Microfluidic communications in characean internodes at neutral and alkaline external pH
Abstract
Intracellular communications mediated by cytoplasmic streaming compensate for the slowness of diffusion on large scale distances. In characean internodes, cyclosis serves to smooth concentration gradients related to local structural distinctions, irregular spotted illumination, and patterned profiles of external pH. In dimly lit Chara cells, the fluidic transmission of reducing equivalents from the spot of bright light incidence to a remote analyzed area transiently elevates the actual yield of chlorophyll fluorescence (F′) under natural acidic zones with little effect on F′ under alkaline bands. Here, the natural formation of alkaline zones was imitated by placing the internodal cell part into a solution with a pH of 9.5. Using PAM microfluorometry, we found that chloroplasts located under an alkaline solution retained the perception of reducing equivalents transported with the fluid flow but, in addition, became responsive to another transportable metabolite that promoted strong quenching of both F′m and F′ fluorescence. The superposition of oppositely directed F′ responses to distinct cyclosis-transported metabolites resulted in the seeming suppression of microfluidic interactions between distant chloroplasts. The action potential generation did not affect F′m fluorescence (an indicator of non-photochemical quenching, NPQ) when the cell was bathed at neutral pH but induced strong NPQ in the high pH solution. We propose that the restricted CO2 supply at high external pH induces the rearrangement of electron transport to alternative pathways, which elevates the background level of NPQ-promoting metabolite (supposedly H2O2), thus enhancing the chloroplast sensitivity to H2O2 portions delivered with the fluid flow from the region subjected to intense local light.
1 INTRODUCTION
Cytoplasmic streaming (cyclosis) is a basic biological property occurring in many plant cells. It is particularly important for giant cells where diffusion rates are insufficient to ensure reliable intra- and intercellular communications (Pickard, 2003; Shimmen, 2007; Tominaga and Ito, 2015; Goldstein and van de Meent, 2015; Lu and Gelfand, 2023). Although the transport role of cyclosis in cell metabolism is generally recognized, its specific functions are not well-known. Cytoplasmic flow helps to transport substances that are unevenly distributed between cell areas having either structural distinctions or subjected to nonuniform light or other physicochemical factors. Giant internodal cells of Characeae exhibiting rapid cytoplasmic streaming are suited perfectly for studying fluidic communications because in the light they are naturally exposed to patterned external pH (pH banding phenomenon; Beilby and Bisson, 2012; Eremin et al., 2019) and since illumination in their habitats is often nonuniform and subject to fluctuations.
Light-dependent formation of alternating acid and alkaline cell regions in characean algae is regarded as an adaptation that facilitates the acquisition of inorganic carbon from slightly alkaline bulk water by converting HCO3− in the acidic zones into uncharged membrane-permeable CO2 molecules (Plieth, Tabrizi and Hansen, 1994; Bulychev et al., 2001; Raven and Hurd, 2012). The clear segregation of H+-excretion and H+-inflow zones under illumination is also typical of lower (abaxial) and upper (adaxial) leaf sides in aquatic plants (Elzenga and Prins, 1989). The cell regions adjacent to zones with slightly acidic external pH (pHo) are better supplied with carbon dioxide than those contacting the alkaline zones (Walker, Smith and Cathers, 1980). Despite different rates of CO2 entry at the acid and alkaline zones in Chara, the uneven distribution of this photosynthetic substrate over the cytoplasmic layer is partly smoothed out by rapid cytoplasmic streaming. However, when the microfluidic delivery of inorganic carbon from CO2-sufficient towards CO2-depleted cell regions is interrupted upon the action potential generation and cyclosis cessation, the heterogeneous distribution of photosynthetic electron transport rates and thermal losses of chlorophyll excitations become tremendously enhanced along the cell length (Krupenina et al., 2008; Bulychev and Krupenina, 2024).
The functional significance of fluid flow is not limited to the lateral transport of inorganic carbon. The substances transported by cyclosis comprise H2O2 produced in excessive amounts upon bright local illumination of Chara internodes (Eremin, Bulychev and Hauser, 2013), as well as reducing equivalents (Bulychev and Komarova, 2015; Bulychev et al., 2021) exported from chloroplasts by the malate valve and triose phosphate translocator (Taniguchi and Miyake, 2012; Selinski and Scheibe, 2019; Fakhimi and Grossman, 2024). The dispersal of reactive oxygen species (ROS) over the whole cell from the site of their local production helps to recruit additional resources for ROS detoxification. On the other hand, the fluidic relocation of excess products of light reactions (NADPH) from brightly lit cell parts to shaded regions may promote net photosynthetic fixation in light-limited areas.
The movement of reducing equivalents with the streaming cytoplasm is evident from the transient increase in chlorophyll (Chl) fluorescence yield F′ (Ft in other notation) in the analyzed area of interest (AOI) following the application of an intense 30-s local light (LL) pulse to a distal region on the upstream cell side (Bulychev and Komarova, 2015). The distance between the place of the LL stimulus and the analyzed area can be as large as 10 mm and possibly more (Bulychev et al., 2021). In this case the peak of F′ fluorescence at room temperature appears after a long lag period, up to 150 s, from the onset of the LL pulse, which suffices for the microfluidic delivery of reducing metabolites from the site of their production to a dim-lit area of the fluorescence assay. The delay between the onset of the LL stimulus and the peak of F′ emission increases upon retardation of streaming at cool temperatures as predicted by the reciprocal relation between travel time to a fixed distance and the velocity of fluid movement. The largest amplitudes of LL-induced F′ transients are observed at short (~1.5 mm) distances between AOI and the point of LL incidence.
Cyclosis-mediated F′ transient responses to illumination of a distal cell site are best pronounced in Chara cell areas underlying acid zones and are small in chloroplasts located under alkaline zones (Bulychev and Komarova, 2017; Bulychev and Rybina, 2018; Bulychev and Krupenina, 2019). It should be noted in this regard that long-distance communication between anchored chloroplasts is a multistep process. It includes the export of excessively produced photometabolites from brightly lit plastids, the lateral fluidic transport of metabolite package along the cell length, the translocator-mediated entry of reducing substances from the streaming cytoplasm into shaded chloroplasts, and the acquisition of imported reductants via the NADH dehydrogenase-like (NDH) complex (Shikanai and Yamamoto, 2017; Ogawa, Suzuki and Sonoike, 2021) with the eventual reduction of the plastoquinone pool and the photosystem II (PSII) quinone acceptor QA. Some of these stages are apparently sensitive to plasma-membrane H+ flows and cytoplasmic pH. The attempts to identify these stages revealed that the steps occurring in source chloroplasts subjected to a bright light pulse, unlike those in sink plastids exposed to dim light, are largely insensitive to H+ fluxes between the cytoplasm and the apoplast. Specifically, the F′ transients induced in low-pHo cell regions after the application of a remote LL pulse were equally large regardless of whether LL was directed to cell sites with high or low surface pH (Bulychev and Rybina, 2018). By contrast, the F′ transients recorded in cell regions with alkaline pHo were small upon directing LL to zones with both acid and alkaline cell surface pH.
The origin of the close relation between the LL-induced F′ transients (F′LL signal), external pH (pHo), and, probably, cytoplasmic pH (pHcyt) is presently unclear. Available data may provide an impression that reducing equivalents fail to enter recipient chloroplasts underlying alkaline zones, and this would prevent plastoquinone reduction and the release of photochemical quenching at the peak of the FLL signal. The induction of non-photochemical quenching (NPQ) caused by the microfluidic transfer of another LL-induced metabolite might also diminish the F′LL signal, but it seemed initially an unlikely option considering the cells were exposed to very low irradiance (10–15 μmol m−2 s−1). However, this possibility cannot be ruled out without experimental tests. In order to elucidate the factors affecting F′ responses to local illumination of a distal cell region, the treatment with elevated external pH in the analyzed area might be useful. It is known that the plasma membrane (PM) of characean internodes placed in alkaline media, with pH between 9 and 11 roughly resembling pericellular pH in the alkaline zones, becomes highly permeable to H+ or OH− and that the pH dependence of the PM electric potential is similar to that of H+-selective electrodes (Bisson and Walker, 1980, 1981; Beilby, 2015). The exposure of internodal cells to high pH imitates the natural occurrence of light-dependent alkaline bands and can be used for studying the impact of high passive H+ conductance of PM on photosynthesis. Since H+ (OH−) distribution across the PM is close to equilibrium at high pHo, no substantial steady-state fluxes of these ions are expected. The imitation of alkaline bands allows analysis of fluorescence parameters at low irradiances that are insufficient for the formation of pH banding patterns in intact cells. No such experiments have been undertaken to date. Unlike the established influence of high pHo on the conductance and membrane potential of PM, the effects of alkaline media on microfluidic transmission of photometabolites remain largely unexplored.
The results of this study show that the replacement of a standard bath solution (pH 7.0) with an alkaline medium (pH 9.5 and 10.6) on a central part of Chara internodal cell greatly reduces the threshold background irradiance sufficient for NPQ development upon local illumination of a distal cell part. A composite shape of F′LL signals at high pHo suggests that the metabolite “package” produced at the site of LL application and delivered by cyclosis to AOI contained two agents. One of them attenuated photochemical quenching (elevated F′) in recipient chloroplasts while the other enhanced NPQ and diminished F′ at low irradiance. The opposite action of two transportable constituents on F′ fluorescence accounted for strong suppression and specific kinetics of F′LL transients under alkaline environment. The delivery of reducing substances to chloroplasts in AOI was actually unaffected at high external pH. The origin of distinct microfluidic communications in zones of H+ excretion and zones of passive H+ influx in photosynthesizing cells has thus been partly clarified.
2 MATERIALS AND METHODS
2.1 Plant material
The alga Chara australis was grown in the laboratory under natural daylight as previously described (Bulychev and Komarova, 2015). Internodal cells measuring 5–6 cm in length and approximately 0.9 mm in diameter were excised and kept in artificial pond water (APW) containing 0.1 mM KCl, 1.0 mM NaCl, and 0.1 mM CaCl2 for at least one day before the measurements. The pH was adjusted to 7.0 with NaHCO3. The isolated internode was placed in a transparent experimental chamber having three compartments (Bulychev, Shapiguzov and Alova, 2023). A narrow central compartment was separated from the large side compartments by air gaps of 4 mm in diameter. At the beginning of the experiments all three pools were filled with standard APW for assaying Chl fluorescence under control conditions. Thereafter, the solution in the central compartment was replaced with a similar solution having a pH of 9.5 or 10.6. The APW with pH 9.5 was prepared by adding 10 mM Tris buffer (Tris-(hydroxymethyl) aminomethan, Serva) or, alternatively, 10 mM carbonate–bicarbonate buffer. Sodium carbonate and sodium bicarbonate were from Sigma Aldrich and Acros Organics, respectively. Bicine (N,N-Bis(2-hydroxyethyl)glycine) was from Serva. Tris-containing APW was prepared on the day of the experiment. The carbonate–bicarbonate buffer was prepared in APW as a 0.1 M stock solution and was diluted tenfold with APW prior to the experiments. The APW with pH 10.6 was obtained by adding Na2CO3 and NaHCO3 at a different molar proportion of these salts (7:1) under the same total concentration (10 mM).
2.2 Chlorophyll microfluorometry
Chlorophyll fluorescence yields F′ (Ft) and F′m were measured by means of a Microscopy-PAM (pulse amplitude modulated) fluorometer (Walz) that was mounted on an Axiovert 25-CFL inverted microscope equipped with a × 32/0.4 objective lens (Bulychev and Rybina, 2018). Fluorescence was assayed in the central compartment on a cell area with a diameter of ~100 μm (area of interest, AOI). During measurements, the internodal cell was exposed to continuous low intensity light. This background light (BGL) was obtained from the upper light source of the microscope and attenuated with blue-green (SZS-22, λ < 580 nm) and neutral density glass filters to the photon flux density of 12–16 μmol m−2 s−1. The modulated measuring light and saturation light pulses were generated with a blue LED installed in the Microscopy-PAM instrument. The output signal of the photomultiplier was processed by the WinControl 3 software (Walz) and displayed on a monitor using a PCI-6024E analog-to-digital converter (National Instruments) and the WinWCP program (Strathclyde Electrophysiology).
2.3 Local illumination of a cell region
In order to reduce the number of experimental variables, the external pH was shifted only in the central compartment comprising AOI, whereas solutions in the side compartments and the cell area exposed to local light remained unaffected. The LL pulse of 30 s duration was applied using a quartz optic fiber (400 μm in diameter) and a computer-controlled LED as a source of white light (photon flux density, 500 μmol m−2 s−1). The tip of the optic fiber was set opposite to a cell portion in the air gap, at a distance of 4 mm from the AOI. Preliminary experiments showed that the illumination of the air-exposed cell part was as effective as the illumination of submerged cell portions. Thus, the replacement of APW with alkaline medium in the central pool had no influence on cell surface pH at the point of LL application. Accordingly, the composition of the cytoplasmic package produced during LL exposure remained presumably identical before and after the replacement of APW with a high pH medium.
2.4 Determination of plasma membrane resistance and membrane potential
The resistance of the PM was determined by passing electric current pulses between Ag/AgCl electrodes fixed in the side pools and the central pool of the experimental chamber comprising the Chara internode. The density of the current pulses was approximately 1 μA cm−2 and the pulse duration was 250 ms. The PM resistance was estimated from the shifts of membrane voltage produced by the injection of current pulses. The shifts of membrane potential were recorded with extracellular electrodes placed in different sections of the experimental chamber. The absolute values of the cell membrane potential were measured by means of glass microcapillary electrodes filled with 1 M KCl using a VAJ-51 electrometer (RTF) and a PCI-6024E ADC interface. The reference electrode was connected to the central chamber compartment via a flexible salt bridge.
2.5 Electric stimulation of internodal cells
The action potentials (AP) were elicited with an electric stimulus using extracellular Ag/AgCl electrodes placed in the central and side compartments of the experimental chamber. Square voltage pulses (3 V, 150 ms) were applied from a digital-to-analog converter (National Instruments) through a 1.2 MΩ load resistance (current density, ~25 μA cm−2).
Velocities of cytoplasmic streaming were measured with a stopwatch by recording the time during which the moving vesicles covered a fixed distance of approximately 1 mm.
Figures illustrate the results of representative experiments performed in at least four replicates on different cells. Traces in figures are either individual or averaged curves calculated from several records, with n indicating the number of replicate measurements. Curves with error bars show averaged traces and standard errors of the means (SEM) obtained from several records on individual or various cells.
3 RESULTS
3.1 Alkaline pHo diversifies the set of microfluidic signals sensed by dimly lit chloroplasts
In a first series of experiments, we measured the LL-induced cyclosis-mediated transients of Chl fluorescence F′ (F′LL signals) before and after the replacement of standard APW with a high-pH (pH 9.5) medium containing 10 mM Tris (Figure 1A). This treatment did not alter the early segment of the F′LL signal but truncated the rear slope of F′ transient (cf. curves 1 and 2), thus diminishing the F′ peak. Upon longer incubation, the negative deflection of F′ fluorescence became prominent following the initial ascending stage (curve 3). These alterations shifted the position of the F′ peak to shorter times compared to that in the untreated cell. The faster achievement of the F′ peak occurred without appreciable acceleration of cytoplasmic streaming.
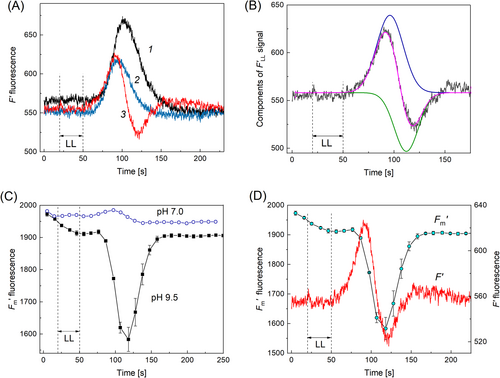
The kinetics of F′LL with positive and negative components points to the occurrence of two metabolites transported with the cytoplasmic flow, one of which elevated and the other diminished the fluorescence yield F′. Only one of these metabolites was sensed by chloroplasts in low light at neutral pHo, while both agents were perceived at pH 9.5. Following this reasoning and by analogy with the elution peaks in chromatography, the composite F′LL response at pH 9.5 to the LL application was suitably approximated with a sum of two time-shifted Gaussian curves having similar bandwidths and approximately equal amplitudes (Figure 1B). The amplitude of the reconstituted positive band at pH 9.5 was nearly equal to the amplitude of F′LL transient at pH 7.0 (Figure 1A and B). Since the cell site exposed to LL was separated by an air gap from the compartment with altered pHo, the composition of metabolites exported into the cytoplasm under LL treatment was supposedly insensitive to pHo variations in the central pool.
The response of the maximal fluorescence yield F′m to the LL stimulus was also modified after the replacement of APW with an alkaline medium. As shown in Figure 1C, local illumination of a distal cell region induced a slight transient increase in F′m under control conditions (curve labeled pH 7.0, standard APW) but strongly quenched F′m after transferring the central cell segment to alkaline APW (curve labeled pH 9.5). It is noteworthy that F′m fluorescence in the cell area exposed to high pH became rather sensitive to saturation pulses. As can be seen in Figure 1C, the onset of the saturation pulse series led to an appreciable reduction in F′m within 50 s, while the starting F′m values were identical at neutral and high pH. The quenching of F′m and F′ developed synchronously (Figure 1D). This observation indicates that the origin of negative F′ deflection resides in the non-photochemical quenching (NPQ) of Chl fluorescence.
3.2 Darkened chloroplasts perceive only one fluidic metabolite at neutral and high pHo
In further tests, to verify that the dip of the F′LL signal originated from non-photochemical quenching of Chl excitations, the background illumination was switched off synchronously with the onset of the LL pulse (Figure 2). The transition from light to darkness excluded photosynthetic electron transport in recipient chloroplasts and its influence on cyclosis-mediated changes of F′ and F′m. When BGL was screened at the moment of switching on the LL pulse, F′ fluorescence decreased gradually toward the Fo level. Nevertheless, in the cell bathed with standard APW, the darkened chloroplasts responded to microfluidic transmission of reducing equivalents by the transient F′ increase that was analogous, though delayed, with respect to the conventional F′LL response (Figure 2A, curves labeled BGL on and BGL off). However, if the experiment was run after the replacement of APW with an alkaline (pH 9.5) solution, the F′LL response developed in different ways depending on the presence or absence of background illumination. In the cells exposed to pH 9.5 and continuous BGL, the F′LL response comprised the positive and negative bands, whereas, after screening the cell from BGL, the F′LL signal contained only one (positive) band (Figure 2B, curves BGL on and BGL off). Remarkably, the amplitude and time position of this band at pHo 9.5 were nearly the same as observed under normal APW (cf. curves BGL off in Figure 2A and B).
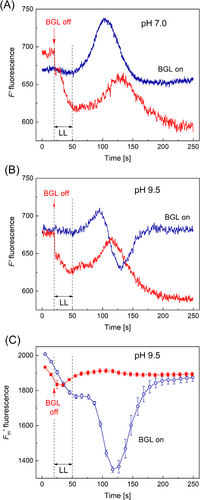
The cyclosis-mediated response of F′m fluorescence to LL at pH 9.5 comprised strong transient quenching under background light but this stage was absent after placing the cell in darkness (Figure 2C). These results support the view that the delayed (negative) peak in the F′LL signal under dim light at pH 9.5 resulted from NPQ caused by a microfluidic passage of LL-induced cytoplasmic component. The lack of the sensitivity of the F′LL signal to the fluidic delivery of this component in darkened cells might be caused by the low background level of this metabolite in the absence of electron transport reactions and by an insufficient increment in its cytoplasmic concentration during the metabolite passage across AOI.
3.3 The delayed component of the F'LL signal at high pHo vanishes after NPQ removal with NH4+
The link between the descending component of the F′LL signal and NPQ at high pH was additionally tested by treating the analyzed cell segment with NH4Cl. Ammonium is known to prevent light-dependent formation of thylakoid ΔpH and energy-dependent quenching of Chl fluorescence (Krause and Laasch, 1987). The neutral deprotonated ammonium form (NH3) readily permeates across the thylakoid membrane and binds luminal protons released during photosynthetic electron transport. The pK for NH3/NH4+ equilibrium is 9.25. Since the membrane-permeable form NH3 is predominant at pH 9.5, we employed the ammonium salt at a low concentration of 0.2 mM. By applying the LL pulse to a distal cell region, we traced its influence on F′m before and after the replacement of a high pHo medium (APW, 10 mM Tris added, pH 9.5) with a similar solution containing 0.2 mM NH4Cl (Figure 3A). In the absence of NH4Cl at high pHo, illumination of the cell region located 4 mm apart from AOI induced transient cyclosis-mediated quenching of F′m (curve 1). These F′m transient changes disappeared after the addition of NH4Cl (curve 2), which was presumably caused by the prevention of ΔpH formation in the thylakoids.
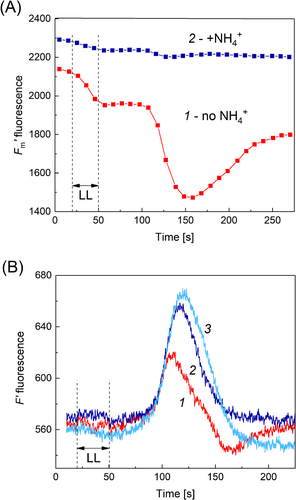
We also examined the influence of NH4+ on cyclosis-mediated F′ transients when the analyzed cell part was kept in the medium with pH 9.5 (Figure 3B). Curve 1 shows the F′LL response comprising the positive and negative F′ deflections, which is a typical shape at high external pH (e.g., Figures 1, 2). The addition of NH4Cl was followed by the removal of a negative component and a substantial increase in the amplitude of F′LL response (Figure 3B, curve 2). Thereafter, the cell was washed for 1 h with standard APW (pH 7.0). In the washed cell, the F′LL signal (Figure 3B, curve 3) was similar to that observed with ammonium salt at pH 9.5. The baseline level of F′ was higher in the presence of NH4Cl than in its absence but this displacement was subtracted in Figure 3B to simplify the comparison of signal shapes. The data in Figure 3 support a view that the negative part of F′LL transient at high pHo is due to non-photochemical Chl fluorescence quenching caused by a metabolite carried in the cytoplasmic stream.
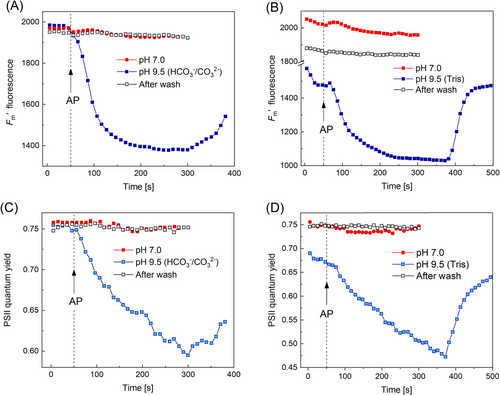
3.4 Effects of high pHo on microfluidic responses are reproduced with a HCO3−/CO32− buffer
In order to find out if the effects of elevated pHo on the F′LL and F′m signals occur in the presence of buffers other than Tris, we examined cyclosis-mediated F′ and F′m responses before and after transferring the cell from standard APW to APW containing 10 mM bicarbonate–carbonate buffer (pH 9.5). These tests revealed pronounced modifications of F′LL and F′m transients that were similar to those observed in Tris-containing medium. It can be seen in Figure 4A, B that, after replacement of APW with a similar medium adjusted to pH 9.5 by a HCO3−/CO32− buffer, the LL pulse induced the negative deflection of F′ concurrent with a profound quenching of F′m fluorescence. At an alkaline pHo F′m became sensitive to a series of saturating light pulses (SP), which was manifested in a substantial F′m decline during the onset of the light pulse series. Strong non-photochemical quenching of F′m induced by repeated SP (at t = 50 s) and the second decrease at t = 130 s caused by the fluidic control were also mirrored by the respective dips in the kinetics of F′ responses to saturation pulses and LL stimuli. By contrast, such F′ and F′m transients were absent if the cell was submerged into standard APW.
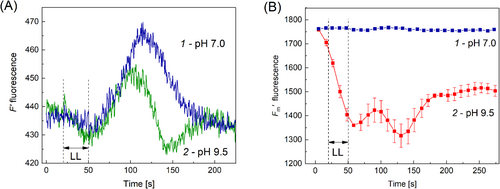
In a further test, we used 10 mM Bicine-NaOH buffer for adjusting the external pH in the alkaline range and found that F′ and F′m changes induced by the LL pulse applied at a distance from AOI were basically similar at pH 9.1 to modifications caused in the presence of Tris buffer. There were both positive and negative F′ deflections in the F′LL response, the negative F′ deflection coincided in time with the NPQ development. Clearly, the above modifications in microfluidic signaling were caused by elevated pH, not by the specific action of Tris added to the bath solution.
The above-described effects of local lighting on F′ and F′m were also observed at higher pH values (pH 10.6) adjusted with a HCO3−/CO32− buffer. As shown in Figure 5, the basic observations at pH 10.6 were similar to those noted at pH 9.5. In the alkaline bath medium, the F′LL signal was suppressed and contained both positive and negative bands (Figure 5A). The maximal fluorescence F′m was strongly quenched by the photometabolite carried with the cytoplasmic flow under background light but was insensitive to this metabolite in the absence of BGL (Figure 5B). The negative deflection in the F′LL signal developed concurrently with NPQ enhancement, i.e., with F′m quenching (Figure 5C). The influence of high pH was reversed after 30 min washing the cell (Figure 5A).
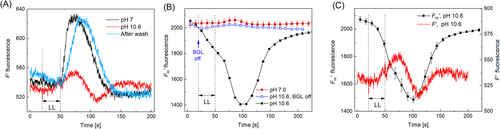
3.5 Implication of cyclosis in Chl fluorescence responses to cell excitation at high pHo
In chloroplasts of cell regions bathed with a HCO3−/CO32− buffer or Tris-containing solution, the F′m fluorescence (an NPQ indicator) and the effective PSII quantum yield (YII) became highly sensitive to the action potential generation (Figure 6). The PM excitation did not affect F′m and the effective quantum yield of PSII-driven electron flow (YII) at neutral pHo, but induced large transient NPQ (Figure 6A, B) and the decline of YII (Figure 6C, D) after the elevation of pHo to 9.5. In this way, the patterns of AP-induced F′m and YII changes in the natural and imitated alkaline bands exhibited apparent similarity.
It should be noted that fluidic interactions between cell parts exposed to neutral (or slightly acidic) and alkaline pHo are engaged in the effects of cell excitation on NPQ and YII. The lateral microfluidic pathway is crucial for inorganic carbon (Ci) acquisition in cell parts submerged in an alkaline medium because the charged forms of Ci (HCO3− and CO32−) cannot enter the cell via lipid bilayer portions of the membrane. A sudden cessation of streaming upon the AP generation interrupts the lateral (cyclosis-mediated) delivery of Ci to chloroplasts in high pHo cell regions from low-pHo regions. The disruption of CO2 supply thus rewires electron transport on the acceptor side of PSI from CO2 assimilation to alternative routes (O2 reduction or the cyclic pathway) that are associated with the thylakoid energization (ΔpH formation) and NPQ development.
While the F′m level was nearly settled at the time range of 200–300 s, the quantum yield YII went on declining (cf. Figure 6A, B and Figure 6C, D). This prolonged decline of YII was caused by the transient increase in actual F′ fluorescence. A similar F′ transient after the AP generation was earlier found in the calcified cell regions predisposed to alkaline band formation at elevated irradiance (Bulychev and Alova, 2022; Bulychev et al., 2023). The effects of high pHo on AP-induced changes in F′m and YII were reverted upon 1-h washing the cell with fresh APW (Figures 6A–6D).
3.6 Effects of high pHo on PM conductance and membrane potential
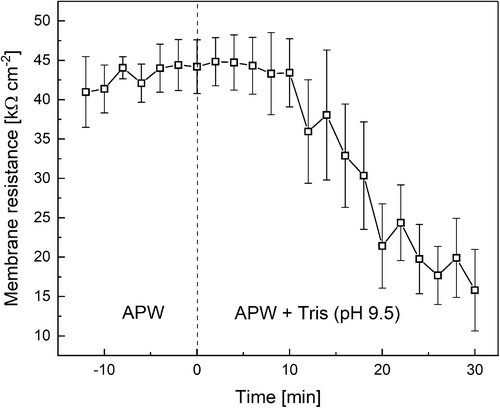
High sensitivity of F′m at alkaline pHo to illumination of a distal cell region, periodic application of saturating light pulses, and the AP generation (Figures 1-3, 6) is probably caused by a modified cytoplasmic composition under the increased alkalinity of the medium. One important factor is that the PM in Chara undergoes the transition to the state with a high conductance for H+ or OH− upon the elevation of pHo to 9.5 or higher (Bisson and Walker, 1981; Beilby and Bisson, 1992; Beilby, 2015).
We checked if the transfer of cells from normal APW to alkaline media in our experiments was indeed associated with the increase in PM conductance. As can be seen in Figure 7, the replacement of neutral APW with Tris-containing alkaline medium (pH 9.5) led to an almost threefold decrease in the membrane resistance Rm after a lag period of ~10 min. A slight initial increase in Rm was noted during the lag period but it was not conspicuous in averaged data. The Rm drop from 44.4 ± 3.2 to 15.8 ± 5.2 kΩ cm2 (Figure 7) is consistent with records by Bisson and Walker (1980, 1981) who observed PM conductance (Gm = Rm−1) values of ~0.32 S m−2 (Rm ~ 30 kΩ cm2) at neutral pH and the increase in Gm up to 0.57–4.6 S m−2 (Rm ≤ 17 kΩ cm2) at pH 11. Thus, our findings are in line with the idea that the PM undergoes the transition at alkaline pH to the state with prevailing H+/OH− conductance and that the transmembrane electric potential is close to the equilibrium potential for H+. The PM electric potentials recorded at pH 9.5 on various internodal cells ranged from −180 to −210 mV (n = 5). The membrane potential measured on four internodal cells at pH 10.6 varied from −170 to −220 mV.
4 DISCUSSION
Long-distance microfluidic communications between immobile chloroplasts in characean algae are manifested differently for cell regions adjacent to natural light-dependent alkaline and acidic zones (Bulychev and Rybina, 2018; Bulychev and Krupenina, 2019). While cyclosis-mediated F′LL transients were large in regions with low pHo, they were small in cell areas featuring high pHo. The present results indicate that the microfluidic transmission and perception of reducing substances released into the cytoplasm upon an LL pulse and responsible for the rising stage of F′LL was actually not suppressed in cell regions underlying external alkaline zones. The apparent decline of F′LL signals at high pHo resulted from the acquired sensitivity of recipient chloroplasts to another cytoplasmic component that also moves with the fluid flow and quenches Chl fluorescence. The enhanced sensitivity to this additional fluid-transported component at pHo ≥9.5 originated presumably from the increased background cytosolic content of a light-dependent metabolite acting as Chl fluorescence quencher, not from a lower threshold of chloroplast–metabolite interaction. Data in Figures 1A, B and 2A, B suggest that the F′ fluorescence rise ascribed to the lateral transport of reducing equivalents in the cytoplasmic flow remained largely unaffected at high pHo but was masked by fluorescence decline under the action of a fluid-carried cytoplasmic metabolite promoting energy-dependent NPQ. The overlap of oppositely directed time-shifted F′ changes resulted in the seeming disorders of microfluidic communication under alkaline bands.
The signaling function of cyclosis is related to the longitudinal transport of inorganic carbon (Ci), reducing equivalents, H2O2, and, possibly, other substances. The cytoplasm whose composition is modified under the action of LL or high pHo travels via fluid flow to downstream cell regions and rearranges photosynthetic electron transport pathways, which modifies photochemical (F′) and non-photochemical (F′m) quenching of Chl fluorescence. In this way the cytoplasmic streaming is involved in regulatory changes of photosynthesis caused by the disturbed Ci supply at elevated external pH or by the altered cytoplasmic composition following irregular lighting (sunflecks). The chloroplasts underlying acidic zones are well supplied with CO2 owing to its permeation from the medium across the PM, whereas the chloroplasts located under the alkaline zones rely mostly on microfluidic delivery of Ci from low-pHo cell regions sufficient in Ci. In the vicinity of alkaline zones, where Ci supply depends on lateral fluid flows, cyclosis cessation during cell excitation results in an acute Ci shortage and the consequent redirection of electron transport pathways from the assimilating to the alternative routes (Figure 6).
Previous studies using methyl viologen (MV) revealed that the AP generation in Chara results in strong non-photochemical quenching associated with the redirection of a CO2-dependent electron flow to O2 photoreduction on the acceptor side of PSI (Krupenina, Bulychev and Schreiber, 2011; Bulychev, Cherkashin and Krupenina, 2024). Such a switchover of electron flows was due to the instant AP-triggered permeation of a divalent cation MV2+ from an external medium into the chloroplasts, where this agent catalyzes the production of O2− and H2O2. Since electron transport to O2 proceeds without ATP consumption, it resulted in the extra energization of thylakoids and NPQ formation, which was eliminated in the presence of a protonophorous uncoupler (Bulychev, Cherkashin and Krupenina, 2024). The effects of PM excitation on F′m fluorescence and the quantum yield YII in cell parts with alkaline pHo (Figure 6) were phenomenologically similar to those observed in the presence of MV. Therefore, it is reasonable to assume that the increasing Ci deficiency in high-pHo chloroplasts after the AP generation may redirect photosynthetic electron transport to O2 reduction or activate cyclic electron transport.
At high pHo strong NPQ development was observed not only after cell excitation (Figure 6) but also after the LL treatment (Figures 1-3). This similarity suggests that local illumination acting in concert with cyclosis-mediated transport facilitates the alternative electron transport in dimly lit chloroplasts, thus enhancing Chl fluorescence quenching and distorting the kinetics of F′LL transients. In consistency with this notion, the dissipation of thylakoid ΔpH in the presence of NH4+ at high pHo resulted in the reversal of NPQ imposed after LL application to a remote cell part (Figure 3). Under CO2 deficiency, the alternative electron transport pathway might be additionally promoted by the release of H2O2 from brightly illuminated plastids at the site of LL incidence (Eremin, Bulychev and Hauser, 2013). It is known from experiments with isolated intact chloroplasts that H2O2 may indirectly act as an electron acceptor with the efficiency comparable to that of methyl viologen (Neubauer and Schreiber, 1989).
The internode parts submerged into alkaline media differ from those surrounded by acid zones in terms of the mode of Ci supply to anchored chloroplasts. A shortage of Ci supply across the PM at high pHo might result in higher rates of O2 photoreduction and elevated content of H2O2 in chloroplasts and the cytoplasm. It is known that H2O2 at low concentrations (~5 μM) induces photochemical and non-photochemical quenching of chlorophyll fluorescence in intact chloroplasts (Neubauer and Schreiber, 1989). It is thus possible that chloroplasts and the cytoplasm of cell areas bathed with a high-pHo medium differ from those bathed with a low-pHo medium by elevated H2O2 content.
Supposing different background H2O2 levels in cell parts adjacent to acid and alkaline zones and considering the S-shaped dependence of F′m fluorescence on H2O2 concentration in isolated intact chloroplasts (Neubauer and Schreiber, 1989), the arrival of H2O2 parcel with the streaming cytoplasm from a brightly illuminated area may exert non-equal effects on Chl fluorescence at neutral and alkaline pHo. At low background concentration of H2O2, the added portion might be insufficient for F′m quenching, while the same portion may produce a stronger impact if the background ROS concentration approaches the effective range. It is also possible that, at a low basal level of cytoplasmic H2O2, this ROS is eliminated by the antioxidant defense system more effectively on the way from LL pulse treatment to AOI than at elevated basal H2O2 content. Then, the H2O2 portion arriving at AOI at an elevated basal level of this ROS would be higher than at a low basal level. Such relations may account for strong NPQ in cell parts bathed with high pHo medium following local illumination of a distal cell region (Figures 1C, 3A) and for the lack of F′m quenching at high pHo after screening the background light (Figures 2C, 5B).
In addition to different cytoplasmic content of Ci and H2O2 in cell areas underlying near-neutral and alkaline environments, substantial differences in PM conductance to H+ (or OH−) between such regions should be taken into account for the interpretation of the effects of high pH media. The results of PM conductance measurements support previous conclusions that the H+/OH− conductance increases substantially upon the transfer of the internodal cell to media with pH ≥9.5 (Beilby, 2015). If the electrochemical equilibrium for H+ is attained at pHo 9.5 due to the dominant H+/OH− conductance of PM (Bisson and Walker, 1980), and the membrane potential is more negative than −180 mV while the electrogenic H+-pump is inhibited (Beilby and Bisson, 1992), the cytoplasmic pH might drop to pH 6.5 or lower. Then, the effects of alkaline media on F′LL transients can be partly ascribed to the acidic shift of pHcyt. Specifically, low pHcyt promotes electron transport from PSI to oxygen (Mehler reaction), which enhances the thylakoid energization (Hormann et al., 1993) and quenches F′m. On the other hand, under the assumption of an electrochemical equilibrium across PM at pHo 10.6 and the membrane potential at the level of −200 mV, the expected difference between pHo and pHcyt is ~3.3 units, which corresponds to the cytoplasmic pH of 7.3. In this case, the pHcyt is close to physiological values, and the shortage of intracellular Ci at pHo 10.6 seems to be the main factor governing the responses of F′m and F′ to local illumination of a distal cell part (Figure 5).
The achievement of electrochemical equilibrium for H+/OH− across the PM at high external pH implies that multiple H+-cotransport systems of characean cells (Beilby, 2015) cannot operate under these conditions. Among these transporters, the symport of H+ and HCO3− might be most relevant for photosynthetic performance, although the functional significance of this system is not commonly accepted (Walker, Smith and Cathers, 1980). Nevertheless, the suppression of H+/HCO3− cotransport under electrochemical H+ equilibrium may contribute to low availability of Ci at high pH, thus rearranging the pathways of photosynthetic electron transport.
The results of this study suggest that circular electric currents maintained between the acid and alkaline cell regions in illuminated characean internodes (Lucas and Nuccitelli, 1980) are not crucial for the effects of AP generation and LL application on the functioning of chloroplasts beneath the alkaline zones. At high pHo the distribution of H+/OH− across the PM is close to equilibrium (Bisson and Walker, 1981), and no circulation of electric current is expected. Despite the lack of circulating currents, the chloroplast fluorescence responses associated with electrical and LL-induced fluidic signaling in the adopted experimental model were similar to those observed in cells with natural pH banding. This finding opens prospects for studying chloroplast performance at high pH and low irradiance, while comparatively high irradiance is needed for the formation of pH banding patterns under natural conditions. It would be interesting to explore the effects of high external pH on the transcellular permeation of metabolites and find out if the nodal complex at high pHo permits the selective cell-to-cell permeation of reducing equivalents while blocking the passage of F′m-quenching agents as it occurs at weakly acid pHo.
AUTHOR CONTRIBUTIONS
Both authors designed and performed the experiments. A. Bulychev wrote the manuscript with the contribution from the coauthor. Both authors approved the final version of the manuscript.
ACKNOWLEDGEMENTS
This work was carried out as part of the Scientific Project of the State Order of the Government of Russian Federation to Lomonosov Moscow State University № 121032500058-7.
FUNDING INFORMATION
The study was conducted under the state assignment of Lomonosov Moscow State University.
Open Research
DATA AVAILABILITY STATEMENT
The authors declare that all data supporting the findings of this study are available in the paper.




