Auxin biodynamics and its integral role in enhancing plant resilience to environmental cues
Abstract
Auxins are essential plant hormones that regulate growth, development, and responses to environmental stressors. Plants frequently encounter challenges such as pests, diseases, high temperatures, drought, and salinity, which necessitate adaptive mechanisms for survival. Auxins modulate stress-responsive signaling pathways by regulating gene expression and interacting with other phytohormones, thereby influencing physiological processes that maintain homeostasis under stress conditions. This review elucidates the molecular mechanisms through which auxins mediate plant responses to biotic and abiotic stresses. The findings indicate that auxins are pivotal in activating defense mechanisms and regulating stress signaling pathways. Differential expression of auxin-related genes has been observed in various crops under stress conditions, underscoring their role in enhancing resistance against pathogens and improving drought tolerance. Additionally, auxins influence root architecture and growth responses, facilitating adaptations such as trichome development for defense against herbivory. Moreover, the interplay between auxin signaling and other phytohormones is crucial for effective stress responses. Overall, auxins play a multifaceted role in enabling plants to cope with environmental stresses by regulating growth and activating defense mechanisms. Understanding these complex signaling pathways involving auxins can inform future research aimed at engineering resilient plant varieties capable of thriving in changing climates. Further studies are needed to clarify the specific functions of auxin in various stress contexts and to develop practical applications for crop improvement.
1 BACKGROUND
Auxins are essential plant hormones that play a critical role in regulating growth, development, and responses to stressors through their metabolism, transport, and signaling mechanisms (Enders and Strader 2015, Liu et al. 2023). Plants are frequently exposed to various biotic and abiotic stresses, including pests, diseases, drought, heat, and salinity, which necessitate the development of adaptive mechanisms for survival (Ali et al. 2018, Khan et al. 2018, Ali et al. 2020a, Yang et al. 2022a). Auxins facilitate these adaptations by modulating gene expression and interacting with other phytohormones, thereby influencing physiological processes that maintain homeostasis under stress conditions. The significance of auxins extends beyond the regulation of growth; they also play a critical role in mediating plant responses to environmental stresses (Jing et al. 2023). Instead, they have evolved intricate signaling pathways that allow plants to perceive environmental cues and respond appropriately. This adaptive capacity is facilitated by the modulation of gene expression significantly influenced by auxins. For instance, during periods of drought or salinity stress, auxin levels fluctuate, leading to alterations in root architecture that enhance water uptake or minimize transpiration. Such modifications are essential for maintaining homeostasis within the plant (Ribba et al. 2020, Gurme et al. 2023). Research has shown that auxins interact with other phytohormones, such as abscisic acid (ABA), jasmonic acid (JA), and salicylic acid (SA), to orchestrate a coordinated response to stress (Mazzoni-Putman et al. 2021). This crosstalk among hormones is critical for activating defense mechanisms against pathogens and managing physiological responses under abiotic stress conditions. For example, auxin signaling pathways can enhance resistance against viral infections in crops by inducing the transcription of specific defense-related genes (Hoffmann et al. 2011, Feng et al. 2015). The interplay between auxin signaling and other hormonal pathways not only determines the plant's ability to respond to immediate threats but also shapes its long-term resilience.
The function of auxins in biotic stress responses is particularly noteworthy. Auxins have been implicated in activating defense mechanisms against herbivores and pathogens through various means, including the modulation of trichome development and the reinforcement of cell walls. Trichomes serve as physical barriers against insect attack, while lignification enhances structural integrity against pathogen invasion. Furthermore, auxin-responsive genes playing significant roles in these processes have been identified, indicating that manipulating auxin levels is a viable strategy for enhancing crop resistance (Gomes and Scortecci 2021, Bao et al. 2024). In addition to their role in biotic stress responses, auxins are also crucial for managing abiotic stresses. For instance, during drought conditions, plants often exhibit increased expression of auxin biosynthesis genes, leading to enhanced production of indole-3-acetic acid (IAA), which is the most prevalent type of auxin (Im Kim et al. 2013). This increase can trigger physiological adaptations such as deeper root growth or altered stomatal behavior to optimize water use efficiency. Similarly, under salinity stress, auxins can modulate root architecture and promote lateral root formation to improve nutrient uptake from saline soils. Furthermore, under heavy metal stress or osmotic pressure from drought or salinity, plants adjust their internal auxin levels through biosynthesis and transport mechanisms (Korver et al. 2018, Yang and Wang 2023). This regulation allows plants to adapt their growth patterns accordingly—either by promoting root elongation or altering leaf morphology—to mitigate the effects of these stresses (Shi et al. 2014, Ding et al. 2015).
Despite the well-documented roles of auxins in mediating plant responses to stress, many aspects remain poorly understood. The complexity of auxin signaling pathways—encompassing biosynthesis, transport, perception, and signal transduction—poses significant challenges for researchers aiming to unravel their precise mechanisms in different stress contexts (Jing et al. 2023). Moreover, variations in auxin response among different plant species add another layer of complexity that must be considered when developing strategies for crop improvement. Recent advancements in molecular biology techniques have enabled researchers to delve deeper into the genetic basis of auxin-mediated stress responses. High-throughput sequencing technologies and gene editing tools such as CRISPR/Cas9 are being employed to elucidate the roles of specific auxin-responsive genes under different stress conditions (Xu et al. 2022, Ali et al. 2023, Yang et al. 2023). These approaches hold promise for identifying key regulatory nodes within auxin signaling pathways that could be targeted for enhancing plant resilience. Despite these advancements in understanding the function of auxins in plant resilience in stress conditions, there remains a significant gap in knowledge regarding the precise molecular mechanisms involved. Many studies have focused on identifying specific genes associated with auxin signaling; however, comprehensive analyses detailing how these genes interact within broader signaling networks are still lacking. The integration of knowledge regarding auxin function with modern breeding techniques presents an exciting opportunity for improving crop performance under stress conditions. By understanding how auxins interact with other signaling pathways and how they influence physiological traits associated with stress tolerance, it may be possible to engineer crops better equipped to withstand biotic and abiotic challenges.
This review aims to provide a comprehensive overview of the molecular mechanisms through which auxins mediate plant responses to biotic and abiotic stresses. Specifically, it seeks to elucidate the crosstalk between auxin signaling pathways and other hormonal responses while highlighting the implications of these interactions for developing strategies aimed at enhancing plant resilience. We identify gaps in the current knowledge regarding the mechanistic roles of auxins in stress mitigation and propose future research directions that could lead to practical applications in crop improvement through targeted manipulation of auxin signaling pathways.
2 AUXIN BIOSYNTHESIS IN PLANTS
IAA is recognized as the most extensively studied natural auxin in plants, playing a crucial function in the regulation of numerous aspects of plant growth and development. The primary precursor for IAA synthesis is L-tryptophan (Trp), which has been identified as an essential building block in the biosynthetic pathways leading to IAA production (Enders and Strader 2015). Recent research has shown that the levels of auxin, including IAA, are regulated through both its production and inactivation (Casanova-Sáez et al. 2021). This regulation is crucial under different stress conditions, such as extreme temperatures, drought, and salinity (Figure 1a-c). The primary pathway for IAA biosynthesis involves converting tryptophan (Trp) into IAA through several proposed mechanisms, including the indole-3-acetaldoxime (IAOx), indole-3-acetamide (IAM), and indole-3-pyruvic acid (IPyA) pathways (Mano and Nemoto 2012). Among these pathways, the IPyA route is recognized as the main pathway for IAA production. This process occurs in two steps: first, tryptophan is deaminated to form indole-3-pyruvic acid through the action of TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS 1 (TAA1), followed by decarboxylation catalyzed by flavin-containing monooxygenases from the YUCCA family, leading to IAA production (Zhao 2014, Cao et al. 2019, Casanova-Sáez et al. 2021). The IAM pathway involves converting tryptophan to indole-3-acetamide by tryptophan monooxygenase, which is then hydrolyzed to IAA and ammonia by indole-3-acetamide hydrolase (Mano and Nemoto 2012). In the IAOx pathway, tryptophan is converted to indole-3-acetaldoxime by cytochrome P450 enzymes (CYP79B2 and CYP79B3), which can be further processed into intermediates such as indole-3-acetonitrile and indole-3-acetaldehyde before being converted to IAA by nitrilase or aldehyde dehydrogenase (Zhao et al. 2002, Zhao 2014). Additionally, the tryptamine pathway begins with the conversion of tryptophan to tryptamine via tryptophan decarboxylase, with subsequent transformations involving amine oxidases and aldehyde dehydrogenases leading to IAA (Mano and Nemoto 2012). The importance of this pathway is highlighted by extensive genetic studies that confirm its conservation throughout the plant kingdom (Morffy and Strader 2020). These pathways illustrate the complexity of IAA biosynthesis in plants, highlighting how different biochemical routes contribute to auxin production under varying physiological conditions.
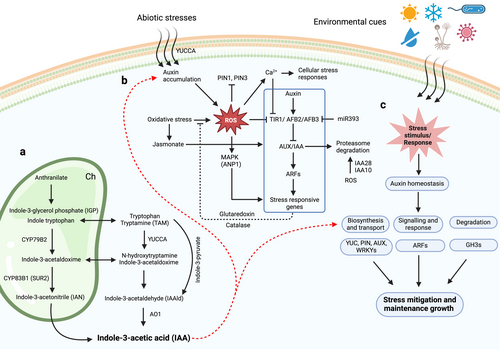
In contrast to these tryptophan-dependent pathways, the tryptophan-independent (Trp-independent) pathway for IAA biosynthesis is an alternative route that allows plants to produce this essential hormone without relying on tryptophan as a precursor (Wang et al. 2015). This pathway was first proposed based on observations from tryptophan auxotrophs in various plant species, including maize and Arabidopsis, indicating that IAA could still be synthesized even when tryptophan is limited (Normanly et al. 1993, Wang et al. 2015). Recent studies have suggested that a cytosolic enzyme known as indole synthase (INS) may initiate the Trp-independent IAA biosynthetic pathway by catalyzing the conversion of indole-3-glycerol phosphate or free indole into IAA (Zhang et al. 2008). This process occurs in cellular organelles such as the cytosol, and its regulation is crucial for maintaining auxin levels during critical developmental stages, such as embryogenesis. This pathway highlights the versatility of plant hormone biosynthesis and the ability of plants to adapt to varying environmental conditions.
3 INTEGRATIVE OVERVIEW OF AUXIN METABOLISM AND TRANSPORT IN PLANT
Auxin levels in plants are regulated through various metabolic inactivation mechanisms, which include conjugation and degradation processes. Auxin can form reversible conjugates that facilitate quick regulation of its active levels. The primary storage forms of auxin consist of amide-linked IAA, ester-linked IAA, and methyl IAA (Casanova-Sáez et al. 2021). Among these, the most common ester-linked variant is IAA-glucose (IAA-Glc), whereas amide linkages occur when auxin is conjugated with amino acids and small peptides. Additionally, the degradation of auxin is essential for maintaining appropriate levels of IAA through an irreversible oxidation process that converts IAA to 2-oxindole-3-acetic acid (oxIAA) (Pěnčík et al. 2013). This conversion is mediated by the DIOXYGENASE FOR AUXIN OXIDATION (DAO) gene, which plays a pivotal role in this regulatory mechanism (Zhao et al. 2013). Moreover, auxin functions by creating concentration gradients between cells, which are essential for driving developmental processes. These gradients result from directional cell-to-cell transport controlled by the influx and efflux carriers in the plasma membrane (Hammes et al. 2022).
AUXIN RESISTANT1/LIKE AUX1 (AUX1/LAX) transporters are responsible for facilitating the influx of IAA into cells, whereas PIN-FORMED (PIN) proteins and ATP-BINDING CASSETTE SUBFAMILY B (ABCB) proteins manage its efflux (Swarup and Bhosale 2019). Recent findings have highlighted that auxin transport plays critical roles during various abiotic stresses, such as high temperature, salt stress, drought, and cold conditions. While in Arabidopsis, four auxin influx carriers—AUX1, LAX1, LAX2, and LAX3—have been identified, each exhibiting specific functions (Péret et al. 2012). The diversity observed in mutant phenotypes among members of the AUX/LAX family indicates diverse roles for these carriers in auxin transport.
Auxin efflux is primarily mediated by PIN and ABCB transporters. Arabidopsis contains eight PIN proteins that are crucial for establishing differential auxin distribution necessary for optimal plant growth and stress responses (Nodzyński et al. 2016). Over the last three decades, substantial progress in genetic and biochemical studies has enhanced our understanding of the mechanisms involved in auxin signaling. The primary nuclear pathway that mediates auxin-regulated gene expression is the TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F-BOX PROTEINS (TIR1/AFBs) pathway. This pathway comprises three key components: the AUXIN RESPONSE FACTOR (ARF) transcription factors, SCF TIR1/AFB receptor complex, and Auxin/IAA repressor proteins (Morffy and Strader 2022). The TIR1/AFB proteins act as F-box proteins integrated into an SKP1-CUL1-F-box (SCF)-type E3 ubiquitin ligase complex, which is crucial for the ubiquitination and subsequent degradation of auxin/indoleacetic acid (Aux/IAA) proteins. Within the TIR1/AFB family, six distinct members have been identified, each exhibiting unique yet overlapping roles in plant development and responses to environmental stimuli (Parry et al. 2009, Salehin et al. 2015). Aux/IAA proteins function as transcriptional repressors and are characterized by three primary domains: a repression domain (DI), a degron domain (DII) necessary for their degradation, and a PB1 domain that facilitates interactions with other Aux/IAAs and ARFs (Morffy and Strader 2022). However, ARF proteins are essential for regulating auxin responses by either activating or repressing the expression of target genes in response to various environmental signals. They contain a B3 DNA-binding domain at their N-terminus, which enables them to bind to specific DNA motifs known as Auxin Response Elements (AuxREs) in the promoters of auxin-responsive genes. The consensus sequence for these elements is TGTCTC, although variations exist, which can affect ARF binding affinity and specificity (Lavy et al. 2016, Li et al. 2023b).
The complexity of auxin signaling is further emphasized by the presence of multiple members within each component of this pathway, allowing for diverse regulatory mechanisms that contribute to the specificity of auxin responses across different plant tissues and developmental stages. Recent research has shown that ARFs can form homo- or heterodimers, which enhances their ability to bind DNA and regulate transcription effectively (Boer et al. 2014). Additionally, the differential expression patterns of TIR1/AFBs, Aux/IAAs, and ARFs across various tissues contribute to the nuanced responses of plants to auxin (Su et al. 2023a), demonstrating how a relatively simple molecule like IAA can trigger a wide range of cellular responses. This intricate signaling network continues to be a focal point for research aiming to understand plant growth regulation and adaptation to environmental changes.
Under the conditions of low auxin concentration, Aux/IAA proteins directly interact with ARFs through their PB1 domain, effectively inhibiting the transcription of genes responsive to auxin (Korasick et al. 2014). When auxin levels rise, it promotes the stabilization of the TIR1/AFB-Aux/IAA co-receptor complex. This stabilization leads to ubiquitylation of Aux/IAAs followed by their degradation via the 26S proteasome. Consequently, this degradation alleviates ARF repression, enabling them to effectively activate transcription. Beyond the SCF TIR1-Aux/IAA nuclear signaling pathway, TMK proteins are also involved in mediating auxin signaling mechanisms (Ang and Østergaard 2023). Furthermore, cytoplasmic-localized AFB1 has been shown to activate auxin responses independently of nuclear gene transcription (Dubey et al. 2023). These non-canonical pathways are essential for facilitating rapid auxin-induced responses but have yet to be explicitly linked to abiotic stress responses. The intricate biosynthesis pathways and signaling mechanisms associated with auxins like IAA provide critical insights into how plants adapt to their environment and regulate growth effectively under varying conditions.
4 AUXIN IN DEFENSE ACTIVATION AND STRESS SIGNALING CROSSTALK
Plants are sessile organisms exposed to various biotic and abiotic stresses, often in combination. To mitigate these stressors and sustain essential life processes, terrestrial plants have evolved adaptive mechanisms for survival (Figure 1c). In response to stress, plants modify their genetic, epigenetic, cellular, and morphological attributes according to environmental cues (Ribba et al. 2020, Gurme et al. 2023). Auxins play a crucial role in environmental signaling and perception, facilitating responses that enable plants to adapt effectively (Feng et al. 2015). They help to maintain homeostasis by regulating specific gene expression in response to environmental changes and the resulting signaling cascades. Additionally, auxins modulate the expression of particular transcription factors and proteins that are involved in stress response (Gomes and Scortecci 2021, Bao et al. 2024). For example, in rice, the stability of auxin receptors modulated by heat shock protein 90 (HSP90) confers plasticity under high-temperature stress (Watanabe et al. 2017). Similarly, alterations in OsIAA20 and OsIAA31 (auxin receptors) increase susceptibility to viral infections (Zhang et al. 2019). The research underscores the significance of auxins in plant homeostasis and survival under stress, while many studies investigated the complex mechanisms involved, which encompasses auxin biosynthesis, transport, flux, activation, and signal transduction (Jing et al. 2023). During stressful conditions, auxin signaling activates defense mechanisms by modulating gene expression cascades and interacting with other phytohormones. Studies indicate that auxins are integral to stress signaling pathways and defense activation. In various cultivated crops, a significant number of auxin-related genes exhibit differential expression (Tables 1 and 2). Ghanashyam and Jain (2009) identified auxin-responsive genes and examined their responses under stress conditions. Among these genes, 239 were induced while 79 were repressed across various stress scenarios. These findings suggest crosstalk between auxins and plant defense mechanisms under stress conditions. Genes belonging to the Aux/IAA, GH3 (Gretchen Hagen 3), and SAUR (small auxin-up RNA) families respond to auxin signaling (Chen et al. 2014).
| Stress | Species | Auxin related genes | Gene expression/observation/mechanisms | References |
|---|---|---|---|---|
| Bacterial infection | Arabidopsis | AtGH3-5/WES1 | Modulate IAA concentration, upregulation of AtPR-1 and AtCBF | (Park et al. 2007) |
| Fungal infection | Maize | ZmARF6, ZmARF18 | Auxin signaling response | (Saidi and Hajibarat 2020) |
| Insect pests | Tomato | SIARF3 | Trichomes development | (Zhang et al. 2015) |
| SlARF4 | Trichomes development, inhibition of SlTHM1 and SlMYB52 | (Yuan et al. 2021) | ||
| Pathogen attack | Arabidopsis | AtYUC8, AtYUC9 | Auxin-mediated cell wall lignification | (Hentrich et al. 2013) |
| Tomato | ACRE189/ACIF1 | Regulates the cell death and defense responses during pathogen recognition | (van den Burg et al. 2008) | |
| Viral infection | Tomato | SlARF8 | Induce auxin signaling | (Werghi et al. 2021) |
| SlARF10 | Tobamovirus infection alters auxin signaling pathways | (Vaisman et al. 2022) | ||
| Rice | OsARF12, OsIAA10 | ARF12-WRKY13 interaction to activate auxin response | (Qin et al. 2020) | |
| OsARF17 | Loss of auxin signaling response | (Zhang et al. 2020) |
| Stress | Species | Auxin related genes | Gene expression/observation/mechanisms | Tissue | References |
|---|---|---|---|---|---|
| Cold | Arabidopsis | IAA14 | Aux/IAA14 regulates microRNA-mediated cold stress response | Root | (Aslam et al. 2020) |
| SgGH3.1 | Overexpression of SgGH3.1 enhances chilling and cold tolerance | Stem and leaves | (Jiang et al. 2021) | ||
| PIN2, PIN3 | Molecular mechanisms of Auxin response under cold stress | Root | (Shibasaki et al. 2009) | ||
| Cucumber | CsARF5 | Hydrogen sulfide improves the cold stress resistance through the CsARF5–CsDREB3 module | Seedlings | (Zhang et al. 2021b) | |
| Rice | OsYUC, OsGH3 | Impairs ABA and IAA biosynthesis and differentially affects cold tolerance | Seedling and panicle | (Du et al. 2013b) | |
| Aux/IAA | Gene regulation during cold acclimation | Whole plant | (Hannah et al. 2005) | ||
| ARFs | Different roles of auxin-responsive genes during reproductive development | panicle and seedling | (Jain and Khurana 2009) | ||
| Drought | Arabidopsis | AtAux/IAA12/19 | AtCPR5-AtAux/IAA12/19 module | Roots | (Nam et al. 2023) |
| AtIAA5, AtIAA6, AtIAA19 | AtDREB2A/B mediated upregulation IAA genes | Stomata | (Salehin et al. 2019) | ||
| AtYUC1, 2, 6 | RAB18, RD22, RD29A, RD29B, DREB2A, and DREB2B. yuc1/2/6 triple mutant | Seedling | (Shi et al. 2014) | ||
| AtSAUR32 | SMALL AUXIN UP RNA32 protein regulates ABA-mediated responses to drought | While plant | (He et al. 2021) | ||
| Masson pine | PmaIAA27, PmaIAA27 | Auxin-related genes could improve drought tolerance | Seedling | (Li et al. 2023a) | |
| Populus spp | YUC16 | YUCCA6 promotes auxin biosynthesis and enhances abiotic stress | Whole plant | (Ke et al. 2015) | |
| Rice | OsPYL5 | PYL5 contributes to drought tolerance in rice by regulating gene expression | Whole plant | (Kim et al. 2014) | |
| OsYUC, OsGH3, OsTIr2, OsAux/IAA, OsARF | Endogenous auxin and jasmonic acid levels are modulated by abiotic stresses | Leaves | (Du et al. 2013a) | ||
| OsARF21 | OsARF21-DRO1 interaction | Root tips | (Uga et al. 2013) | ||
| OsGH3 | Auxin homeostasis in mediating plant responses to abiotic stresses | Whole plant | (Zhang et al. 2009) | ||
| Sorghum | SbGH3-1, 2, 4, 5, 12, 13 | SbGH3-13, and SbLBD32 | Whole plant | (Wang et al. 2010) | |
| Heat | Arabidopsis | AtTIR1, AtAFB2 | TIR1, AFB2, and HSP90 | Seedlings | Wang et al. (2016) |
| CYP79B, TAA1 | Roles of gibberellins and auxin in this regulatory process | Stem | (Stavang et al. 2009) | ||
| NPA | Biosynthesis of GAs and auxin was regulated by temperature | Hypocotyl | (Franklin et al. 2011) | ||
| Barley | ARF6, ARF8, ARF13, ARF17 | MicroRNAs (miRNAs) involved in heat stress response | Whole Plant | (Song et al. 2019) | |
| Tobacco | NtARF1, NtARF2 | Overexpression of the OsPT8 phosphate transporter enhanced tobacco tolerance to high-temperature stress | Root | (Kruszka et al. 2014) | |
| Heavy metal | Apple | MdIAA24 | Improve antioxidant capacity and reduce Cd absorption | (Wang et al. 2019) | |
| Arabidopsis | AtIAA14/SLR1 | Adventitious root formation root growth | Roots | (López-Bucio et al. 2015) | |
| Rice | OsMYB-R1 | Auxin-SA signaling | Roots | (Tiwari et al. 2020b) | |
| Nutrient deficiency | Arabidopsis | AtTAA1, AtYUCCA8 | Increase auxin accumulation, Activation of ARF8, increases N uptake | Root hairs | (Jia et al. 2023) |
| AtIAA14-ARF7/19 | Auxin-mediated transcription of LBD16/29, increases P uptake | Roots | (Okushima et al. 2007) | ||
| AtNIT1, AtNIT2, AtYUC4 | Auxin mediates plant responses to salt stress through specific genes | Roots | (Cackett et al. 2022) | ||
| Osmotic | Maize | ZmARF2, ZmARF31 | Interaction with ZmHAK1, root development under low P | Roots | (Sheng et al. 2020) |
| ZmGH3 | GH3 family genes mediate auxin-related responses to abiotic stresses | Whole plant | (Feng et al. 2015) | ||
| Tomato | SIPIN1 | Mechanical regulation influences auxin-mediated growth via specific gene expression | Shoot | (Nakayama et al. 2012) | |
| Wheat | TaGH3-I, TaGH3-II, TaGH3-III | Elucidate the roles of GH3 genes in mediating auxin responses and stress tolerance mechanisms | Leaves, Roots | (Jiang et al. 2020) | |
| Salinity | Apple | MdIAA8, MdIAA9, MdIAA25 | Overexpression of IAAs | Callus | (Li et al. 2022) |
| Arabidopsis | AtbZIP17 | Reduction in root stem meristem size and root growth inhibition | Roots | (West et al. 2004) | |
| AtAUX1 | SMB-AUX1 interaction module promotes halotropism under salt stress | Roots | (Zheng et al. 2024) | ||
| ARF7, ARF19 | Lateral root inhibition | Roots | (Zolla et al. 2010) | ||
| AtAFB3 | AFB3 enhances salt stress tolerance through gene expression modulation | Roots | (Garrido-Vargas et al. 2020) | ||
| AtIAA17 | ARF functions in regulating gene expression related to plant growth and stress tolerance | Roots | (Liu et al. 2024) | ||
| AtWRKY46 | WRKY46 and ABA interaction | Roots | (Ding et al. 2015) | ||
| Cucumber | CsYUC | CsYUC plays a crucial role in regulating both stress tolerance and flower development | Roots, leaves, and flowers | (Yan et al. 2016) | |
| Maize | ZmGH3 | GH3 family genes mediate auxin-related responses to abiotic stresses | Whole plant | (Feng et al. 2015) | |
| Rice | OsGH3 | TLD1/OsGH3.13 regulate auxin levels and enhance drought tolerance | Whole plant | (Zhang et al. 2009) | |
| Sorghum | SbGH3-1, 2, 4, 5, 12, 13 | SbGH3-13, and SbLBD32 | Whole plant | (Wang et al. 2010) | |
| Shade | Arabidopsis | AtYUC2, AtYUC5, AtYUC8, AtYUC9 | Specific genes and their interactions in mediating plant responses to stress | Seedlings | (Sessa et al. 2005) |
| AtTAA1 | Auxin synthesis through a new tryptophan-dependent pathway contributes to shade avoidance in plants | Cotyledons and hypocotyl | (Tao et al. 2008) | ||
| AtYUC2, AtYUC5, AtYUC8, AtYUC9 | Auxin responsiveness, mediated by specific gene expressions, drives contrasting growth adaptations in different plant organs | Lamina and petiole | (de Wit et al. 2015) | ||
| AtYUC2, AtYUC5, AtYUC8, AtYUC9 | Intricate genetic and molecular mechanisms through auxin biosynthesis | Leaf and hypocotyl | (Müller-Moulé et al. 2016) |
Auxin crosstalk enhances resistance against viral infections, and alterations in auxin signaling can induce resistance to such infections (Naseem et al. 2015). Numerous auxin-related genes involved in defense against biotic stress have been identified in various crops (Table 1). Additionally, auxins regulate trichome development across different plant species, serving as physical barriers against insect attacks (Zhang et al. 2015, Li et al. 2021). In tomato, auxin-related genes such as AIIs and ARFs are implicated in trichome formation. Specifically, the auxin response factor SlARF4 regulates trichome development in response to arthropod pest attacks, with plants overexpressing SlARF4 exhibiting a higher density of trichomes, suggesting that SlARF4 enhances trichome density in tomatoes, aiding defense against pests. Similarly, epigenetic modulation of SlARF8 disrupts auxin signaling, increasing susceptibility to tomato spotted wilt virus (TSWV), while SlARF10a has been reported to confer resistance against tomato brown rugose fruit virus (ToBRFV) infection (Werghi et al. 2021, Vaisman et al. 2022).
The GH3 gene family is crucial for maintaining hormonal balance in plants by conjugating excess levels of IAA, SA, and JA to amino acids (Jiang et al. 2020). This process is vital during hormone-related signaling and in response to various stress conditions. Bacterial infections induce the Arabidopsis AtGH3-5/WES1 gene, which modulates endogenous IAA concentration while increasing the expression of AtPR-1 and AtCBF to enhance resistance (Park et al. 2007). Jagadeeswaran et al. (2007) suggest that AtGH3-mediated defense also involves the SA-signaling pathway, while Tiwari et al. (2020b) reported that the crosstalk between auxin and SA is mediated by OsMYB-R1. In rice, OsARF12 interacts with OsIAA10 to enhance antiviral defense against rice dwarf virus by binding to the promoter of the auxin response element of OsWRKY13 and activating its transcription (Qin et al. 2020). Previous studies have shown that the WRKY26 gene promotes auxin homeostasis (Ding et al. 2015). OsARF17 confers resistance against the black-streaked dwarf virus: knockout mutants of Osarf17 exhibit severe symptoms and increased susceptibility to the virus (Zhang et al. 2020). In maize, ARF6 and ARF18 are upregulated by Colletotrichum graminicola and Fusarium verticillioides infection, potentially acting as infection receptors (Saidi and Hajibarat 2020). The SlARF8 gene provides resistance against viral infections by inducing auxin signaling: disruption of SlARF8 activity via miRNA167a and epigenetic modification of its promoter reduces auxin accumulation, leading to increased sensitivity to viral infections (Werghi et al. 2021).
Auxins regulate various physiological processes in response to stress conditions (Zou et al. 2019, Zhang et al. 2022). Several auxin-related genes involved in defense against abiotic stress have been identified across different crops (Table 2). Auxins induce the expression of RAB18, RD22, RD29A, RD29B, DREB2A, and DREB2B genes under drought stress conditions; these genes enhance the production of antioxidant enzymes and reactive oxygen species (ROS), thereby improving drought resilience (Shi et al. 2014). Ding et al. (2015) demonstrated that antagonistic effects between WRKY46 and ABA elevate auxin levels in roots during salt stress in Arabidopsis while regulating both auxin homeostasis and ABA signaling. Chromate (Cr) stress inhibits primary root growth; however, AtIAA14/SLR1 promotes adventitious root formation under Cr stress through auxin transport and signaling pathways (López-Bucio et al. 2015). Overexpression of OsMYB-R1 confers tolerance under drought and Cr stress conditions by controlling auxin-SA signaling (Tiwari et al. 2020a). Under drought conditions, AtDREB2A/B enhances the activity of IAA genes, such as AtIAA5, AtIAA6, and AtIAA19; higher expression levels maintain glucosinolate levels while enhancing plant resilience under stress, whereas loss of activity results in defective stomatal movement (Sakuma et al. 2006, Salehin et al. 2019). Nam et al. (2023) observed that the AtCPR5-AtAux/IAA12/19 module regulates lateral root development through auxin signal transduction; however, under drought conditions, reduced expression of AtCPR5 disrupts normal root growth due to decreased auxin signaling and accumulation of IAA12/19.
Additionally, ultraviolet (UV) radiation-induced oxidative stress in plants like tobacco (Nicotiana tabacum) and duckweed (Lemna sp.) has been found to increase peroxidase activity, leading to the oxidation of IAA and subsequent instability of auxin levels (Jansen et al. 2001). Similar effects were observed in pea (Pisum sativum) seedlings exposed to cadmium (Cd) stress, where peroxidase activity oxidized IAA, impacting its stability (Ostrowski et al. 2016). In grapes (Vitis vinifera), peroxidase-mediated oxidation of IAA has also been documented during root initiation and development (Vatulescu et al. 2004). However, the oxidation of IAA may not be entirely selective; an enzyme DIOXYGENASE FOR AUXIN OXIDATION (DAO) has been identified, which catalyzes the conversion of IAA into its oxidized form, oxIAA. This enzymatic reaction is significant for auxin metabolism and regulation (Mellor et al. 2016, Porco et al. 2016).
During osmotic stress, plants modulate auxin biosynthesis and transport to maintain homeostasis effectively. Upregulation of auxin biosynthesis genes enhances resilience against stress conditions (Jing et al. 2023). Cackett et al. (2022) demonstrated that overexpression of the auxin biosynthesis genes NIT1, NIT2, and YUC4 enhances tolerance under salt treatment. Tolerance induction via YUC family genes has also been reported under salinity conditions in Cucumis sativus (Yang et al. 2022b). Overexpression of the F-box gene AFB3 increases salinity stress tolerance in Arabidopsis (Garrido-Vargas et al. 2020). Jiang et al. (2020) reported that wheat contains three major categories of auxin-conjugating enzymes—TaGH3-I, TaGH3-II, and TaGH3-III)—that are potentially involved in regulating stresses like salt or osmotic stresses. In lower plants like Physcomitrella patense, GH3 inactivation leads to higher tolerance under salt stress (Koochak and Ludwig-Müller 2021). Auxin carrier genes PIN1, PIN3, PIN7, and CcPIN are downregulated by salt stress, affecting transport capabilities during such stresses (Yang et al. 2022b). To facilitate auxin redistribution under salt stress conditions, PINOID plays a critical role; loss of PID activity impairs both the redistribution and polar transport of auxins effectively (Wang et al. 2019). Overexpression of IAA genes MdIAA8, IAA9, and IAA25 has also conferred salt tolerance in apple callus tissue cultures (Li et al. 2022). A recent study by Zheng et al. (2024) demonstrates that auxins physically participate in halotropism by directing roots away from high-salinity areas toward more favorable conditions. The SOMBRERO (SMB) module regulates the expression of the auxin carrier AUX1 for proper redistribution within the lateral root cap. This SMB-AUX1-auxin module plays a crucial role in avoiding salinity-induced stress effects altogether (Sohail et al. 2024). The role of auxin under heavy metal stress has also been elucidated. In apples, overexpression of MdIAA24 enhances tolerance to Cd stress by improving antioxidant capacity and reducing Cd absorption. This overexpression also increases tolerance to alkaline stress in apples (Wu et al. 2019, Wang et al. 2021). Overall, these findings suggest that auxin plays an important role in mitigating both biotic and biotic stresses via stress perception and mitigation by activating or deactivating various pathways. However, detailed analyses are needed to provide a clear understanding of the mechanistic roles of auxin in stress mitigation within cultivated crops.
5 THE INTERACTION OF AUXIN WITH OTHER PHYTOHORMONES
Auxin promotes plant growth and development while interacting extensively with other hormones, modulating their levels and functions (Mazzoni-Putman et al. 2021). This interaction is crucial during stress responses, where hormonal balance dictates a plant's adaptability and survival. For example, auxin stimulates root development, while cytokinin promotes shoot growth, creating an antagonistic relationship essential for balanced plant architecture. In vitro studies show that auxin enhances root formation, whereas cytokinin inhibits it (Rivas et al. 2022). In vivo studies reveal that auxin levels inversely correlate with cytokinin levels; auxin application can rapidly decrease cytokinin biosynthesis (Nordström et al. 2004). This relationship suggests that under stress conditions like drought or nutrient deficiency, hormone modulation significantly impacts plant resilience and growth (Shi et al. 2014).
Auxins are also associated with ethylene, another critical hormone in stress responses. Exogenous auxin enhances ethylene production by inducing genes related to ethylene biosynthesis (Yue et al. 2020). This interaction is vital during stress, as ethylene mediates responses such as fruit ripening and stress tolerance. Conversely, ethylene inhibits both lateral and basipetal transport of auxin (Negi et al. 2008), indicating a feedback mechanism that modulates auxin distribution during stress. Additionally, auxin stimulates gibberellic acid (GA) synthesis, which is essential for developmental processes. Basipetally transported auxin is crucial for producing active gibberellins GA1 and GA3 in barley (Wolbang et al. 2004). Gibberellins promote the degradation of DELLA repressor proteins that inhibit growth, and disruption in auxin transport can hinder GA-mediated DELLA degradation, highlighting auxin's influence on GA signaling pathways during stress adaptation (Claeys et al. 2014). The interplay between auxin and brassinosteroids (BRs) is also significant; both hormones facilitate root gravitropic curvature in maize (Xuan and Beeckman 2021), with treatments leading to similar transcript accumulation associated with growth regulation under stress. Furthermore, the interaction between auxin and ABA is particularly important during stress responses like drought. ABA levels rise under stress, decreasing free IAA levels while increasing esterified IAA conjugates in plants like muskmelon (Agehara and Leskovar 2012). This shift prioritizes survival over growth, as exogenous ABA inhibits lateral root formation, emphasizing its role as a negative regulator of auxin's effects on root development during stress (Lu et al. 2019). Overall, auxins play a central role in modulating interactions with other phytohormones, such as cytokinins, ethylene, gibberellins, brassinosteroids, and ABA, during stress responses. These interactions are vital for regulating growth patterns and physiological adaptations that enhance plant resilience under adverse conditions.
Several mutants with defects in auxin response also exhibit impaired responses to other phytohormones, indicating potential cross-talk among hormonal pathways. For example, mutations affecting components of the SCF (Skp1-Cullin-F-box) complex lead to compromised responses to various hormonal stimuli (Yu et al. 2007). The SCF complex is crucial in the ubiquitin-proteasome pathway for degrading specific proteins involved in hormone signaling. Many mutants with disrupted auxin responses exhibit pleiotropic defects, such as axr1 mutants cannot effectively modify CUL1 with RUB (rubellin), resulting in resistance against root elongation inhibition from auxins as well as other hormones like cytokinin and ethylene (Dharmasiri et al. 2007). RUB modification is crucial for the functionality of active SCF complexes involved in hormone responses beyond just SCF TIR1 and auxin. SCF COI1 is essential for JA signaling (Ren et al. 2005), indicating how disruptions in auxin signaling can affect responses to JA. The RUB E2 enzyme-defective mutant rce1 shows resistance to both auxin and JA while overproducing ethylene (Larsen and Cancel 2004), suggesting a compensatory mechanism that alters growth and stress responses. Additionally, reduced expression of CSN5 from the COP9 signalosome or RBX1, which enhances RUB to CUL1, can impair responses to both auxin and JA (Hind et al. 2011). Mutants such as axr1 and cand1 also resist hypocotyl elongation inhibition by the ethylene precursor ACC when grown in darkness (Cheng et al. 2004). In dark conditions, apical hook formation—an auxin-mediated differential growth response promoted by ethylene— is significantly reduced in cand1 mutant plants (Mazzella et al. 2014), indicating the importance of the interaction between auxin and ethylene for proper growth regulation. Interestingly, while rce1 mutants are defective in auxin response (Yang et al. 2012), they overproduce ethylene and exhibit an exaggerated triple response phenotype, illustrating how hormonal crosstalk can lead to unexpected developmental outcomes.
Different phytohormones can influence the activity of SCF activity in distinct manners. Specifically, the degradation of repressive proteins occurs in response to auxin, gibberellins, and JA (Fu et al. 2002, Chico et al. 2014, Yang et al. 2016), while ethylene prevents the degradation of transcriptional activator EIN3 via SCF EBF1/EBF2 complexes (Chico et al. 2014). This complexity underscores the intricate regulatory networks governing plant hormone signaling, emphasizing that mutations affecting one pathway can have cascading effects on others. Understanding these interactions provides valuable insights into mechanisms underlying plant development and stress responses. In addition to factors influencing SCF function, several proteins with less clearly defined roles in auxin response exhibit altered reactions to other hormones and environmental conditions. Evidence suggests that protein phosphorylation plays a significant role in mediating responses to various stimuli, including auxin. Treatments with auxin, SA, wounding, and salt increase mitogen-activated protein kinase (MAPK) activity in Arabidopsis roots (Ichimura et al. 2000). Specifically, kinase inhibitors can block the auxin-induced activation of a reporter gene, highlighting MAPK signaling importance in auxin response. Furthermore, constitutively active forms of ANP family members from Arabidopsis MAPKs reduce the responsiveness of an auxin-inducible reporter gene construct in protoplasts (Mockaitis and Howell 2000). This kinase family positively regulates oxidative stress responses; notably, exposure to hydrogen peroxide inhibits the induction of auxin-responsive reporter genes.
Similarly, a constitutively active form of tobacco MAPK NPK1 diminishes both the responsiveness of a reporter gene to auxins while providing resistance to freezing, heat, and salt stresses (Ichimura et al. 2000). These findings indicate kinases can modulate auxin responsiveness positively or negatively. Additionally, a mutant identified due to its altered response to an auxin transport inhibitor—roots curl in NPA 1 (rcn1)—is deficient in a subunit of protein phosphatase 2A (Blakeslee et al. 2008). This mutant exhibits increased IAA transport and overproduces ethylene (Vandenbussche et al. 2003), complicating its hormonal interactions. Notably, rcn1 also shows defects in responses to NPA (N-1-naphthylphthalamic acid), gravity, and ABA (Rashotte et al. 2001). The application of protein phosphatase inhibitors produces similar gravitropic defects in wild-type Arabidopsis. Furthermore, the IAA-response mutant ibr5 is deficient in a dual-specificity phosphatase (Johnson et al. 2015). This mutant displays resistance to both auxins and ABA along with phenotypes such as fewer lateral roots and longer primary roots compared to wild type—characteristics indicative of impaired overall auxin response. Auxin signaling components also participate in environmental cue responses; for instance, Aux/IAA and ARF proteins play significant roles in mediating light responses in plants. Gain-of-function mutations in the shy2/iaa3, axr2/iaa7, and axr3/iaa17 mutants result in enhanced de-etiolation in dark conditions (Tian et al., 2003). Additionally, the constitutive photomorphogenic mutant long hypocotyl 5 (hy5) can be partially restored by overexpressing AXR2/IAA7 (Cluis et al. 2004), indicating a complex interplay between these proteins in light signaling and developmental processes.
Additionally, the mutant hookless1 (hls1) fails to form an apical hook in darkness—a phenotype suppressed by mutations in ARF2 responsible for auxin response (Lim et al. 2010). The Arabidopsis mutant abscisic acid insensitive 3 (abi3) was identified based on its germination phenotype; it is deficient in a transcription factor important for ABA signaling (Hu et al. 2019). In addition to its resistance to ABA-induced effects on germination, abi3 also exhibits resistance to lateral root proliferation induced by auxins as well as lateral root repression caused by NPA treatment. Interestingly, ABI3:GUS reporter accumulation is stimulated by exposure to auxins suggesting ABI3's involvement in integrating ABA with auxin signaling pathways. Certain mutants with defects in auxin transport also show altered responses to other stimuli. For example, the auxin influx mediator AUX1 demonstrates resistance not only to specific auxins but also to ethylene's effects on growth regulation (Vandenbussche et al. 2010). The rcn1 mutant has increased transport along with various developmental phenotypes influenced by hormonal interactions. Additionally, mutations within the TRANSPORT INHIBITOR RESPONSE gene lead not only to low-auxin phenotypes but also modified responses towards light ethylene cytokinin gibberellins (Desgagné-Penix et al. 2005). These observations underscore the complexity of hormonal interactions within plants, highlighting protein phosphorylation's significance as a regulatory mechanism integrating various signaling pathways involved throughout plant growth adaptation processes. Understanding these intricate networks will provide insights into how plants adapt their development according to environmental challenges.
6 AUXIN AND REACTIVE SPECIES CROSSTALK
Auxin and reactive oxygen species (ROS) play pivotal roles in regulating plant responses to abiotic stresses and enhancing tolerance (Figure 1b) (Ali et al. 2020a). In a previous study, Zhao et al. (2012) highlighted a significant correlation between hydrogen peroxide (H₂O₂) levels and auxin distribution in rice subjected to Cd stress. The study found that H₂O₂ influences the expression of critical genes such as OsYUCCA, OsPIN, OsARF, and OsIAA, which are essential for auxin biosynthesis, transport, and signaling pathways. This suggests that H₂O₂ may act upstream in the auxin signaling cascade (Zhao et al. 2012). Additionally, Hu et al. (2013) demonstrated that Cd stress leads to ROS-induced oxidative damage in rice plants, resulting in decreased levels of IAA, a primary form of auxin. Furthermore, H₂O₂ can activate specific MAPK pathways in Arabidopsis, particularly the Arabidopsis NICOTIANA PROTEIN KINASE 1-LIKE PROTEIN KINASE (ANP1), which negatively regulates auxin activity during oxidative stress conditions (Kovtun et al. 2000). Interestingly, plants with catalase deficiencies exhibit altered auxin levels due to a high accumulation of H₂O₂, which may result from enhanced auxin conjugation or increased transport rates of auxin (Tognetti et al. 2012, Sharma et al. 2015).
Furthermore, auxin signaling plays a critical role in the plant's adaptive response to oxidative stress by influencing redox metabolism in Arabidopsis (Iglesias et al. 2010). Specifically, auxin modulates the levels of ROS, which are crucial for signaling pathways that help plants manage oxidative stress. Through this interaction, auxin not only contributes to stress tolerance but also regulates the expression of antioxidant enzymes, thereby enhancing the plant's ability to cope with environmental challenges. In short, the interaction between auxin and reactive oxygen species is complex and multifaceted (Parveen et al. 2022). Both components are critical for regulating plant growth and stress responses. Their crosstalk influences not only auxin signaling pathways but also plays a vital role in the adaptation mechanisms of plants facing environmental challenges. Understanding these interactions can provide insights into improving plant resilience against abiotic stresses through targeted agricultural practices or genetic modifications.
Building upon the intricate interactions between auxin and ROS, recent research indicates that the signaling pathways of auxin and nitric oxide (NO) interact significantly to regulate various stress responses in plants. For example, NO has been shown to inhibit the activity of IAA oxidase, leading to reduced degradation of auxin in Medicago truncatula under Cd stress, thereby enhancing the plant's tolerance to this heavy metal (Xu et al. 2010). Additionally, interactions between NO and auxin have been observed in rice root systems, where they work together to alleviate the toxic effects of both Cd and arsenic (As) (Piacentini et al. 2020). In Triticum aestivum's root apices treated with aluminum, exposure to sodium nitroprusside (SNP), a NO donor, resulted in increased IAA levels (He et al. 2012, Faria-Lopes et al. 2019). Conversely, during copper stress in Arabidopsis seedlings, auxin and NO were found to negatively regulate each other's signaling pathways, influencing morphological responses (Faria-Lopes et al. 2019).
In conditions of iron (Fe) deficiency, wild-type Arabidopsis has been observed to have increased levels of both auxin and nitric oxide (NO), coinciding with increased activity of root ferric-chelate reductase (FCR) (Chen et al. 2010). The application of inhibitors targeting auxin transport and NO biosynthesis resulted in suppression of FCR activity, indicating a regulatory relationship. Further investigations using NO-deficient mutants demonstrated that auxin functions upstream of NO in activating FCR (Chen et al. 2010). Recent studies have also indicated that sucrose and γ-aminobutyric acid (GABA) can modulate FCR activity in conjunction with auxin and NO under Fe deficiency conditions (Lin et al. 2016, Guo et al. 2020). In tomato plants, NO has been shown to play a role in regulating root branching in response to Fe deficiency, acting downstream of auxin signaling (Jin et al. 2011). Additionally, a similar interaction between auxin and NO has been documented in Oryza sativa, where both hormones collectively regulate lateral root formation while inhibiting the elongation of seminal roots during Fe deficiency (Sun et al. 2017). Beyond metal stress responses, NO has been found to enhance auxin-mediated adventitious root formation and alleviate growth inhibition in Suaeda salsa seedlings subjected to waterlogging stress (Chen et al. 2016). This crosstalk between auxin and NO is vital for plant adaptation during abiotic stresses, as it not only governs growth responses but also strengthens tolerance mechanisms against environmental challenges. Understanding these intricate signaling networks is crucial for developing innovative strategies aimed at improving crop resilience and productivity under stress conditions.
7 AUXIN REGULATES PLANT GROWTH UNDER STRESS CONDITIONS
Auxin and its distribution significantly affect vascular development, apical dominance, and growth responses in plants (Gomes and Scortecci 2021). Plant growth and development depend on precise auxin levels and their distribution over time and space. Consequently, numerous systems regulate auxin levels to effectively govern plant growth and reproduction. These processes include de novo biosynthesis, transport, degradation, and conjugation-deconjugation (Woodward and Bartel 2005). Stress signals in plants modulate IAA levels by altering gene expression and the activity of auxin-balancing enzymes activity. Both biotic and abiotic stresses lead to reductions in plant metabolism and survival, resulting in significant losses in crop productivity (Wang et al. 2013). For example, abiotic stresses significantly influence the expression and activity of enzymes involved in the reversible conjugation of auxin. Key enzymes in this process include auxin conjugate synthetases from the GH3 family, UDP-glucosyltransferases (UGT), and auxin amidohydrolases such as IAR and ILL (Rampey et al. 2004, Mateo-Bonmatí et al. 2021, Luo et al. 2023). These enzymes play critical roles in the conjugation and subsequent release of auxin, thereby modulating its availability during hormone-related signaling and stress responses. Additionally, the regulation of biosynthesis-related genes is also affected by these environmental stressors, highlighting the complex interplay between abiotic stress and auxin homeostasis. However, plants typically adjust their internal cellular environment through hormonal homeostasis. When subjected to stress, these conditions profoundly affect plant growth, survival, and productivity (Jing et al. 2023). Auxins have been reported to regulate growth and development throughout the plant life cycle; however, their role becomes even more critical under stress conditions. Studies have shown that stress responses modulate auxin biosynthesis and signaling pathways to reprogram growth and development (Korver et al. 2018). Auxins act as chemical messengers that modulate gene expression through a short nuclear pathway, involving various transcription factors (TFs), including auxin response factors (ARFs) (Liu et al. 2014).
During stress conditions, auxin responses and signaling help plants mitigate stress through auxin homeostasis. Once the stress is overcome, maintaining normal growth and development becomes crucial. Several reports have highlighted the role of auxin in regulating and sustaining growth affected by various stresses (Zheng et al. 2024). However, limited information is available regarding salt stress mitigation via auxins (Ryu and Cho 2015). Salt stress leads to decreased growth, indicating changes in IAA biosynthesis levels and distribution (Kazan 2013). Increased salt stress hyperactivity enhances YUCCA3-mediated auxin production (Jung and Park 2011). Furthermore, auxin distribution and redistribution are associated with changes in root architecture under salt stress conditions (Sohail et al. 2024). Recent studies have shown that manipulating YUCCA expression through CRISPR/Cas9 can significantly improve drought and salinity tolerance in crops by enhancing auxin production, thereby promoting root development and stress response mechanisms (Cao et al. 2019, Marzi et al. 2024). For example, overexpression of OsYUC4 in rice has been linked to increased auxin biosynthesis and improved salinity tolerance, highlighting the potential of YUCCA genes in stress adaptation (Marzi et al. 2024). Similarly, the GH3 gene family, which encodes auxin-amido synthetases, is involved in the regulation of auxin homeostasis by conjugating IAA to amino acids, thus modulating its availability and activity within plant tissues. However, knockout of specific GH3 genes has been shown to alter auxin levels, leading to enhanced stress tolerance. For example, the overexpression of OsGH3-2 in rice resulted in improved cold stress resistance while also affecting ABA levels, indicating a complex interplay between auxin and abscisic acid in stress responses (Du et al. 2012).
Moreover, after waterlogging stress, soybean roots at an early vegetative stage produced a novel auxin-amidohydrolase enzyme that releases free auxin, promoting the development of adventitious roots. Auxin redistribution in the root cap, mediated by AUX1 under the influence of SMB, causes angular bending of the growing root away from salinity. In other words, auxins physically modulate root growth and movement to avoid stress while maintaining growth (Zheng et al. 2024). OsARF21 interacts with DRO1 to modulate cell elongation in the root tip area, facilitating downward growth in response to gravity while providing tolerance under drought stress (Uga et al. 2013). Recent studies reported that salt stress reduced primary and lateral root growth while decreasing auxin activity due to the disorganization of PIN1 protein interactions with serotonin (Mukherjee and Bhatla 2023). Exogenous application of auxin potentially restores normal root growth and development. Under low nitrogen availability, TAA1 and YUCCA8 increase auxin accumulation in root hairs (Jia et al. 2023). This accumulation activates ARF8/8, stimulating the RHD6-LRL3 module to elongate root hairs under low nitrogen conditions (Jia et al. 2023). Low phosphorous stress is modulated by IAA14-ARF7/19 through the auxin-mediated transcription of LBD16/29 in roots. LBD homologs regulate the expression of EXP genes, which promote lateral root growth and emergence (Lee et al. 2013). Similarly, PHR1/MYB responds to phosphorus starvation induced by ARF7/19 (Puga et al. 2017). OsARF16 regulates phosphorus uptake and homeostasis in rice; however, Osarf16 mutant roots lose sensitivity and are unable to absorb phosphorus effectively (Shen et al. 2013). OsARF16 modulates cytokinin-mediated inhibition of phosphate transport and signaling in rice. In maize, ARF genes provide tolerance under low phosphorus and potassium conditions; for example, ZmARF2 interacts with ZmHAK1 and ZmARF31 (Sheng et al. 2020). Overexpression of ZmARF4 modulates root development under low phosphorus availability in Arabidopsis by regulating the expression of AtRNS1, AtDER, and AtANS (Li et al. 2022). In Arabidopsis, AtACS2 and AtWRKY46 downregulate AtGH3.1, AtGH3.5, and AtGH3.9 genes involved in auxin-amino acid conjugation. Downregulation of these genes maintains free IAA levels in roots, which supports efficient lateral root development (Ding et al. 2015, Han et al. 2019).
The role of auxin in regulating plant developmental processes under biotic stress remains elusive. Several reports have described how auxin supports development during pathogen attacks; however, these studies highlight the development of secondary organs such as trichomes and cell wall lignification as mechanisms to evade pathogen attack (Zhang et al. 2015, Srinivasan 2024). In tomato plants, ARF3 promotes trichome development under pathogen stress; trichome development is regulated by auxins, providing mechanical protection (Zhang et al. 2015). Similarly, AIIs and ARF4 are also involved in trichome development in tomato against arthropod attacks (Yuan et al. 2021). In Arabidopsis, during pathogen stress, IAA mediates cell wall lignification to maintain normal growth. Overexpression of YUC8 and YUC9 induces auxin signaling, which leads to cell wall lignification (Hentrich et al. 2013). Overall, these results suggest that auxin plays an important role in stress mitigation while also being crucial for maintaining normal growth under stressful conditions. The role of auxin is well elucidated concerning root development in response to osmotic stress conditions; however, it remains less clear for other types of stresses. Future, research should focus on understanding the role of auxin in sustaining growth and development under various stresses. This knowledge could enable researchers to manipulate the auxin signaling and homeostasis to develop resilient cultivars that can better withstand changing climate conditions.
8 STRATEGIES FOR ENHANCING PLANT RESILIENCE AND PRODUCTIVITY IN CHALLENGING ENVIRONMENTS THROUGH THE REGULATION OF AUXIN-MEDIATED STRESS RESPONSES
Plant resilience and productivity face significant challenges from extreme temperatures, water deficits, and high salt levels (Krasensky and Jonak 2012, Ali et al. 2020b). These conditions impede photosynthesis and cell development. Photosynthetic activity is crucial for plant growth and survival, necessitating a complex system of long-distance and short-distance communication to regulate photosynthesis in response to these challenges. Stress increases chloroplast-derived ROS generation due to reduced CO2 availability or decreased photosynthetic metabolism (Li and Kim 2022). Chloroplasts generate more ROS in plant cells during daylight, as investigated by Bhatt et al. (2021). This preferentially oxidizes proteins and lipids while impairing electron transport chain recycling (Nishiyama et al. 2004). The mechanisms by which auxins mediate stress responses are multifaceted and crucial for plant adaptation to environmental challenges. Understanding these mechanisms provides insights into enhancing crop resilience against various stresses through targeted manipulation of auxin signaling pathways.
8.1 Enhancing plant resilience against biotic stress
Auxin signaling has evolved from a common ancestor in bryophytes to serve multiple functions in vascular plants. In land plants, auxin signaling coordinates multicellular development, including cell and tissue polarity modulation, rhizoid growth, and overall plant growth (De Smet et al. 2011). This signaling pathway plays a dual role in plant defense; it promotes growth while also modulating responses to biotic stressors. IAA modulates other hormonal pathways to enhance the plant's response to herbivore attacks and strengthens plant defenses against various attackers (Erb et al. 2012, Machado et al. 2016). Additionally, indole-3-butyric acid (IBA) and other auxins have been shown to protect chrysanthemum plants against pests such as thrips and leaf miners, demonstrating functions beyond root development (Mouden et al. 2020). The mechanical defense of plants against herbivores and pathogens relies on auxin's role in lignin synthesis and cell wall fortification (Huang et al. 2013). Increased herbivory elevates auxin levels, driving anthocyanin production, which serves as a chemical deterrent and indirectly promotes ethylene synthesis, which are essential for effective plant defense (Machado et al. 2016, Tenorio-Berrío et al. 2018). Furthermore, auxins interact with microbial biocontrol agents to enhance plant defenses. For example, IAA produced by microorganisms influences plant physiology and encourages defensive mechanisms such as root growth and basal protection against diseases. In interactions with Fusarium head blight (FHB), Pseudomonas fluorescens stimulates auxin synthesis in barley tissues, enhancing disease resistance (Mainali and Nyaupane 2023). During viral infections in tomato leaves, auxin regulates genes such as ARFs to protect the plant from biotic stress (Bouzroud et al. 2018). Microarray analyses of rice plants demonstrate that various auxin-induced and -suppressed gene families respond to biotic stress. Specifically, genes such as GH3, Aux/IAA, SAUR, and ARF were upregulated in plants infected by M. grisea and S. hermonthica compared to non-infected controls (Ghanashyam and Jain 2009). Viral infections can lead to symptoms such as leaf curling, loss of apical dominance, and reduced growth—phenomena that often mimic mutations affecting auxin synthesis and signaling pathways. Numerous auxin-responsive genes in Arabidopsis are altered following tobacco mosaic virus (TMV) infection (Padmanabhan et al. 2008). The TMV replicase interacts with IAA26 to facilitate TMV movement across cells; this interaction inhibits IAA26 and other proteins (IAA18/IAA27) from accessing the nucleus. Consequently, IAA18, IAA26, and IAA27 cannot downregulate auxin-responsive gene expression, resulting in abnormal plant morphology post-TMV infection (Padmanabhan et al. 2006).
8.2 Role of auxins in mitigating drought stress in plants
Transcriptional patterns under drought stress indicate that auxin signaling plays a pivotal role in drought stress responses. Drought conditions can either upregulate or downregulate numerous auxin-responsive genes across species, such as Arabidopsis, rice, and sorghum (Wang et al. 2010, He et al. 2021). In rice under drought stress, auxin biosynthesis genes, including anthranilate synthase (AS), and tryptophan (Trp) synthesis, are suppressed. These genes are only marginally activated by cold and heat stress. The YUCCA gene family, producing flavin monooxygenases converting indole-3-pyruvic acid (IPA) to IAA, also exhibits comparable suppression and activation during stress (Tillmann 2021). Recent findings suggest that many Aux/IAA proteins may serve as hubs for ABA signaling pathways. Loss-of-function mutants iaa5, iaa6, and iaa19 exhibit reduced drought tolerance due to direct targeting by DREB/CBF proteins (Salehin et al. 2019). Overexpressing OsIAA18 and OsIAA20 enhances drought tolerance in rice (Zhang et al. 2021a). The wheat Aux/IAA gene TaIAA15-1A regulates ABA-related genes to promote drought tolerance (Su et al. 2023b). Tognetti et al. (2012) found that drought and salt stress enhanced UDP-glucosyltransferase (UGT74E2) expression. This enzyme adds glucose molecules to its substrate, IBA. Drought-induced ABA-mediated stomatal closure involves SAUR proteins like SAUR32. Activation of YUC7 correlates with increased drought tolerance in Arabidopsis; recent research also links YUC6 to potato drought resilience (Kim et al. 2014). By comprehensively addressing these aspects of auxin-mediated stress responses, we gain valuable insights into enhancing crop resilience against diverse environmental challenges.
8.3 Enhancing plant resilience against osmotic stress
In Arabidopsis, extreme osmotic stress increases IAR3 transcript levels, a response that is crucial for the plant's adaptation to stress conditions (Kinoshita et al. 2012). Interestingly, it did not affect the transcription of other amidohydrolases like ILL5. Auxin receptors, including TIR1/AFBs, ABP1, and SKP2A, play vital roles in mediating auxin signaling cascades, which regulate various aspects of plant growth and development. The tir1 afb2 mutant exhibits lower levels of hydrogen peroxide and superoxide anions while showing greater antioxidant enzyme activity, which suggests enhanced oxidative stress tolerance. This finding highlights the importance of auxin signaling in managing oxidative stress responses.
Functional analysis of miR393 suggests it controls TIR1- and AFB2-mediated responses to osmotic stress (Li et al. 2016). The interaction between miR393 and auxin receptors suggests a complex regulatory network that fine-tunes plant responses to osmotic challenges. Moreover, studies have shown that auxin signaling is intricately linked to other hormonal pathways, particularly ABA signaling, which is critical for stomatal regulation during drought conditions (Sharma et al. 2023). For example, ABA enhances the expression of genes involved in root growth and water uptake, while auxins modulate these pathways to optimize plant resilience under osmotic stress (Rowe et al. 2016). Recent research has demonstrated that auxin transporters such as PIN proteins also play a crucial role in osmotic stress responses by facilitating the redistribution of auxins within plant tissues. This redistribution can enhance root development and improve water absorption efficiency during water scarcity (Zhou and Luo 2018). Additionally, the upregulation of certain Aux/IAA genes under osmotic stress conditions indicates their potential role as negative regulators of auxin signaling, which may help to balance growth and stress responses (Shani et al. 2017). Furthermore, studies have shown that plants with enhanced expression of specific Aux/IAA proteins exhibit improved tolerance to osmotic stress by maintaining higher levels of photosynthetic activity and reducing oxidative damage. By integrating these findings into breeding programs or genetic engineering approaches, it may be possible to develop crop varieties with enhanced resilience to osmotic stress. In short, the evidence suggests that TIR1/AFB receptors are essential for plant growth and environmental adaptation under osmotic stress conditions.
8.4 Role of auxins in promoting salt stress tolerance in plants
High salt concentrations present significant challenges to plant growth, primarily through osmotic stress, which adversely affects root systems. While mild salt stress (less than 50 mM) can stimulate lateral root growth by enhancing auxin distribution and promoting root plasticity, excessive salt concentrations (more than 100 mM) severely restrict root development, leading to reduced nutrient and water uptake (Zolla et al. 2010). Furthermore, Wang et al. (2009) demonstrated that auxin-signaling mutants such as axr1, axr4, and tir1 exhibit reduced lateral root development, highlighting the critical role of auxin signaling in root architecture under saline conditions. The salt overly sensitive (SOS) pathway is essential for Arabidopsis to enhance its resistance to salt stress. Ji et al. (2013) showed that this pathway regulates ion homeostasis and osmotic adjustment, which are crucial for maintaining cellular function under high salinity. Interestingly, after three hours of salt treatment, the cucumber YUC genes CsYUC11 and CsYUC10a are repressed, indicating a potential feedback mechanism in auxin biosynthesis under salt stress. In contrast, overexpression of YUC11in Arabidopsis has been linked to improved salt tolerance through enhanced root elongation and increased lateral root formation (Yan et al. 2016). Recent research has identified the auxin-responsive gene TaSAUR75 is being suppressed under salt stress conditions. Overexpression of TaSAUR75 in Arabidopsis has been shown to significantly improve salt tolerance by increasing root length and survival rates during saline exposure (Guo et al. 2018). This suggests that TaSAUR75 may play a pivotal role in mediating auxin responses that enhance plant resilience to salinity.
8.5 Auxins improve cold stress resistance in plants
Cold stress significantly limits species distribution and impacts plant survival and productivity. This stress encompasses chilling temperatures (0-12°C) and freezing (<0°C), both of which are prevalent among various plant species. Freezing temperatures can cause severe damage to cell membranes, while chilling temperatures restrict growth and metabolism processes (Kidokoro et al. 2022). To adapt to cold conditions, plants have evolved several acclimation mechanisms; for example, calcium channels detect cold through the CHILLING TOLERANCE DIVERGENCE 1 (COLD1) protein, while the CBF/DREB1 transcription factors activate cold stress response genes known as COR genes (Ma et al. 2015). Auxin response regulation is integral to the complex molecular mechanisms involved in cold adaptation. Cold stress influences auxin levels based on plant species, developmental stage, and physiological context. For example, cold temperatures suppress gravitropic growth in both stems and roots of Arabidopsis and rice, indicating a disruption in normal growth patterns due to altered auxin signaling (Shibasaki et al. 2009, Du et al. 2013a). Additionally, auxin analogs have been shown to induce Brassica napus to accumulate freeze-protective metabolites under cold stress conditions, thereby enhancing its resilience. Recent studies indicate that cold exposure increases auxin levels in Triticum aestivum L. (Wang et al. 2023), which may contribute to enhanced growth responses under low-temperature conditions. Furthermore, cold stress significantly impacts the expression of auxin efflux carriers, ARF transcription factors, and Aux/IAA transcription repressors in both Arabidopsis and rice. For example, cold treatment enhances the expression of rice YUC family genes while decreasing the expression of the OsGH3 family gene, which is involved in auxin homeostasis (de Freitas et al. 2019). The sensitivity of Arabidopsis to exogenous IAA is influenced by the overexpression of SgGH3.1; this alteration also affects the expression of AtCBF1–AtCBF3 gene, which are crucial for chilling tolerance (Jiang et al. 2021). Moreover, cold stress inhibits intracellular trafficking of the PIN2 and PIN3 efflux carriers for auxins, distributing root development and gravitropic responses. This disruption can lead to increased susceptibility to cold stress, as observed in Arabidopsis mutants lacking the transcriptional repressor IAA14 (Aslam et al. 2020). Conversely, overexpression of the CsARF5 transcription factor gene has been shown to enhance chilling tolerance in cucumber plants, indicating its potential role in mediating auxin responses under cold stress conditions (Zhang et al. 2021b). In summary, auxins play a crucial role in promoting cold stress resistance through their involvement in various physiological and molecular processes. By elucidating these mechanisms further, we can develop targeted strategies for enhancing plant resilience to cold stress, ultimately improving crop productivity and survival in changing climates.
9 CONCLUSION AND FUTURE PERSPECTIVE
This review emphasizes the critical role of auxins in mediating plant responses to both biotic and abiotic stresses. Auxins serve not only as growth regulators but also as essential signaling molecules that activate defense mechanisms necessary for survival under adverse conditions. The complex interplay between auxin signaling pathways and other phytohormones is vital for maintaining homeostasis while enabling adaptive responses. Future research should focus on elucidating the specific molecular pathways through which auxins mediate these responses, particularly crosstalk with other hormonal pathways, such as SA and JA, which play complementary roles in plant defense. Additionally, reducing auxin degradation and formulating auxin-based bio-stimulants could provide practical solutions for enhancing crop performance under stress conditions. Advancements in genetic engineering could yield crop varieties with improved resilience against environmental stresses by manipulating both biosynthesis and signaling pathways related to auxins. Integrating omics technologies—such as transcriptomics, proteomics, and metabolomics—will provide comprehensive insights into dynamic changes occurring in auxin-related gene expression during stressful conditions. By deepening our understanding of these mechanisms mediated by auxins, researchers can significantly contribute to developing resilient cultivars capable of thriving in increasingly variable environments, thereby ensuring food security amid changing climatic conditions.
AUTHOR CONTRIBUTIONS
Conceptualization of the project and manuscript drafting, M.A and L.S; contribution to the editing and proofreading of the manuscript draft, M.A.K, and A.A, S.H and J.S; All authors have read and agreed with the published version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by a grant from the National Natural Science Foundation of China (Grant No. 32100286).
DECLARATION OF GENERATIVE AI AND AI-ASSISTED TECHNOLOGIES IN THE WRITING PROCESS
During the preparation of this work the authors used ChatGPT 3.5 tool in order to improve readability and language. After using this tool/service, the author(s) reviewed and edited the content as needed and take(s) full responsibility for the content of the publication.
Open Research
DATA AVAILABILITY STATEMENT
Not relevant to this review




