Male sterility-induced parthenocarpy arose during tomato domestication
Abstract
The huge diversity of cultivated tomatoes is the result of a long process of domestication followed by intensive breeding. Breeding efforts have been focused on increasing fruit size and on the diversification of fruit phenotypes. The formation of seedless (parthenocarpic) fruits in tomato plants is an interesting trait for growers, providing a mechanism to overcome fertilization failure under unfavourable environmental conditions. Early anther or pollen ablation is an effective strategy to promote parthenocarpy in tomato plants and was proven to be effective in several tomato cultivars. Whether this is an ancestral trait or was acquired during domestication and breeding is unknown. In this study, we evaluated the formation of parthenocarpic fruits in the cultivated tomato and the wild relative Solanum pimpinellifolium through the generation of male-sterile mutants. Only cultivated tomatoes, but not Solanum pimpinellifolium plants, produced seedless fruits. Expression analyses showed that parthenocarpy correlates with the activation of fertilization-independent gibberellin biosynthesis in the ovaries. When compared with wild relatives, modern tomato cultivars present small deletions in the promoter of these genes that could account for the differences in gene expression that ultimately trigger parthenocarpy. Our results suggest that seedless fruit production was actively repressed in the absence of pollination in the ancestral tomato lineages.
1 INTRODUCTION
Modern tomato cultivars are the result of a complex process of domestication followed by intensive breeding. Domestication started in the American continent when wild populations of Solanum pimpinellifolium (SP) and Solanum lycopersicum var. cerasiforme (SLC) moved to Mesoamerica, where Solanum lycopersicum was domesticated. Then, tomato plants were taken to Europe around the 16th century, where a collection of landraces originated (Bai and Lindhout, 2007; Blanca et al., 2022; Razifard et al., 2020). More recently, since the 20th century, extensive breeding has led to a huge variety of cultivars showing variation in plant architecture and, especially, in fruit shapes, colours, and sizes.
Domestication correlates with substantial gene loss, which causes, among other effects, the reduction of anti-nutritional compounds in fruits. Among the traits associated with the domestication process, the reduction of style length was a key transition to favour self-pollination in domesticated tomatoes. The phenotype of short style in cultivated tomatoes is associated with a deletion in the promoter of the putative transcription factor Style2.1 (Chen et al., 2007). A second stigma exertion gene, SE3.1 (Stigma Exertion 3.1), has been identified, and loss-of-function alleles of this gene seem to have been selected during tomato domestication (Shang et al., 2021). Both genes contributed to the transition from exerted to inserted stigmas that improved the efficiency of self-pollination. Other important loci are involved in domestication traits related to fruit shape and size and locule number. Mutations in the genes OVATE, SUN, LOCULE NUMBER (LC), and FASCIATED (FAS) are responsible for the large variation of fruit shape and morphology characteristic of cultivated tomato, and OVATE and FAS mutations are also partial loss-of-function alleles (Rodríguez et al., 2011).
In tomato plants, as in many self-pollinated species, pollen and ovary maturation are coordinated, ensuring efficient pollination and fertilization. After anther dehiscence, pollen grains are released into a receptive stigma where they germinate, producing pollen tubes that eventually will reach the ovules. Upon fertilization, the ovary changes from a static stage to a fast-growing condition for the newly developing fruit (Serrani et al., 2008). The phytohormones auxin and gibberellin act after fruit set promoting ovary growth and rapid expansion into fruits. In the absence of fertilization, ovaries shortly enter senescence, a process that seems to be mediated by ethylene (Shinozaki et al., 2018). Ovary growth can be artificially induced in the absence of pollination by external treatments with several hormones (Molesini et al., 2020), leading to the formation of seedless fruits. In tomato, GA signaling is integrated through a single DELLA protein, and loss-of-function plants showed parthenocarpic fruit development, suggesting that SlDELLA restrains ovary growth in the absence of pollination-derived signals (Bassel et al., 2008; Hu et al., 2018; Martí et al., 2007). Additional mechanisms involved in ovary restrain seem to be mediated by the developing anthers as suggested by the formation of parthenocarpic fruits in male-sterile tomato genotypes (Ampomah-Dwamena et al., 2002; Medina et al., 2013; Okabe et al., 2019; Rojas-Gracia et al., 2017; Salazar-Sarasua et al., 2022). Interestingly, the repressive signal seems to decrease gradually upon stamen maturation since manual emasculation of flowers does not trigger parthenocarpic fruit growth.
Despite the apparent paradox of developing seedless fruits in terms of reproductive success, parthenocarpy has been reported to occur in nearly 100 Angiosperm taxa, including wild and non-fruit crop species (Picarella and Mazzucato, 2019). It has been suggested that partial parthenocarpic fruit production could represent an adaptative trait to reduce seed predation in wild species, as suggested for Pasticana sativa (wild parsnip), Pistacea lentiscus, or Bursera morelensis (Ramos-Ordoñez and Arizmendi, 2011; Zangerl et al., 1991). In the case of B. morelensis, which produces brightly coloured fruits, parthenocarpy also seems to increase attractiveness for birds that oversee seed dispersal (Ramos-Ordoñez and Arizmendi, 2011).
In this study, we evaluated whether male-sterility-induced parthenocarpy observed in Solanum lycopersicum (cultivated tomato) is an ancestral character or was acquired during the domestication that originated the modern tomato cultivars. The tomato DEFECTIVE IN TAPETAL DEVELOPMENT AND FUNCTION1 (SlTDF1) gene was identified and selected as a target of CRISPR/Cas9 editing to produce male-sterile plants in both S. lycopersicum and the wild relative Solanum pimpinellifolium. Male sterility triggered parthenocarpy in tomato but not in the mutant lines obtained in the wild relative. We employed expression analyses and in silico promoter scanning to evaluate the role of gibberellin metabolism and signalling in the occurrence of parthenocarpy only and specifically in domesticated tomatoes. The parthenocarpic trait correlated with changes in regulatory regions in the promoters of genes involved in gibberellin biosynthesis. Domesticated genotypes carry small deletions in putative binding sites of transcription factors that might occur at early stages during tomato domestication. Moreover, such knowledge provides candidate genes to modify fruit initiation, leading to a new generation of parthenocarpic varieties in crops.
2 MATERIALS AND METHODS
2.1 Plant material and growth conditions
Plants of tomato (Solanum lycopersicum; cultivars Moneymaker, Microtom, M82, and P73), Solanum pimpinellifolium (accession LA1670), and Solanum lycopersicum var. cerasiforme (accessions GBV006768 and GBV007931 from COMAV collection, Valencia, Spain) were grown in the greenhouse at 25–30°C day and 18–20°C night. Natural light was supplemented with Osram lamps (150 μmol m−2 s−1, Powerstar HQI-BT, 400 W) to obtain a 16/8 h light/dark photoperiod. Plants were grown in pots with a mix (50/50) of medium-weight peat and perlite n°3 and irrigated daily with Hoagland solution.
2.2 Phylogenetic analysis
For the phylogenetic analysis, TDF1 homologs were identified using the protein sequence (A0A654FBL4) by a sequence homology search with the tool tBLASTn in the NCBI NR database for the species Solanum lycopersicum, Solanum tuberosum, and Arabidopsis thaliana. Multiple sequence analysis was performed using MEGA X (Kumar et al., 2018). The phylogenetic tree was constructed using the Neighbor-Joining method, and the bootstrap node support (10000 replicates) is shown next to the branches. Accession numbers of the sequences used in the analysis are listed in Supplemental Table S1.
2.3 Expression analyses
2.3.1 RNA extraction, cDNA synthesis, and qRT-PCR
Total RNA was extracted from different tomato tissues (roots and apices from 2-week-old seedlings, young leaves, flower buds, and dissected ovaries) using RiboZol RNA extraction buffer (VWR) and following the manufacturer's instructions. DNase-treated total RNA was used to obtain cDNA using the Primer Script 1st strand cDNA synthesis kit (Takara) and qRT-PCR experiments were carried out using MasterMix qPCR ROX PyroTaq EvaGreen 5x (CmB) using sequence-specific primers and SlACT8 gene as a reference. Relative expression of the genes was calculated according to the 2-ΔCt method with three biological replicates. The list of the oligonucleotides is included in the supplementary data (Table S2).
2.3.2 RNA in situ hybridization
Floral samples at different development stages were collected and immediately fixed in FAE (3.7% formaldehyde; 5% acetic acid; 50% ethanol) at 4°C overnight. The fixative was replaced by 70% ethanol, and samples were stored at 4°C until processing. Next, samples were embedded in paraffin using an automated tissue processor (Leica TP1020; Leica Biosystems). Serial sections (8 μm) were obtained and used in the hybridization to localize the presence of transcripts. To generate RNA probes for the SlTDF1 transcript, a fragment of 403 bp was amplified by PCR using primers SlTDF1-IS-For and SlTDF1-IS-Rev and cloned into a vector under the T7 promoter. RNA probes labelled with digoxigenin were obtained using a T7 RNA polymerase and the DIG RNA labelling mix (Roche). Tissue processing, hybridization, and colourimetric signal development were previously described (Gómez-Mena and Roque, 2018).
2.4 Subcellular location of the SlTDF1 protein
To determine the subcellular location of SlTDF1, the complete cDNA sequence was amplified by PCR using SlTDF1-ATG-For and SlTDF1-cDNA-Rev primers (789 bp) and cloned into the pEarlyGate104 binary vector (Earley et al., 2006), generating a SlTDF1-YFP fusion protein. The construct was introduced into Agrobacterium strain C58C1 and used for agroinfiltration of 4-week-old Nicotiana benthamiana leaves. Leaf disks of the plants were analyzed two days after infiltration using confocal microscopy. A 35S-GFP construct was agroinfiltrated in parallel and was used as a control that shows the pattern of fluorescence of the free GFP.
2.5 Generation of CRISPR/Cas9 construct, tomato transformation, and selection of mutant alleles
Mutant alleles in the SlTDF1 gene were generated using CRISPR/Cas9 technology with a single 20-nucleotide guide (5’-GCTCAGGTCTTTGCTGAGTA-3′) complementary to the genomic sequence of Solyc03g059200. The final SlTDF1-CRISPR-Cas9 construct was generated using the Golden Braid 4.0 cloning system (Vazquez-Vilar et al., 2016) and contains three modules: the gene-specific sequence under the pU6-26 promoter fused to the gRNA scaffold, the coding sequence of Cas9 under a 35S promoter (p35S:hCas9:tNos), and the nptII gene module (pNos:nptII:tNos) that confers kanamycin resistance in plants. The construct was introduced in tomato plants by genetic transformation using Agrobacterium tumefaciens (LBA4404 strain) and cotyledon explants as starting material (Ellul et al., 2003). Transformants were obtained using in vitro culture methods and selected as kanamycin-resistant plants that were transferred to the greenhouse. The ploidy of the plants was determined by flow cytometry, and only diploid plants were maintained for further analysis. The same protocol was used to transform both Solanum lycopersicum and Solanum pimpinellifolium plants.
2.6 Histology of anther development
Flower buds of different sizes were sequentially collected to obtain a complete serial collection of the developmental stages of the anther and the male gametophyte in wild-type and mutant plants. Flowers were fixed in FAE (3.7% formaldehyde; 5% acetic acid; 50% ethanol) at 4°C overnight. After the removal of the fixative, the flowers were embedded in acrylic resin (Technovit 7100; Kulzer) according to the manufacturer's instructions. Histological sections (1–2 μm) were obtained and stained with Toluidine Blue to visualize the tissular structure of the anther.
2.7 Hormone treatments
Treatments were done as previously described (Medina et al., 2013). To prevent self-pollination, flowers were emasculated one day before anthesis. Gibberellic acid (GA3, Duchefa) solution was applied to emasculated ovaries with 10 μL of a GA3 solution (0.3 mM GA3, 10% methanol, 1% Tween 80) one day before anthesis. This treatment was repeated three times at three-day intervals. Pacobutrazol (Duchefa) was added to the watering solution at a final concentration of 1 mM and used to water the plants every three days.
2.8 Promoter analyses
S. pimpinellifolium LA2093, LA1670, LA1237, and LA1589 and S. lycopersicum Heinz 1706 (SL4.0), M82, MoneyBerg, and Microtom were compared to look for genomic differences in promoters corresponding to GA biosynthesis, signalling and inactivation genes. Both genomes and their annotations were loaded into Integrative Genomics Viewer (IGV) v. 2.16.2 (Robinson et al., 2011; Thorvaldsdóttir et al., 2013), and 1000 bp were selected upstream from the translation start site (ATG), then aligned using ClustalW v.1.8 (https://www.genome.jp/tools-bin/clustalw). Regions with insertions or deletions were then analyzed using Tomtom and MEME from The Meme Suite web server v.5.5.3 (Bailey et al., 2015) to discover and compare motifs to existing motifs found in plants and present in the JASPAR 2022 database (Castro-Mondragon et al., 2022). After confirming the insertions or deletions of interest, the promoters of S. galapagense ZY56, S. peruvianum LA3858, S. chilense LA1972, and S. pennellii LA0716 were scanned and compared.
2.9 Repression assays
2.9.1 Construct assembly
TCX2 (Solyc12g007180), BCP1 (Solyc04g008380), and CDF2 (Solyc05g007880) transcription factors were amplified by PCR and cloned into the intermediate vector pCR8 (ThermoFisher Scientific). Then, they were introduced into the binary destination vector pEG100 under a CaMV 35S promoter through a gateway recombination reaction (https://gatewayvectors.vib.be/).
The promoter regions of GA20ox1 and GA3ox2 were amplified by PCR and cloned into the pUPD2 vector for GoldenBraid assembly. The first module was assembled into the pDGB3α1 destination vector and consisted of the cloned promoter region fused to a pmini35S (GB0050) to allow transcription, the luciferase CDS (GB0096) as a reporter and the tNOS terminator (GB0037). In a successive multipartite reaction, this module was assembled with the GB0160 part (containing the Renilla and P19 transcriptional units) into the final destination vector pDGB3Ω1 and introduced into the Agrobacterium strain C58C1. The list of the oligonucleotides used is included in the supplementary data (Supplemental Table S3).
2.9.2 Luciferase assays
Luciferase assays were conducted as previously described (Vazquez-Vilar et al., 2021) with minor modifications. Briefly, 4-week-old Nicotiana benthamiana leaves were co-infiltrated with different construct combinations. Five days after inoculation, three leaf discs were excised from independent agroinfiltrated leaves of the same plant and frozen in liquid nitrogen. Homogenized leaf discs were extracted with 150 μL of Passive Lysis Buffer (Promega) and centrifuged for 10 minutes at 13000 xg at 4°C. The supernatant was used as the working plant extract. Fluc/Rluc were determined following the Dual-Glo Luciferase Assay System (Promega) protocol using 10 μL of working plant extract, 40 μL of LARII, and 40 μL of Stop&Glo Reagent. Samples were loaded onto a Corning 96-well flat white-bottom plate (Sigma-Aldrich) and measured using an Infinite M Plex plate reader (TECAN) with a 2 ms delay and a 10 ms measurement time.
2.10 Statistical analyses
Statistical analyses were done using IBM SPSS 29. Normality, variance and independence of the samples were tested, and means were compared using a Student's t-test for parametric variables.
3 RESULTS
3.1 Identification of the TDF1 ortholog in tomato plants
In Arabidopsis, maize, and rice, several transcription factors involved in pollen development have been identified (Marchant and Walbot, 2022). Among them, the Arabidopsis TDF1 gene encodes an R2R3 MYB transcription factor essential for tapetal differentiation and function (Zhu et al., 2008). In tdf1 mutants, abnormal tapetal cell vacuolation leads to microspore degradation, resulting in complete male sterility. TDF1 function is conserved between distant species such as Arabidopsis, maize, and rice, among others, and therefore is a good target gene to obtain male sterile plants by gene editing strategies.
The putative tomato ortholog of TDF1 was identified through a sequence homology search using the Arabidopsis protein sequence At3g28470 as bait. The best hit obtained corresponds to the gene Solyc03g059200, considered the least-diverged ortholog, which we renamed as SlTDF1 (Solanum lycopersicum TDF1). The protein comprises 263 amino acids and contains the conserved R2R3 MYB domains that characterize this subfamily and that correspond to the putative DNA binding domain (Figure S1). Two putative nuclear localization signals (NLS) were also identified, flanking the MYB domains (Figure S1). Phylogenetic analysis using TDF1-like proteins from different plant species and other R2R3-type MYB proteins showed a close relationship between Arabidopsis TDF1 (At3g28470) and SlTDF1 (Solyc03g059200) that grouped in a cluster together with the previously described TDF1 orthologs of rice and barley (Cai et al., 2015; Hua et al., 2023) (Figure 1A; Table S1).
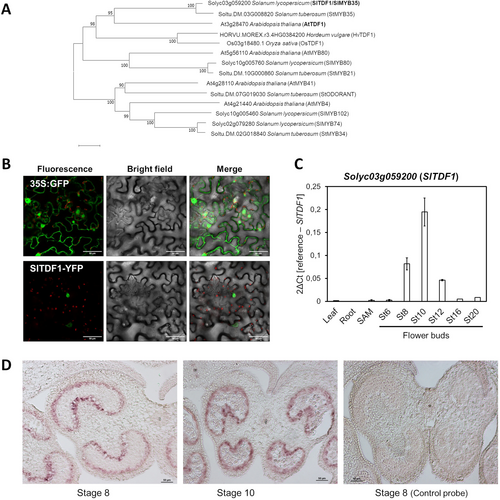
We investigated the subcellular localization of the SlTDF1 protein using a construct that contains a translational fusion of SlTDF1 to YFP driven by a constitutive promoter (35S promoter from CaMV). The 35S:GFP construct was used as a control for the infiltration experiments in Nicotiana benthamiana leaves. In the control plants, the protein was located in both the cytoplasm and the nucleus of the epidermal cells (Figure 1B). In contrast, we observed transient expression of the chimeric SlTDF1-YFP protein exclusively in the nucleus of the cells (Figure 1B), in agreement with its putative role as a transcription factor involved in the regulation of gene expression.
The expression pattern of SlTDF1 was analyzed using qRT-PCR and in situ hybridization essays. First, total RNA was obtained from different tissues and developmental stages of tomato plants, and the expression of the gene was analyzed using qRT-PCR. The transcript of the gene was mainly detected in floral buds from floral stage 8 (pre-meiotic) to anthesis with a maximum of expression at floral stage 10 (tetrad of micropores). Conversely, the TDF1 transcript was hardly detectable in seedlings and young leaves (Figure 1C). In situ hybridization experiments were performed using a transcript-specific riboprobe to determine the temporal and spatial pattern of expression during anther and pollen development. The expression of the gene was visible from floral stage 8 after anther layers differentiated associated with the tapetum and remains intense after meiosis (Figure 1D).
3.2 Knockout of SlTDF1 leads to male sterility and seedless fruit formation in Solanum lycopersicum
CRISPR/Cas9 technology was used to generate loss-of-function mutations in the SlTDF1 gene of tomato. The commercial cultivar Moneymaker was used as the genetic background. Using online target-designing tools, we selected a single target region on the distal part of the first exon of the gene (Figure 2A). A construct with the required elements to edit the gene was generated (see methods) and introduced in Agrobacterium tumefaciens to transform tomato plants. Primary T0 transformants were obtained after organogenesis under kanamycin selection and, after elongation and rooting, 22 independent diploid plants were transferred to the greenhouse. To identify CRISPR/Cas9-induced changes in SlTDF1 sequence, a fragment of 559 bp flanking the targeted sequence was amplified by PCR and sequenced. The chromatograms were analyzed using the TiDe (Brinkman et al., 2014) and ICE v2 CRISPR Analysis (https://ice.synthego.com/#/) tools that estimate the frequency and type of edition (insertions and deletions of bases) obtained in the targeted gene. Only diploid plants with a percentage of edition over 80% (Supplemental Table S4) were further analyzed at the floral stage to assess the phenotypical effect of the mutations in the development of the anthers. All the remaining lines (9) showed collapsed anthers devoid of pollen and were hand pollinated to maintain the mutant plants.

The F2 populations of two of the crosses were grown in the greenhouse and genotyped by sequencing to select stable mutant plants that did not contain the CRISPR/Cas9 construct. These plants correspond to the mutant alleles Sltdf1-1 and Sltdf1-2 used during this research and showed deletions of 1 and 2 nucleotides, respectively (Figure 2B). The mutations introduced changes in the open reading frame of the sequence, leading to a premature stop codon that would modify the length of the putative transcript and protein (Figure 2B). At anthesis, the anthers of the mutant plants showed collapsed locules that did not contain viable pollen (Figure 2C). Male sterility and aberrant anther development have been associated with the production of seedless fruits in tomato plants (Hao et al., 2017; Medina et al., 2013; Okabe et al., 2019; Rojas-Gracia et al., 2017). Sltdf1 mutant plants produced fruits with reduced size and weight and, as expected, the fruits did not contain any seeds (Figure 2D).
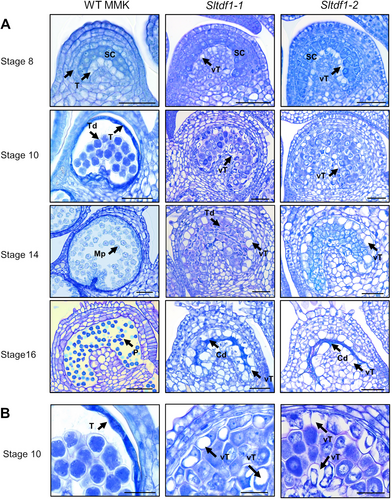
The SlTDF1 gene was anticipated to be involved in pollen development according to its pattern of expression and to previously functional studies of homolog genes in Arabidopsis and rice (Cai et al., 2015; Zhu et al., 2008). Therefore, the effect of the mutations of the gene in the process of male gametogenesis was analyzed using histological sections of anthers in different stages of development (Figure 3). Wild-type and mutant anthers were undistinguishable during the early stages until floral stage 8 (premeiotic), when tapetal cells from the inner tapetum showed abnormal vacuolation in the mutant anthers. Shortly, wild-type sporogenous cells underwent meiosis, appearing as tetrads enclosed by a callose envelope and separated from a dense tapetum. Callose degradation results in the separation of microspores into the locule that appear as individual cells in the following stages. In contrast, at stage 10 the mutant anthers showed vacuolated tapetum surrounding microspore mother cells that seem to have initiated meiosis (Figure 3B). In the subsequent stages, tetrads are visible in the mutant anthers, but meiocytes are unable to separate and start to degenerate and collapse. The highly vacuolated tapetum is still visible in the mutant anthers at floral stage 16 where all the content of the locule appears as a dense debris of cell material (Figure 3). Both mutant alleles showed complete male sterility, confirming that SlTDF1 is essential for pollen formation in tomato plants.
3.3 Effect of SlTDF1 mutation in genes controlling tapetum development and function
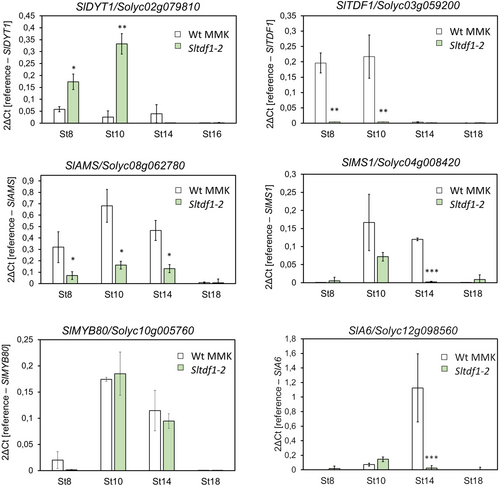
In Arabidopsis plants, TDF1 was described as part of a genetic pathway for the control of tapetum development and function. The transcription factors involved in the pathway (DYT1-TDF1-AMS-MYB80-MS1) show sequential and partially overlapping expression patterns during anther development (Zhu et al., 2011). DYT1 (DYSFUNCTIONAL TAPETUM1) and AMS (ABORTED MICROSPORES) genes encode bHLH transcription factors (Sorensen et al., 2003; Zhang et al., 2006), while MS1 (MALE STERILITY1) encodes a protein with a PHD-finger motif that also acts as a transcriptional regulator (Ito and Shinozaki, 2002; Lou et al., 2018). We analyzed the effect of SlTDF1 gene mutation in the expression of the putative gene homologs that constitute this tapetum-specific pathway in tomato plants. Homologs have been described for SlDYT1/ Ms1035 gene (Solyc02g079810) (Jeong et al., 2014; Jung et al., 2020) and SlAMS (Solyc08g062780) (Bao et al., 2022). MYB80 (formerly known as MYB103 or MS188) and MS1 from Arabidopsis were used to identify the corresponding tomato SlMYB80 (Solyc10g005760) and SlMS1 (Solyc04g008420) homologs.
To obtain information on the role of the SlTDF1 gene in the network of genes regulating tapetum development in tomato plants, we analyzed how the mutation affects gene expressions using qRT-PCR. We analyzed four floral stages from the pre-meiotic stage (stage 8) to anthesis (stage 18). The expression of SlDYT1 showed an important up-regulation at floral stages 8 and 10 in mutant plants where SlTDF1 is nearly indetectable (Figure 4), in agreement with the proposed repressive role of TDF1 on DYT1 expression (Lou et al., 2018). On the other hand, we detected strong downregulation of SlAMS and SlMS1 in the absence of SlTDF1, while the SlMYB80 transcript level remained unchanged (Figure 4). Besides the genetic network involved in tapetum development, we analyzed the expression of Solyc12g098560 (SlA6), homolog to the Arabidopsis A6/MEE48 gene involved in callose dissolution (Damayanti et al., 2019; Hird et al., 1993). The expression of this gene is highly downregulated at floral stage 14 in the mutant background (Figure 4), in agreement with the failure observed in microspore separation that leads to anther collapse and male sterility (Figure 3). In Arabidopsis, expression analyses in different mutant backgrounds suggested that A6 acts downstream of MYB80 (Zhang et al., 2007), although it does not seem to be by direct regulation (Phan et al., 2011). In agreement with these results, SlMYB80 expression is not sufficient to activate SlA6 expression in the Sltdf1 mutant background.
3.4 Knockout of the TDF1 homolog in the wild species Solanum pimpinellifolium
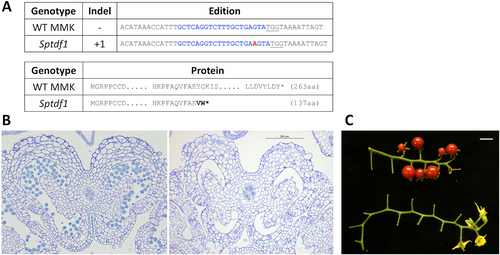
After the characterization of the SlTDF1 gene and mutant lines, we generated knock-out plants in the S. pimpinellifolium (SPI) background. SPI is a full wild species from which the cultivated tomato varieties are proposed to have been derived by domestication (Blanca et al., 2022). The existence or absence of a link between male sterility and parthenocarpy in wild species would shed light on the origin of this trait. To obtain male sterile SPI lines we used the same construct as for S. lycopersicum given that it targets an identical sequence in the S. pimpinellifolium genome. After in vitro transformation, a single mutant line showing a high rate of edition (over 80%) was obtained and it was hand-pollinated using wild-type pollen. Only homozygous mutant plants from the following generations were used for phenotyping. The mutant line contained an insertion of a nucleotide (adenine) that caused a shift in the frame of the transcript (Figure 5A). The introduction of a premature stop codon would result in the production of a truncated protein and, therefore, can be considered a null mutant allele. The mutant lines showed complete male sterility and did not produce pollen (Figure 5B), showing similar developmental defects to the ones observed in Sltdf1 mutant alleles (Supplemental Figure S2). This includes premature vacuolation of the tapetum, absence of callose degradation, microspore degeneration, and anther locule collapse (Supplemental Figure S2). However, in contrast with the phenotype of the lines obtained in the S. lycopersicum background, these plants never set fruits (Figure 5C), suggesting that the production of seedless fruits is not an ancestral trait in the Solanaceae species.
3.5 Changes in the regulation of gibberellin-related genes in the ovary of male sterile plants and its relationship with parthenocarpy
Auxins and gibberellins are two key hormones that regulate ovary growth during fruit initiation. Several independent results also highlight a predominant role of gibberellins in the formation of parthenocarpic fruits in male sterile plants (Hao et al., 2017; Medina et al., 2013; Okabe et al., 2019; Rojas-Gracia et al., 2017; Takei et al., 2019). All these results have been obtained from different tomato cultivars that originated after domestication and breeding.
To investigate the role of gibberellins in parthenocarpic fruit development in tdf1 mutants, we treated plants with the gibberellin biosynthesis inhibitor Paclobutrazol (PCB). PCB treatment suppressed parthenocarpic ovary growth in Sltdf1 mutants (Supplementary Figure S3), indicating that gibberellins are required for parthenocarpic fruit development in Sltdf1 mutants.
To get insight into the molecular mechanisms that connect male sterility with parthenocarpy in modern tomato cultivars, we studied the transcriptional changes that occur in the ovary in both parthenocarpic and non-parthenocarpic male-sterile lines obtained in S. lycopersicum and S. pimpinellifolium. In tomato plants, the network of genes involved in GA biosynthesis and response is well characterized (Chen et al., 2016; Ezura et al., 2023; Martí et al., 2007). We analyzed the expression of enzymes involved in GA biosynthesis (GA 3-oxidases; SlGA3ox and GA 20-oxidases; SlGA20ox) and GA deactivation (GA 2-oxidases; GA2ox); GA sensing (GA receptors GID) and GA response (DELLA/PROCERA) in ovaries before and after flower anthesis. GA20ox genes rise after flower opening in the wild-type parental lines (Figure 6) except for GA20ox2. In comparison with S. lycopersicum, the level of expression of some genes involved in GA biosynthesis (GA20ox3 and GA3ox2) increased at higher levels in the wild species. Interestingly, the parthenocarpic genotypes (S. lycopersicum background) showed constitutive high expression before and after flower anthesis, except for GA20ox2 (Figure 6). The effect is more conspicuous in the GA20ox1 and GA3ox2 genes. Regarding genes involved in GA deactivation (GA2ox genes), the pattern of expression was similar in parthenocarpic and non-parthenocarpic genotypes regardless of the genetic background (Supplemental Figure S3). On the other hand, the transcript level of two of the three GID1 analyzed (GID1b1 and GID1b2) showed a high level of expression before and after flower opening in the parthenocarpic genotype (Figure 6) in contrast with the activation mediated by pollination in wild-type plants.
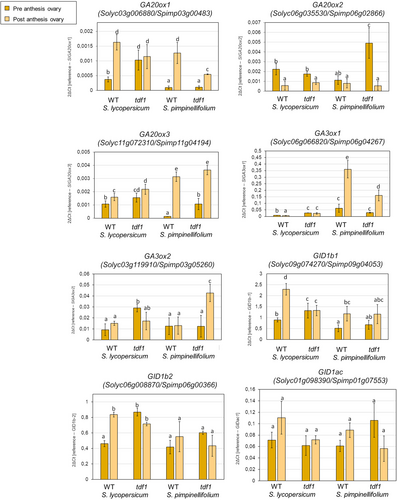
In summary, early activation of GA biosynthesis genes (SlGA20 and SlGA3 oxidases) and of GA receptors (SlGID1b1 and SlGID1b2), which are activated after pollination in wild-type tomato plants, could be related to parthenocarpic fruit development in male-sterile SlTDF1 mutants. This early activation of GA-related genes is not present in unpollinated S. pimpinellifolium plants or male-sterile Sptdf1 mutants, which do not produce parthenocarpic fruits. Indeed, treatment of Sptdf1 mutants with GA3 induced parthenocarpic ovary growth (Supplemental Figure S3B), confirming that parthenocarpy can be achieved by increasing GA levels in the ovary also in S. pimpinellifolium.
3.6 Analysis of GA-related promoter variations associated with the parthenocarpic trait
GA20ox and GA3ox enzymes are involved in the last steps of bioactive GAs biosynthesis. The expression of these genes is regulated at the transcriptional level in response to developmental and environmental signals, but also some transcription factors involved in the direct regulation of this pathway have been identified in several species (reviewed by (Hedden, 2020). In tomato plants, changes in promoters (usually deletions) that affect the binding of TFs account for the acquisition of novel traits during tomato domestication (Ye et al., 2017; Zhu et al., 2023).
We analyzed the promoters of the genes involved in GA synthesis and response in the available S. lycopersicum (Heinz 1706) and S. pimpinellifolium (LA2093) backgrounds, looking for changes in these sequences that could affect the binding of regulatory factors (see methods). Using this in silico analysis, we identified polymorphisms in the promoters of five genes that disturb the sequence of seven putative binding sites (Supplemental Table S5). To confirm the presence of this polymorphism we sequenced these regions from a collection of genomic DNA from two wild species (Solanum pennellii, SPE, and Solanum pimpinellifolium, SPI), two semi-domesticated Solanum lycopersicum var. cerasiforme (SLC) accessions and four domesticated Solanum lycopersicum var. lycopersicum (SLL) cultivars (Microtom, Moneymaker, P73, M82). Parthenocarpy has been reported in these four cultivars associated with male sterility (Hao et al., 2017; Rojas-Gracia et al., 2017; Salazar-Sarasua et al., 2022). The SLC accessions are part of a collection of 121 S. lycopersicum var. cerasiforme from Ecuador, Peru, Mexico, and several countries from Mesoamerica that show extensive diversity in fruit, flower, and vegetative traits (Mata-Nicolás et al., 2020). The two accessions (GBV006768 y GBV007931) were classified according to their geographical origin into the SLC_Ecuador and SLC_Mexico groups, the first being the more diverse SLC population and the closest to S. pimpinellifolium (Mata-Nicolás et al., 2020).
The results showed that two of the genes analyzed, SlGA20ox1 and SlGA3ox2, presented deletions in the promoter regions (Figure 7) that seem to correlate with domestication and parthenocarpy. The promoter of the SlGA20ox1 gene showed deletions on putative binding sites described in Arabidopsis for the BASIC PENTACYSTEIN (BCP) transcription factors (Petrella et al., 2020). A second binding for TCX2-like proteins (Andersen et al., 2007) presented a deletion of twelve nucleotides in the two semi-domesticated S. cerasiforme accessions and the four domesticated tomato cultivars (Figure 7A). In the promoter of SlGA3ox2, we identified a small deletion of three nucleotides in a putative binding site for CYCLING DOF FACTOR (CDF)-like proteins (Corrales et al., 2017) in all modern tomato cultivars. Interestingly, only one of the semi-domesticated accessions (GBV006768) showed the deletion (Figure 7B). In summary, the tree putative binding motifs identified in the promoters of genes involved in GA biosynthesis are present in the tomato wild relatives S. pennellii and S. pimpinellifolium and showed deletions in domesticated S. lycopersicum varieties (SLL and SLC).
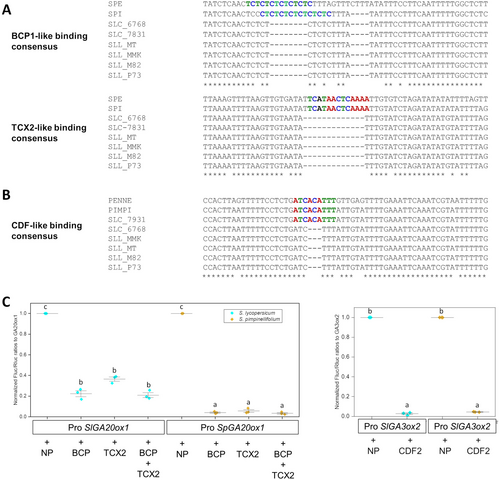
We analyzed the presence of these binding motifs in other tomato accessions and wild species using an in silico approach to determine whether the deletion occurred during domestication (Supplemental Table S6). The putative binding motifs for BCP1-, TCX2- and CDF-like proteins were present in all the analyzed wild species but were deleted in the semi-domesticated S. lycopersicum var. cerasiforme and all analyzed cultivars of S. lycopersicum. This suggests that the identified promoter deletions arose during domestication.
To determine whether these polymorphisms affect the binding of transcription factors, we analyzed the impact of the deletions on LUC expression driven by these promoters. For the GA20ox1 promoters, we tested the binding of BCP1 (Solyc04g008380) and TCX2 (Solyc12g007180) individually and in combination (Figure 7C). When targeting the promoter from S. pimpinellifolium, there was almost complete repression with both transcription factors, indicating that they bind to the promoter and effectively repress transcription. In contrast, the SlGA20ox1 promoter from SL retained transcriptional activity even in the presence of the two repressors, with relative transcriptional activity (RTA) ranging from 20% to 40%. In the case of the GA3ox2 promoters, CDF2 (Solyc05g007880) was able to fully repress expression regardless of the presence of polymorphism. In summary, BCP1 and TCX2 act as strong repressors of transcription driven by the SpGA20ox1 promoter. However, deletions in the GA20ox1 promoter of S. lycopersicum reduce binding affinity, allowing partial transcription to be maintained.
4 DISCUSSION
4.1 Role of SlTDF1 in tapetal cell function and pollen development in tomato
TDF1 orthologs were isolated and characterized in Arabidopsis and two crops: rice and barley (Cai et al., 2015; Hua et al., 2023; Zhu et al., 2008), suggesting strong functional conservation in monocot and dicot plants. The tomato SlTDF1 gene (Solyc03g059200) characterized here is the first ortholog described in a plant bearing fleshy fruits. Mutations in these genes disturb the development of the tapetum, the innermost somatic cell layer that surrounds the sporogenous tissue, resulting in male sterility. It has been reported in Arabidopsis that TDF1 is necessary for correct tapetal differentiation and subsequent PCD (Wu et al., 2023), which correlates to incorrect morphology and absence of degradation in tapetal cells of the Sltdf1 mutants. TDF1 homologs encode MYB-type transcription factors, one of the largest family of transcription factors in plants. In Solanum lycopersicum, 127 MYB genes have been identified and classified into 18 subgroups based on phylogenetic analyses and domain similarity (Li et al., 2016). The R2R3-MYB subclass is specific to plants and the most abundant, with 122 members in tomato, with reported functions in diverse processes, including plant defence against abiotic and biotic stresses and the formation of developmental structures of the plant such as glandular trichomes (Chalvin et al., 2020), stomata, reproductive floral organs (Ma et al., 2012; Makkena et al., 2012), and seeds (Zhang et al., 2013).
SlTDF1 corresponds to the uncharacterized SlMYB35 protein, which, according to our study, has a key role in pollen formation in tomato plants. The gene was specifically expressed in tapetal cells at the early stages of anther development, regulating the progression of tapetum and, by extension, microspore formation. According to the phylogenetic analysis of MYB genes in S. lycopersicum, SlTDF1/SlMYB35 is closely related to SlMYB80, and they have a similar pattern of expression in developing flower buds (Li et al., 2016). In Arabidopsis, mutations in TDF1 or MYB80 (also named MYB103/MS188) genes show similar tapetal defects leading to microspore arrest and degeneration (Zhang et al., 2007; Zhu et al., 2008), suggesting that these genes are putative paralogs. In tomato floral buds, we detected normal levels of expression of SlMYB80 in the absence of SlTDF1 function (Figure 4), suggesting that both genes are regulated independently during tapetal cell development. The obtaining and study of double mutant plants in these two genes could shed some light on the possible redundant functions of SlTDF1 and SlMYB80.
Tapetum development and function require the coordinated activation of a network of transcription factors, including DYT1-TDF1-AMS-MS188/MYB80-MS1 genes (Wei and Ma, 2023). DYT1 encodes a putative basic helix–loop–helix (bHLH) transcription factor and is a direct regulator of TDF1 expression (Gu et al., 2014). Downstream in this network, TDF1 activates AMS expression by the direct binding of sequences in the AMS promoter. In addition, TDF1 and AMS physically interact to regulate common target genes, forming a feed-forward loop during anther development (Lou et al., 2018). Here, we showed that in the absence of SlTDF1 function, the expression of SlDYT1 increases sharply in the early flower stages (Stage 8 and 10; Figure 4), supporting the existence of feedback regulation of SlDYT1 by SlTDF1, also suggested in Arabidopsis (Lou et al., 2018). In contrast, the expression of SlAMS, SlMS1, and SlA6 (a putative callase) was highly reduced in Sltdf1 mutants (Figure 4), suggesting that the expression of these genes is dependent on SlTDF1 function. These results confirm the strong conservation of the genetic network among distant flowering plant species.
4.2 Parthenocarpy and domestication in tomato plants
In tomato flowers, self-pollination is favoured by the relative disposition of the reproductive floral organs, with the stamens arranged in a cone around the inserted stigma. However, fertilization of the ovules is not a strict requirement for fruit development since pollen tube penetration into the ovary triggers fruit set (Tran et al., 2023). Fruit setting in the absence of fertilization is an attractive trait for the food industry and consumers and, therefore, has attracted significant research attention. The formation of parthenocarpic fruits could be achieved in tomato by several means, including exogenous treatment with hormones to the unpollinated ovaries, knockout of specific genes, or transgenic studies (Joldersma and Liu, 2018; Sharif et al., 2022). The role of the stamens in the control of ovary growth emerged from the study of mutants in B-type MADS-box genes required for petal and stamen identity. Mutants in the tomato APETALA 3 (AP3) homolog TAP3/STAMENLESS show distorted stamens that never produce pollen and develop parthenocarpic fruits (Gómez et al., 1999; Quinet et al., 2014). Similarly, a loss of function mutation in the B-class gene PISTILLATA (PI) in Malus domestica (apple tree) results in parthenocarpic fruit development (Yao et al., 2001). More recently, several genes involved in different steps during male gametogenesis have been identified in tomato plants. Mutations in HYDRA/SPOROCYTELESS-like (Hao et al., 2017; Rojas-Gracia et al., 2017), SlTPD1 (Salazar-Sarasua et al., 2022), SlAMS (unpublished results) and SlTDF1 (this work) cause male sterility and parthenocarpy suggesting that repressive signals originated during male gametogenesis could inhibit the growth of the unpollinated ovary before fertilization.
In this study, we evaluated the occurrence of male-induced parthenocarpy in the wild relative S. pimpinellifolium by obtaining and analyzing mutants devoid of pollen (Figure 5). Unlike the mutants obtained in the commercial cultivar (Figure 2), fruit-set was never observed, suggesting that parthenocarpy triggered by male sterility could be a trait acquired during domestication. Parthenocarpy is a trait extensively observed in horticultural crops from a variety of plant families, including Cucurbitaceae, Solanaceae, and Rosaceae (Sharif et al., 2022). However, a bibliographic survey reported the occurrence of parthenocarpy in 96 Angiosperm taxa, including many wild species, suggesting a possible adaptative role for parthenocarpy (Picarella and Mazzucato, 2019). In tomato, several so-called natural sources of parthenocarpy were obtained from crosses between cultivated and wild relatives in the last decades of the last century (Gorguet et al., 2005). Two of the genes have been cloned so far, the parthenocarpic gene Pat-k corresponds to a mutant allele in the SlAGAMOUS-LIKE 6 (SlAGL6) locus, an E-class MADS-box protein involved in ovule development (Takisawa et al., 2018) and pat-2 contains a recessive mutation in a zinc finger homeodomain protein (Takisawa et al., 2020). Still, most of the parthenocarpic genotypes seem to be the result of an exceptional combination of genes after wide hybridization between wild and cultivated tomato species rather than the modification of individual genes (Picarella and Mazzucato, 2019). This idea is also in agreement with a higher proportion of parthenocarpy associated with polyploid species (Picarella and Mazzucato, 2019).
Regardless of its origin, many parthenocarpic genotypes have a common feature showing increased levels of hormones in the ovary, mainly gibberellins (GAs) and auxins (Fos et al., 2000; Goetz et al., 2007; Martí et al., 2007; Medina et al., 2013; Okabe et al., 2019). Several studies showed that endogenous phytohormone levels correlate with the transcriptional modulation of genes involved in hormone biosynthesis or response. In tomato plants, the transcription level of genes encoding GA20-oxidases, and especially SlGA20ox1, increases dramatically after pollination and fruit growth (Rebers et al., 1999; Serrani et al., 2007). Similarly, several parthenocarpic genotypes show precocious activation of this GA biosynthetic gene in immature or unpollinated ovaries before anthesis (Martí et al., 2007; Medina et al., 2013; Okabe et al., 2019; Takei et al., 2019). GA20-oxidases and GA3-oxidases catalyze the last steps to produce bioactive GAs. In tomato plants, pollination activates the transcription of GA20ox in the ovary but not GA3ox genes, suggesting that the activity of GA20ox enzymes is the limiting factor in unpollinated ovaries (Serrani et al., 2007). We detected specific deregulation of SlGA20ox1 expression in the ovary of the male-sterile sltdf1 mutants, showing constitutive activation of the gene in unpollinated and immature ovaries before flower anthesis (Figure 6). To a minor extent, SlGA3ox2 expression is also constitutively activated in the ovary of the parthenocarpic plants. This has also been observed in other parthenocarpic mutants, where the expression of SlGA3ox1 and SlGA3ox2 increased in the ovary at different stages of development (Niu et al., 2024; Rojas-Gracia et al., 2017). While SlGA3ox2 is not activated in the ovary after pollination, it seems to be important for parthenocarpy triggered by male sterility. Interestingly, both genes showed changes in putative regulatory motifs located in the promoter regions (Figure 7). However, only the changes in the regulatory motifs of the GA20ox1 promoter seem to influence the expression when coupled with the corresponding transcription factors, suggesting that binding affinity to these transcription factors is reduced due to the deletions in the putative binding sites acquired during domestication. Notably, although the changes detected in the SlGA3ox2 promoter are comparatively smaller than those observed in the SlGA20ox1 promoter, they have been stabilized in current tomato varieties. However, we did not observe that this deletion causes a reduction in SlCDF2-mediated regulation of the gene, although it could have an effect in a natural genomic context. This points to a higher contribution of the SlGA20ox1 gene to parthenocarpic fruit development.
Globally, our results suggest that changes in the regulatory regions of multiple rather than loss-of-function mutations in single genes may have been acquired during domestication, favouring parthenocarpic fruit growth under special developmental or environmental circumstances that compromise normal fruit development. Several traits selected during the domestication of crops are caused by changes in the regulatory regions of promoters or in the regulation of protein activity. For instance, the transition from exerted to inserted stigmas in cultivated tomatoes is partly mediated by a mutation in the promoter of Style2.1 that results in a downregulation of the gene, which was selected during domestication and breeding, favouring self-pollination and improved fruit production (Chen et al., 2007). For other traits, such as plant architecture or fruit size, alleles with changes in promoters or protein activity that confer quantitative trait modifications seem to have been favoured during domestication (Soyk et al., 2017; Xu et al., 2015). This implies that not only loss of function mutation but also changes in the genome that affect gene expression or protein activity have been acquired during the domestication and breeding of modern tomato cultivars.
GA homeostasis is highly regulated during plant growth and in response to environmental conditions. The amount of bioactive GAs depends on a tight balance between GA synthesis and catabolism. Several families of transcription factors, including HD-ZIP, TCP, AP2/ERF, bHLH, and WRKY are involved in the transcriptional regulation of GA biosynthetic genes. In tobacco (Nicotiana tabacum), a KNOX homeodomain protein (NTH15) was found to directly control the expression of a GA20 oxidase (Ntc12 gene) through the binding of sequences located in the first intron (Sakamoto et al., 2001). Similarly, the MADS-box protein SVP (SHORT VEGETATIVE PHASE) inhibits flowering in Arabidopsis by the direct regulation of AtGA20ox2 expression (Andrés et al., 2014). In this study, we identify changes in the promoters of two tomato genes involved in GA biosynthesis (SlGA20ox1 and SlGA3ox2) between wild and domesticated tomato species (Figure 7). Additionally, we confirmed that the modifications in the SlGA20ox1 promoter, but not in SlGA3ox2, affect transcriptional regulation by altering the binding of regulatory proteins, specifically BCP1 and TCX2 transcription factors (Figure 7C). The function of the Arabidopsis TCX proteins TSO1, TCX2/SOL2, and TCX3 has been associated with the control of the cell cycle in different organs and tissues regulating stem-cell-specific gene networks (Clark et al., 2019; Simmons et al., 2019). TCX2 coordinates cell division regulating stem-cell-specific gene networks in the root and shoot apex. According to the expression Arabidopsis atlas, TCX2 is expressed in the ovary of the flower, but the function of the protein during the reproductive phase has not been investigated. Regarding BCP proteins, they belong to a still poorly characterized transcription factor family with roles in meristem size maintenance and seed development (Petrella et al., 2020; Simonini and Kater, 2014). Interestingly, BCPs act as direct regulators of cytokinin responses in the meristem binding to the promoter of the ARR7 (ARABIDOPSIS RESPONSE REGULATOR 7) gene (Simonini and Kater, 2014). Five tomato CDF homologs have been identified, and three of them (SlCDF2, SlCDF4, and SlCDF5) are expressed at high levels during flower and fruit development (Corrales et al., 2014). The authors propose a function for SlCDFs in the regulation of flowering time and the response of tomato plants to abiotic stresses. The function of these transcription factors in the control of ovary growth in tomato plants needs to be confirmed by further investigation. The results obtained in this study suggest that the parthenocarpic trait could be implemented by breeders through the complete deletion of the identified binding domains for these repressor proteins and may serve as a promising biotechnological target for developing tomato varieties with enhanced parthenocarpic capacity.
AUTHOR CONTRIBUTIONS
CG-M designed the research and wrote the grant that founded the project. BS-S, ER, CG-S, CG, and JG-S performed the experiments. AB supervised the bioinformatic analyses. CG-M wrote the manuscript. LAC and JPB supervised the study and reviewed the manuscript. All the authors agreed with the final version of the manuscript.
ACKNOWLEDGMENTS
We thank Diego Orzáez for providing GoldenBraid parts and Maricruz Rochina for expert technical assistance during the project.
FUNDING INFORMATION
This work was supported by grant PID2021-123705OB-I00 funded by MCIN/AEI/ 10.13039/501100011033 and by FEDER “A way of making Europe”.
DISCLOSURES
The authors declare that there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are contained in the manuscript or the supporting material available online.




