCl solution, followed by a second treatment on the 28th day. Ten days following the onset of stress, plants were harvested, and biometric parameters, including root length, shoot length, and dry weight, were recorded. Additionally, stress markers, chlorophyll content, antioxidant enzyme activity, and sodium-potassium levels were analyzed in the harvested plant tissues.
2.4.2 Under Natural Conditions
The effect of bioformulations on the growth of pigeon pea subjected to salinity stress was assessed in the nursery of the Indian Institute of Technology Delhi, India. Seed sowing was carried out in June 2023, when the average temperature was 31°C (range: 22°C–41°C) and the average relative humidity was 67%. Harvesting took place in April 2024 under an average temperature of 39°C (range: 18°C–40°C) and 48% average humidity. Seeds coated with bioformulation were sown in pots containing approximately 8 kg of non-sterile soil. Plants were treated with salt solution after 20 days of sowing, once seedlings were fully established. Salinity stress was imposed by irrigating the plants with 400 mL of 200 mM NaCl solution twice, that is, on the 21st and 31st days after sowing. Following each salt treatment, plants were irrigated with water to prevent salt buildup while ensuring sufficient hydration. The sampling was done at the flowering stage, and biometric parameters, viz. root length, shoot length, and dry weight of the plants, were recorded. For both controlled and natural conditions, each treatment included eight biological replicates, with each replicate consisting of individual pot containing a single plant.
2.5 Stress Markers and Photosynthetic Pigment Estimation in Plants
Electrolyte leakage (EL) percentage, and proline and malondialdehyde contents were measured in the shoot tissues of pigeon pea plants following the methods of Hniličková et al. (2019), Hodges et al. (1999), and Bates et al. (1973), respectively. Chlorophyll content was estimated by homogenizing 100 mg of tissue in chilled 80% acetone. The absorbance of the extract was measured at 663 and 645 nm, and chlorophyll content was calculated as described by Bruuinsma (1963). All biochemical analyses were conducted using samples from three biological replicates.
2.6 Defense Enzyme Activity in Plants
Activities of defense enzymes were determined by homogenizing shoot tissue (0.5 g) in 5 mL of potassium phosphate buffer (0.1 M; pH 7) containing EDTA (0.1 mM), 1% polyvinyl pyrrolidone, phenylmethanesulfonyl fluoride (100 μM), and 0.3% dithiothreitol. The homogenate was centrifuged, and the resulting supernatants were used for enzyme assays. Superoxide dismutase (SOD) activity was assessed using a reaction mixture containing phosphate buffer (40 mM; pH 7.8), enzyme extract (0.1 mL), nitroblue tetrazolium (75 μM), ethylenediamine tetraacetic acid (0.1 mM), and riboflavin (2 μM). The mixture was incubated under a fluorescent light for 30 min, with a blank sample (reaction mixture without enzyme extract) kept in the dark. Absorbance was measured at 560 nm (Beauchamp and Fridovich 1971). Ascorbate peroxidase (APX) activity was determined following the method of Nakano and Asada (1981). Catalase activity was measured by monitoring the decrease in H2O2 absorbance at 240 nm in a reaction mixture containing enzyme extract, phosphate buffer (50 mM; pH 7) and H2O2 (20 mM) (Aebi 1984). These activities were performed using samples from three biological replicates.
2.7 Estimation of Sodium and Potassium Ion Contents in Plants
Sodium and potassium contents in shoot and root tissues of pigeon pea plants were estimated following the protocol of Horneck and Hanson (1998). In brief, predried powdered tissue (200 mg) was digested in 15 mL acid mix of perchloric acid and nitric acid (3:10) overnight at room temperature. Samples were further digested at 150°C and diluted to 50 mL using distilled water. The resulting filtrate was collected, and Na+ and K+ ions were detected using Flame Photometer. Analysis was done using samples from three biological replicates.
2.8 Statistical Analysis
Statistical analysis was done using one-way ANOVA followed by Duncan's multiple range test in SPSS 16.0 to determine significant differences among treatments. Additionally, an unpaired t-test was carried out in PRISM to evaluate treatment-specific variations.
3 Results
3.1 Growth-Promoting Potential of B. haynessi in the Host Plant, Pigeon Pea
The impact of inoculation of plant growth-promoting bacterial strain SD2 on the growth of pigeon pea plants was evaluated first under controlled conditions (Figure S1). Bacterization of pigeon pea seeds with SD2 resulted in improved growth of plants (Figure S1a). The results displayed significantly enhanced biometric parameters, including shoot length (46.97%), root length (22.91%), fresh weight (43.82%), and dry weight (132.35%) in the SD2-treated plants compared to uninoculated control (Figure S1b,c).
3.2 Metabolite Profiling of Cell-Free Supernatant of SD2
Metabolite profiling of the CFS from SD2 identified a diverse array of compounds belonging to various classes, including alkaloids, amino acids, fatty acids, indanes, vitamins, alcohols, and terpenoids, contributing to different biological functions (Table 1). Notably, metabolites linked to plant stress tolerance were detected, such as pyridoxine, pyrimidoxine, isoleucine, indane, nicotinic acid, coumarin, proline, melatonin, and DL-lysine. Moreover, metabolites involved in plant growth promotion, such as 4-methyl-5-thiazole ethanol, cinnamic acid, DL-tryptophan, and methyl indole-3-acetate, were also detected in CFS. Several metabolites with antibacterial, antifungal, nematicidal, and algicidal properties, including acetophenone, docosonamide, nicotinyl alcohol, crotetamide, hexadecanamide, 3-(propan-2-yl)-octahydropyrrolo[1,2-a]pyrazine-1,4-dione, caryophyllene oxide, styrene, and tryptoline, were produced by SD2. Moreover, two metabolites, oleamide and glutamic acid, which play a potential role in regulating the interaction between plants and microbes in the rhizosphere, were also detected.
| S. No. | Metabolite | Class | Peak area | m/z | Function | References |
|---|---|---|---|---|---|---|
| 1. | Pyridoxine | Vitamin | 3,439,536,824 | 170.08101 | Cofactor of carbohydrate, amino acid, and fatty acid metabolism. Plant protection against oxidative stress | Palacios et al. 2014 |
| 2. | 4-Methyl-5-thiazoleethanol | Alcohol | 3,381,326,366 | 144.04768 | Degradation product of thiamine and crucial for metabolism of purine | Lau et al. 2015 |
| 3. | Indane | Indanes | 2,605,117,927 | 119.08558 | Antimicrobial, antioxidant, and free radical scavenging activity | Meng et al. 2023 |
| 4. | Cinnamic acid | Phenolic acid | 2,493,325,505 | 148.0253 | Stimulates proliferation of lateral and adventitious roots | Steenackers et al. 2019 |
| 5. | Pyridoxamine | Vitamin B6 | 1,495,968,362 | 169.09695 | Plant protection against stress conditions | Parra et al. 2018 |
| 6. | DL-Tryptophan | Amino acid | 1,101,450,992 | 205.09698 | Auxin precursor | Yasmin et al. 2017 |
| 7. | Acetophenone | Ketone | 1,017,311,001 | 121.06481 | Antimicrobial activity | Gutiérrez-Luna et al. 2010 |
| 8. | Methyl indole-3-acetate | — | 972325424.6 | 190.08603 | Phytohormone. Plant growth promoting property | Wang et al. 2022 |
| 9. | Styrene | — | 943547184.9 | 122.0965 | Nematicidal compound | Luo et al. 2018 |
| 10. | 4-Methyl-5-thiazoleethanol | Alcohol | 752488224.1 | 144.04768 | Thiamine metabolism | Wang and Xie 2022 |
| 11. | Cyclo (leucylprolyl) | Alpha amino acid | 609644832.5 | 211.14389 | Antibacterial property | Teregulova et al. 2024 |
| 12. | L-valine | Amino acid | 529324403.3 | 118.08643 | Growth and survival of bacteria | Picchi et al. 2021 |
| 13. | Docosanamide | Fatty acid amide | 521660712.4 | 340.35673 | Antifungal activity | Zeyad et al. 2022 |
| 14. | Isoleucine | Amino acids | 442414035.3 | 132.1019 | Precursor of Jasmonic acid Isoleucine. Plant defense response against abiotic and biotic stresses | Li et al. 2021 |
| 15. | Nicotinyl alcohol | Pyridines | 441846800.7 | 110.06024 | Antimicrobial activity | Alrumman et al. 2019 |
| 16. | Crotetamide | — | 638526714.4 | 227.1753 | Antimicrobial and antioxidant properties | Nchabeleng et al. 2024 |
| 17. | Hexadecanamide | Fatty acid amide | 394752425.3 | 265.26296 | Detected in antagonist Bacillus subtilis and Pseudomonas sp. metabolite fraction | Bharose and Gajera 2018 |
| 18. | 3-(propan-2-yl)-octahydropyrrolo[1,2-a]pyrazine-1,4-dione | — | 363933676.5 | 196.12823 | Detected in chloroform fraction of Streptomyces sp. showing antifungal activity against Candida albicans | Nirwati et al. 2022 |
| 19. | Caryophyllene oxide | Terpenoid | 239553222.9 | 203.17917 | Antifungal activity | Hilgers et al. 2021 |
| 20. | Caprolactam | Amide | 340333495.8 | 114.09154 | Produced by plant growth-promoting Citrobacter amalonaticus and Bacillus safensis | Shen et al. 2021 |
| 21. | Oleamide | Fatty acid amide | 274384508.6 | 282.27893 | Fatty acids enrich rhizosphere and modulate plant-microbe interaction | Mashabela et al. 2022 |
| 22. | 8-hydroxyquinoline | Alkaloids | 220987118.7 | 146.05971 | Antimicrobial, insecticidal and antiparasitic activity | Elkhwaga et al. 2023 |
| 24. | Trans-3-Indoleacrylic acid | Indole | 164183956.1 | 188.07043 | Antimicrobial activity | Boonbangkeng et al. 2022 |
| 25. | 3-amino-2-phenyl-2H-pyrazolo[4,3-c]pyridine-4,6-diol | — | 162386621.2 | 243.08734 | Detected in the methanolic extract of biocontrol agent Paraburkholderia fungorum | Munakata et al. 2022 |
| 26. | Nicotinic acid | Vitamin | 86311810.66 | 124.0394 |
Production of cofactor involved in cellular oxidation–reduction reaction Stress management in plants |
Palacios et al. 2014 |
| 27. | Benzaldehyde | Aromatic aldehyde | 88457906.01 | 107.04933 | Insecticidal, antioxidant and antimicrobial compound | Ullah et al. 2015 |
| 28. | Coumarin | Lactone | 80595303.86 | 334.14731 |
Abiotic and biotic stress management in plants Mediate interaction between plants and belowground microbiome |
Stringlis et al. 2019 |
| 29. | Proline | Amino acid | 80266772.99 | 116.07076 | Reactive oxygen species scavenging, cellular structure stabilization and regulation of cell redox homeostasis in plants under stress | Srivastava and Srivastava 2020 |
| 30. | N-Acetyltyramine | Tyramine alkaloid | 79464771.34 | 180.10176 | Antioxidant and antimicrobial activity | Driche et al. 2022 |
| 31. | Tryptoline | Alkaloid | 78209060.9 | 173.10715 | Algicidal compound | Zhang et al. 2016 |
| 32. | Melatonin | Indoleamine | 73935581.62 | 215.11772 | Plant growth regulator and protecting plants against reactive oxygen species | Jiao et al. 2016 |
| 33. | L-(−)-Methionine | Amino acid | 68700531.7 | 150.05826 | Precursor of various plant growth regulators and important biomolecules (cofactors, polyamines, and vitamins) | Rafique et al. 2022 |
| 34. | DL-Glutamic acid | Amino acid | 61975226.09 | 148.06035 | Regulates the composition of plant-associated microbiome | Kim et al. 2021 |
| 35. | DL-Lysine | Amino acid | 54676670.09 | 147.11275 | Regulates plant response under stress by inducing tryptophan metabolism and jasmonate signaling pathway | Yang et al. 2020 |
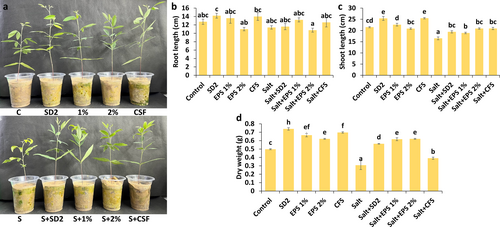
3.3 Effect of Different Formulations on Biometric Parameters of Pigeon Pea Under Salinity Stress Condition
The study examined the effect of cell-based (SD2) and metabolite-based (CFS and EPS) formulations on the growth parameters of pigeon pea plants grown under salinity stress (Figure 1a–d). Salinity stress significantly reduced physical attributes such as shoot length and dry weight (Figure 1c,d), with a reduction of 23.25% and 38.77%, respectively, compared to non-stressed control plants. Stressed plants treated with formulations displayed improved health, which was evident in significantly higher shoot length and dry weight than non-treated stressed plants. The shoot length of the formulation-treated plants remained comparable across all formulations under stress conditions. Among the treatments, the EPS-based formulation showed the highest increase in dry weight (EPS 1% [100.48%] and EPS 2% [102.00%]), followed by SD2 (82.83%) and CFS (26.97%) formulations when compared to untreated stressed plants. Root length remained unaffected by salinity stress and treatments, regardless of growth conditions (Figure 1b).
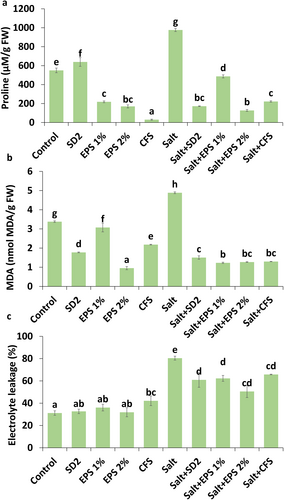
3.4 Stress Markers and Chlorophyll Content in Pigeon Pea Plants Under Salinity Stress
The level of osmoprotectant proline was found to be enhanced by 77.52% in pigeon pea plants subjected to salinity stress compared to control plants (Figure 2a). However, stressed plants treated with formulations exhibited reduced proline levels in the shoot compared to untreated stressed plants. The most significant reduction in proline was observed in plants treated with 2% EPS, followed by those treated with SD2, CFS, and 1% EPS formulations. Similarly, MDA content, a marker of oxidative stress, was significantly elevated in salt-stressed plants (44.80%) relative to control plants (Figure 2b). Treatment with formulations effectively alleviated the impact of salt stress, reducing MDA levels by 69.26%, 75%, 73.97%, and 73.56% in plants treated with SD2, 1% EPS, 2% EPS, and CFS, respectively, compared to untreated stressed plants. Electrolyte leakage, another stress indicator, was also markedly higher in salt-stressed plants (80.43%) than control plants (31.16%) (Figure 2c). A notable reduction of electrolyte leakage was observed in all formulation-treated plants under stress conditions.
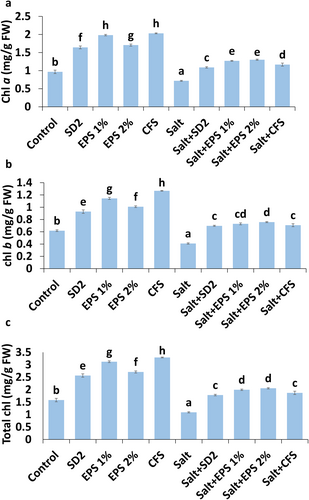
Both cell-based and metabolite-based formulations positively influenced the chlorophyll content of plants under both non-stressed and stressed conditions. Under control conditions, the highest increase in total chlorophyll content was observed with the CFS-based formulation (108.135%), followed by 1% EPS (97.24%), 2% EPS (71.32%), and SD2 (62.28%) formulations. Salinity stress significantly reduced photosynthetic pigments in pigeon pea plants, with decreases of 25.71% in chlorophyll a, 33.76% in chlorophyll b, and 31.14% in total chlorophyll, compared to the control (Figure 3a–c). Among different treatments, 2% and 1% EPS-based formulations significantly enhanced the levels of chlorophyll a (81.29% and 76.83%), chlorophyll b (84.97% and 78.21%), and total chlorophyll (88.73% and 83.26%) in stressed pigeon pea plants. Additionally, the CFS and SD2 formulations enhanced total chlorophyll content in stressed plants by 71.78% and 63.70%, respectively, compared to untreated stressed plants.
3.5 Sodium and Potassium Ion Levels in Pigeon Pea Plants Grown Under Salinity Stress
Differences in the level of sodium content were observed in pigeon pea plants subjected to saline conditions with different treatments (Figure 4a). Untreated plants grown under salinity accumulated significantly higher sodium levels in shoots (2.59 ppm) and roots (1.23 ppm) compared to control plants. All formulation treatments led to a notable reduction in shoot sodium ion levels. The maximum reduction was recorded in plants treated with SD2 (38.75%) and CFS (37.02%), followed by 2% (24.07%) and 1% EPS (22.76%) compared to non-treated stressed plants. In root tissues, SD2-treated plants showed a marked decrease in sodium ion content, whereas other treatments resulted in either comparable (1% EPS) or enhanced sodium levels (2% EPS and CFS) in plants under saline conditions. Salinity stress also significantly reduced potassium levels in both root and shoot compared to control plants (Figure 4b). Different treatments significantly improved the potassium ion content in shoot by 59.37% (SD2), 74.02% (1% EPS), 70.95% (2% EPS), and 94.48% (CFS) relative to untreated stressed plants. Similarly, potassium ion levels in root tissues were enhanced across treatments, with 2% EPS-treated plants exhibiting the highest potassium ion content in roots.
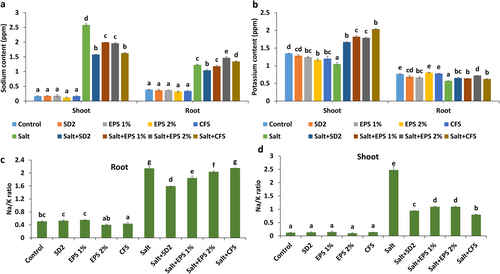
The ratio of sodium and potassium ions was elevated in both shoot and root tissues of pigeon pea plants exposed to saline conditions compared to non-stressed plants (Figure 4c,d). In shoot, treatment with SD2 and its CFS significantly reduced the sodium/potassium ion ratio, followed by plants treated with EPS formulations, compared to untreated stressed plants (Figure 4c). In roots, a higher sodium/potassium ion ratio was observed in plants treated with 2% EPS and CFS, while a lower ratio was recorded in plants treated with SD2 and 1% EPS formulations (Figure 4d).
3.6 Defense Enzyme Activity in Pigeon Pea Plants Grown Under Salinity Stress Condition
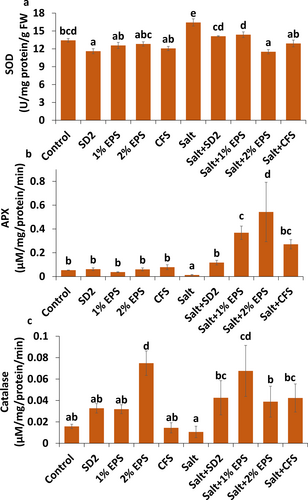
Superoxide dismutase activity in plants was increased by 22.35% in response to salt stress compared to control plants (Figure 5a). However, a significant reduction in the level of SOD was observed in stressed plants treated with formulations compared to untreated stressed plants. The greatest reduction was observed in plants treated with 2% EPS (29.96%), followed by CFS (21.43%), SD2 (14.06%), and 1% EPS (12.48%). In contrast, the activities of catalase and ascorbate peroxidase (APX) were significantly reduced under salinity stress, with a reduction of 75.81% and 32.75%, respectively, compared to control plants (Figure 5b,c). Formulation treatments notably enhanced these enzyme activities in stressed plants. The 2% EPS-based formulation resulted in the highest increase in APX activity (4107.78%), followed by 1% EPS (2754.15%), CFS (2001.47%), and SD2 (808.21%) treatments compared to stressed plants. Similarly, the maximum improvement in catalase activity was recorded in plants treated with the 1% EPS formulation, showing a 535.84% increase under salt-stress conditions.
3.7 Effect of Different Formulations on Growth of Pigeon Pea Under Natural Conditions
The impact of bioformulations on the growth of pigeon pea plants under salt stress in natural conditions was evaluated by measuring root length, shoot length, and dry weight (Table 2). Results showed no significant difference in root length among treatments, regardless of growth conditions. However, plants treated with SD2 showed significantly higher root length than other treatments. Shoot length increased by 32.52%, 26.54%, 38.25%, and 24.16% in plants treated with SD2, 1% EPS, 2% EPS, and CFS, respectively, under non-saline conditions compared to the control. The impact of salinity stress was not observed on the shoot length of the non-treated salt-stressed plants compared to the control. However, plants treated with 1% and 2% EPS showed significantly higher shoot length than both non-inoculated control and stressed plants. The dry weight of the stressed plants declined by 60.50% as compared to non-stressed plants. The plants treated with CFS bioformulation showed a significantly similar dry weight as stressed plants. However, treatment with SD2, 1% EPS, and 2% EPS resulted in an increment in dry weight by 106.37%, 181.42%, and 150.09%, respectively, under saline conditions compared to non-treated stressed plants.
| Treatments | Root length (cm) | Shoot length (cm) | Dry weight (g) |
|---|---|---|---|
| Control | 11.80 ± 1.93a | 83.73 ± 2.36a | 12.66 ± 4.49cd |
| SD2 | 19.93 ± 2.92c | 110.96 ± 8.29bcd | 17.00 ± 2.16e |
| 1% EPS | 16.46 ± 2.64abc | 105.96 ± 10.57bc | 11.33 ± 0.47bcd |
| 2% EPS | 13.90 ± 1.87ab | 115.76 ± 0.75cde | 11.33 ± 2.62bcd |
| CFS | 13.70 ± 0.56ab | 103.96 ± 3.49bc | 8.00 ± 0.81ab |
| Salt | 13.50 ± 2.40ab | 93.40 ± 7.14ab | 5.33 ± 0.47a |
| Salt+SD2 | 17.63 ± 3.97bc | 97.60 ± 19.93abc | 11.00 ± 5.71bcd |
| Salt+1% EPS | 13.40 ± 0.92ab | 127.46 ± 1.88de | 15.00 ± 1.63de |
| Salt+2% EPS | 15.76 ± 1.35abc | 132.80 ± 2.47e | 13.33 ± 0.94cde |
| Salt+CFS | 12.40 ± 2.19ab | 96.66 ± 5.61abc | 9.33 ± 0.94abc |
- Note: Different letters above bars denote statistically significant differences at p = 0.05.
4 Discussion
Salinity stress poses a critical threat to global agriculture, severely impairing plant growth, disrupting nutrient uptake, and leading to significant yield losses in affected regions. Conventional methods to combat salinity are often unsustainable and cost-intensive (Shrivastava and Kumar 2015). In contrast, formulations using beneficial microbes such as PGPR and EPS offer a natural and effective solution and represent a transformative, eco-friendly approach (Srivastava et al. 2024). These natural amendments not only enhance plant resilience to salt stress but also promote sustainable agriculture by improving soil health and reducing dependency on chemical inputs (Navarro-Torre et al. 2023). Henceforth, in light of the increasing challenge of soil salinization, the present study aimed to evaluate the potential of different formulations developed using PGPR (SD2) and its metabolites (EPS and CFS) in enhancing plant tolerance to salinity stress.
We earlier demonstrated that bacterial strain SD2 withstood high salinity levels and exhibited several key plant growth-promoting traits, including phosphate solubilization, auxin production and biofilm formation in the presence and absence of NaCl (Srivastava et al. 2024). PGPR and its metabolites have gained significant attention for enhancing plant growth and stress resilience (Marra et al. 2022). Bacteria synthesize a range of secondary metabolites, including amino acids, vitamins, carbohydrates, lipids, and fatty acids, that contribute to stress tolerance, plant development, and plant-microbe interaction, and exhibit antimicrobial properties (Krishnamoorthy et al. 2022). Metabolomic profiling of the CFS from the bacterial strain SD2 revealed the presence of diverse compounds, such as fatty acids, vitamins, amino acids, alkaloids, and phenolics.
The detrimental impact of salinity stress on plant growth has been well studied (Zuzunaga-Rosas et al. 2024). In this study, plants subjected to salt stress exhibited lower shoot length and dry weight of the plants compared to those grown under control conditions. The decline in growth parameters under stress can be associated with water loss, inhibited cell division, reduced total biomass, and a decreased number of leaf-bearing organs (Jamshidi Goharrizi et al. 2020). Salt-tolerant microorganisms with plant growth-promoting properties in the rhizosphere are highly beneficial for alleviating salt stress and promoting plant growth in saline environments (Kumawat et al. 2023). The present study also found improved growth of pigeon pea plants treated with SD2-based bioformulation, underscoring their efficacy in promoting plant growth. The improved biometric parameters of salt-stressed pigeon pea plants treated with CFS-based bioformulation demonstrate the beneficial impact of these metabolites on stress management and growth. PGPR contributes to plant growth and stress alleviation through different mechanisms, including the production of vitamins and antibiotics (Mekonnen and Kibret 2021). The interplay between amino acids, defense priming in plants, and growth is well established (Cai and Aharoni 2022). These molecules contribute essentially to metabolic and signaling pathways vital for plant health and resilience. The presence of different amino acids, including tryptophan, isoleucine, valine, methionine, glutamic acid, and proline, in CFS might have been involved in improving growth and stress tolerance in pigeon pea plants under salt stress conditions. Shah et al. (2022) similarly found CFS-mediated salt stress mitigation in soybean plants, reporting improved fresh and dry weight of shoot and increased leaf area under stress. Moreover, the enhanced plant growth observed with EPS-supplemented bioformulation under salt stress may be attributed to the cation-binding properties of EPS, which form a physical barrier around roots, limiting salt availability to the plants (Kumar, Kumar, et al. 2020).
Survival of plants under salinity stress relies on several key mechanisms, including the accumulation of osmoprotectants, induction of antioxidant enzymes, and maintenance of ion homeostasis (Gupta and Huang 2014). Proline, an osmoprotectant produced by plants in response to salt stress, helps regulate photosynthesis, protect cell membranes, and quench hydroxyl radicals. Its elevated accumulation indicates a higher extent of stress in plants (Jain et al. 2013; Hayat et al. 2012). In this study, the elevated level of proline in stressed plants reflected the detrimental effect of salt, while the reduced proline accumulation in bioformulations-treated plants demonstrates the effectiveness of the treatments in mitigating salt-induced damage. MDA, a byproduct of lipid peroxidation, and EL are commonly used markers to assess the extent of cell damage caused by stress on plants (Hnilickova et al. 2021; Soltabayeva et al. 2021). Notably, plants exposed to salinity stress showed a significant increase in the level of MDA and EL, indicating a higher level of stress-mediated damage to the plants. The protective role of all bioformulations was observed in terms of reduced lipid peroxidation and EL of the plants exposed to saline conditions, representing a remarkable impact of SD2, CFS, and EPS on the higher membrane stability and reduced oxidative damage to the plants (Mousavi et al. 2022). Liu et al. (2022) also witnessed reduced MDA levels and EL in salt-stressed maize plants bacterized with EPS producing halotolerant Pseudomonas simiae.
During salt stress, the excess production of reactive oxygen species (ROS) is common in plants. ROS are highly reactive molecules, and when present in higher concentrations, they trigger destructive processes that cause significant cellular damage in plants (Kesawat et al. 2023). In response, plants activate various antioxidant enzymes as part of their defense mechanism to scavenge ROS and prevent stress-induced cellular damage. SOD serves as the first line of defense, catalyzing the dismutation of superoxide radicals into hydrogen peroxide and molecular oxygen (Cavalcanti et al. 2004; Mishra et al. 2023). Increased SOD activity is linked to the adaptive response of plants grown under saline conditions. The present study also observed a higher level of SOD in stressed pigeon pea plants not treated with bioformulations. However, SOD activity alone is insufficient to fully protect plants from ROS; other antioxidant enzymes responsible for the removal of hydrogen peroxide are crucial for imparting tolerance (Arora et al. 2002; Kavian et al. 2022). The lower activity of APX and catalase in salt-stressed plants indicates their sensitivity to saline conditions. Conversely, increased APX and catalase activity in bioformulation-treated stressed plants highlights the substantial role of SD2 and its metabolites (CFS and EPS) in eliminating hydrogen peroxide and reducing the membrane damage caused in a saline environment, thereby protecting plants against oxidative stress.
Under salinity stress, Na+ translocates from roots and accumulates in the aerial parts of plants via xylem, leading to toxicity. This accumulation disrupts the Na+/K+ balance, inhibiting cytosolic functions and interfering with enzymes crucial for respiration and photosynthesis (Jacoby et al. 2016; Bhat et al. 2020). Additionally, K+ play an important role in cell homeostasis, elongation, photosynthesis, and osmotic regulation in plants, but higher levels of Na+ disrupt the K+ uptake in plants (Kumar, Singh, et al. 2020). Enhanced accumulation of K+ is a key mechanism to mitigate the harmful effects of Na+ (Etesami and Beattie 2018). The higher accumulation of Na+ in pigeon pea plants under salt stress is in congruence with the previous study that reported elevated Na+ levels in liquorice under high salt conditions (Shen et al. 2022). However, different formulations, including SD2, EPS, and CFS, significantly reduced Na+ accumulation in shoots, with the Salt+SD2 and Salt+CFS treatments showing the most pronounced reductions. Interestingly, these treatments also led to a higher accumulation of Na+ in roots, suggesting possible ion sequestration in roots and restricted translocation to the aerial plant parts. This differential distribution highlights a potential mechanism of salt stress mitigation via localized Na+ compartmentalization in roots.
On the contrary, the lowest accumulation of K+ in salt-stressed plants suggests the antagonistic relationship between Na+ and K+ uptake. Bioformulation treatments reversed this trend, especially Salt+CFS and Salt+EPS 2%, which significantly improved shoot K+ levels and restored the Na+/K+ balance. The observed changes suggest that PGPR and their derivatives may enhance selective K+ uptake or reduce Na+ toxicity, possibly through stabilization of membrane transporters or indirect hormonal regulation. While we did not directly assess transporter gene expression, these physiological responses point toward improved ionic homeostasis under bioformulation treatment.
Salinity stress led to a significant reduction in the levels of chlorophyll a, b, and total chlorophyll in plants compared to control plants. This decline in photosynthetic pigments aligns with the increased accumulation of Na+ and the subsequent decrease in K+ under salt stress. The elevated levels of Na+ in plants disrupt osmotic balance and impair chloroplast function, which in turn contributes to chlorophyll degradation and reduced photosynthesis (Lu et al. 2023). Conversely, the impact of different formulations on improved K+ uptake and reduced Na+ levels in shoots further enhanced the level of chlorophyll content in plants, suggesting that the mitigation of ionic toxicity helped preserve chloroplast integrity and pigment synthesis.
All the formulations, including cell-based (SD2) and metabolite-based (EPS and CFS), significantly improved the growth of pigeon pea plants under stress conditions in a controlled environment; their real-world effectiveness was further validated under natural conditions. This step ensures that the observed benefits extend beyond controlled environments, confirming their potential for practical agricultural use. Unlike controlled conditions, the effectiveness of the CFS-based formulation was reduced under natural conditions. The dry weight of the CFS-treated stressed plants was significantly similar to the untreated stressed plants. The inefficiency of the CFS-based formulation may be attributed to the instability of CFS to different environmental conditions, such as susceptibility to high temperature and salt concentrations (Pellegrini et al. 2020). In contrast, both SD2 and EPS-based bioformulations had a substantial impact on the growth of pigeon pea plants under stress conditions. Among both, the study found the bioformulation comprised of 1% EPS to be the most effective under natural conditions, followed by 2% EPS and SD2 bioformulations. However, this was based only on plant growth attributes for the experiment under natural conditions. Future work could encompass the quantification of other fitness parameters, including stress markers. Also, by employing a method to track the applied strain as bioformulation, its enhanced survival and thereby efficiency can be ascertained in the next experiments.
5 Conclusion
In conclusion, this study highlights the potential of metabolite-based formulations, particularly those utilizing EPS, as sustainable and eco-friendly strategies to improve crop resilience under saline conditions. Both cell-based and metabolite-based formulations demonstrated significant improvements in plant growth, stress tolerance, and biochemical markers in pigeon pea plants. While CFS-based formulation was effective only under controlled conditions, its limited performance in natural environments emphasizes the need for further optimization to improve its efficacy in natural conditions. In contrast, EPS-based formulation improved plant growth and stress mitigation, making them a viable alternative to cell-based formulations. These findings open new avenues for the development of metabolite-based next-generation formulations. In the future, studies should focus on intensifying these formulations for field applications and assessing their stability and function in different environmental conditions. In addition, the functional efficacy of metabolite-based formulations should be evaluated after storage to assess their efficiency over extended periods.
Author Contributions
S.Sri.: methodology, formal analysis, investigation, writing – original draft. A.B.: investigation. V.C.A.: investigation. S.Sha.: conceptualisation, supervision, project administration, writing – review and editing.
Acknowledgments
S.Sri. wishes to thank IIT Delhi for fellowship. Funding received from ANRF is acknowledged (CRG/2023/000193). S.Sha. wishes to thank the TATA Transformation prize in Food Security (2023) and the Batch of 1980 Chair Professor position for the support. The authors acknowledge the central instrumentation facility of Delhi University, South Campus, for LC–MS analysis.
Open Research
Data Availability Statement
Data sharing are not applicable.




