Green light induces Solanum lycopersicum JA synthesis and inhibits Botrytis cinerea infection cushion formation to resist grey mould disease
Abstract
Light signals are prevalent and influence the survival strategies of both plants and the pathogenic fungi that infect them. In this study, we found that green light inhibits the infectivity of Botrytis cinerea on Solanum lycopersicum. Through transcriptome analysis and validation of S. lycopersicum leaves infected with B. cinerea, we discovered that green light enhances the synthesis of jasmonic acid and its related metabolites by upregulating the expression of OPR3 and JAR1 in S. lycopersicum. Additionally, green light boosts the activity of antioxidant enzymes like peroxidase, catalase, and ascorbic acid peroxidase in S. lycopersicum to combat tomato grey mould. Conversely, green light inhibits the expression of plant-induced colonization onset genes, mitogen-activated protein kinase genes, and the formation of infection cushions in B. cinerea. Our findings provide insights into the role of environmental green light signals in the interaction system between plants and phytopathogenic fungi.
1 INTRODUCTION
Botrytis cinerea is a necrotrophic plant pathogen capable of infecting more than 1,400 plant species (Bi et al., 2023). The US Department of Agriculture estimates that B. cinerea causes agricultural losses of 10 to 70%, amounting to millions of dollars in Europe annually, leading to crop losses of approximately 10 to 70% both before and after harvest (Dean et al., 2012; Elad & Stewart, 2007). The B. cinerea, known for its significant destructive impact on agricultural production, is ranked as the second most prevalent plant pathogenic fungus (Dean et al., 2012). Although fungicides can control grey mould caused by B. cinerea, many have lost their effectiveness due to the tendency of B. cinerea to mutate (Williamson et al., 2007). Thus, new methods are urgently needed to prevent and control tomato grey mould.
Upon infecting a host, B. cinerea can produce conidia dispersed through air disturbances (Yu & Fischer, 2019). Once these conidia settle on the host's surface, they can develop into critical pathogenic structures known as infection cushions (ICs) (Schumacher, 2017). These structures play a pivotal role in infection and colonization by secreting various substances, including cell wall degrading enzymes and toxins, which facilitate the pathogen's invasion and establishment within the host tissue (Emmett & Parbery, 1975; Schumacher, 2017). Pathogenic analysis of some B. cinerea mutants indicates the important role of IC in their infection of plants (Canessa et al., 2013; Choquer et al., 2021; De Vallée et al., 2019). In the B. cinerea intermediate stages of infection (about 36–48 hpi), the conidia germ tubes or more elongated hyphae differentiate simple appressoria and IC (Bi et al., 2023). In addition, at this stage, the plant-induced colonization outset genes (PIO) peak in expression during the outset of colonization (Gioti et al., 2006). After successful colonization on the surface of the host, B. cinerea can continue to infect the host's tissues based on this stage. However, the role of light signals, especially green light, in the formation of IC during B. cinerea infection has not been studied yet.
Light signals are ubiquitous in the environment and play a crucial role for plant pathogens, which rely on their hosts for nutrition, these signals often indicate alterations in the broader environmental conditions (Yu & Fischer, 2019). Consequently, hosts must modify their survival strategies to adapt to these impending changes (Corrochano, 2019). For instance, B. cinerea typically produces conidia in light and sclerotia in darkness (Schumacher, 2017). Moreover, light signals can control the production of melanin via the high-osmolarity glycerol (HOG) pathway in B. cinerea, which is one of the three Mitogen-activated protein kinase (MAPK) signal transduction pathways in fungi (Turrà et al., 2014), to responding the changes in the external light environment (Liu et al., 2011). Light signals also regulate the pathogenicity of phytopathogenic fungi. Research indicates that light signals regulate the synthesis of deoxynivalenol (DON) by Fusarium graminearum (Ansari et al., 2014). In B. cinerea, pathogenicity varies significantly between dawn and dusk under different photoperiods (Hevia et al., 2015), and the light receptor White Collar Complex (WCC) and the light-responsive complex VELVET in B. cinerea play roles in regulating pathogenicity (Canessa et al., 2013; Müller et al., 2018). The above results show light signals regulate the pathogenicity of plant fungi. However, research on the impact of light signals, particularly green light, on the pathogenicity of plant pathogenic fungi remains limited.
The jasmonic acid (JA) signalling pathway is crucial for plant resistance to necrotic fungi (Yang et al., 2012). Numerous reports examine how red and far-red light regulates the JA signalling pathway under various light conditions. For instance, low R: FR ratios can weaken JA signalling through the antagonism between DELLAs and JAZs (Chico et al., 2014; Hou et al., 2010; Leone et al., 2014; Machado et al., 2017; Yang et al., 2012) and could directly regulate the production of bioactive JAs via the phyB–PIF transcription module (Fernández-Milmanda et al., 2019). Furthermore, research on Arabidopsis thaliana shows that green light stimulates the synthesis of plant JA (Sato et al., 2015). These results suggest that light signals play a role in regulating the JA signalling pathway in plants. However, the interaction between non-red light signals and the JA signalling pathway requires further investigation.
Solanum lycopersicum, one of the crucial horticultural crops, is highly susceptible to B. cinerea infection throughout its growth stages, including flowering, fruiting, and post-harvest, leading to the outbreak of S. lycopersicum grey mould disease (Bi et al., 2023; Dean et al., 2012). Grey mould has become a major fungal disease in facilities, affecting S. lycopersicum cultivation (Shu et al., 2021). In this study, we aimed to reveal molecular insights into how green light inhibits B. cinerea infection in S. lycopersicum by analyzing the transcriptomes of both species under varying light conditions.
2 MATERIALS AND METHODS
2.1 Plants growth conditions
The seeds of Solanum lycopersicum cv. m82 were large-fruited determinate cultivars sourced from the Jiang Yina laboratory at East China Normal University. These seeds were sown in pots and allowed to germinate under Philips White Power LED lights within an environmentally controlled growth chamber. The growth conditions were meticulously maintained for a period of four weeks, with day and night temperatures set at 25 and 20°C, respectively. The air humidity was consistently regulated at 65%. Additionally, the plants were exposed to a long-day photoperiod, consisting of 16 hours of light and 8 hours of darkness, with a photosynthetically active radiation (PAR) level of 150 μmol m-2 s−1.
To examine the effects of green and white light on the interaction between B. cinerea and S. lycopersicum, the plants, once reaching the vegetative growth stage, were inoculated with the conidial suspension. They were then exposed to Philips Green or White Power LED lighting conditions, consisting of a 16-h Green or white light/8-h dark photoperiod at 150 μmol m−2 s−1 photosynthetically active radiation (PAR), for nine days.
2.2 Fungal growth conditions
The wild-type B. cinerea strain B05.10, originally described by (Büttner et al., 1994) was sourced from the Xu Ling laboratory at East China Normal University. The cultivation of B. cinerea strains was conducted on potato dextrose agar (PDA) medium. These strains were incubated at a temperature of 25°C in Percival incubators. The incubators were equipped with cool white fluorescent tubes, providing a light intensity of up to 100 μmol m−2 s−1 and covering a wavelength range of 400–720 nm.
2.3 Virulence assay
The virulence assay was conducted with appropriate modifications as previously described by (Lian et al., 2018). To produce conidia, B. cinerea was cultivated for two weeks under light conditions at 25°C on a PDA plate. Conidia were collected using 5 mL of double-distilled water (ddH2O), and 10 μL droplets of the resulting conidial suspension, with a final concentration of 1 × 10^6 conidia/ml, were applied to the surface of vegetative growth stage S. lycopersicum leaves, and the leaves inoculate with B. cinerea suspension were then exposed to Philips Green or White Power LED lighting conditions, consisting of a 16-h Green or white light/8-h dark photoperiod at 150 μmol m−2 s−1 photosynthetically active radiation (PAR), and been placed in a controlled environment with 90% relative humidity for 12 hours post-inoculation (hpi). The lesion area at the inoculation site was subsequently measured using Image J software.
2.4 Total RNA extraction and RT-qPCR
To investigate the effects of green light on gene expression related to B. cinerea infection in S. lycopersicum, leaves were collected at the vegetative growth stage after being infected with a 10 μL droplets 1 × 10^6 conidia/ml suspension of B. cinerea for two days. Total RNA was isolated using the TRIzol reagent (Invitrogen) following the protocol outlined by (Canessa et al., 2013), For the RT-qPCR assay, RNA was extracted using the RNAiso Plus kit (TaKaRa), and cDNA synthesis was performed with the PrimerScript RT Reagent Kit with gDNA Eraser (TaKaRa) according to the manufacturer's instructions. The RT-qPCR procedure was conducted as described by (Hevia et al., 2015). Additionally, the preprocessed RNA-Seq reads were aligned to the reference genomes of S. lycopersicum and B. cinerea using the Bowtie, TopHat, and Cufflinks programs.
2.5 Transcriptome analysis
The DEGs were identified from RNA-Seq data with the cut-off of corrected P-value < .0.1. Analyses of the biological information of DEGs were performed as previously described. The database used in the analyses included EggNOG (evolutionary genealogy of genes: Non-supervised Orthologous Groups, http://eggnogdb.embl.de/#/app/home), a database of orthologous groups of genes, Gene Ontology Consortium, an international standard classification system for gene function, including biological processes, cellular components, and molecular function (http://www.geneontology.org/), KEGG (http://www.genome.jp/kegg/), Swiss-Prot (http://web.expasy.org/docs/swiss-prot_guideline. html), NR (ftp://ftp.ncbi.nlm.nih.gov/blast /db/), and Pfam (http:// pfam.xfam.org/).
The raw transcriptome data has been uploaded to NCBI (https://www.ncbi.nlm.nih.gov/sra/PRJNA1193267).
2.6 Plant hormone measurements
To analyze the endogenous phytohormones in Solanum lycopersicum leaves during the vegetative growth stage, samples were collected after 12 h of exposure to either white light (WL) or green light (GL). The extraction and quantification of jasmonic acid (JA), salicylic acid (SA), auxin (IAA), the ethylene intermediate product amino cyclopropanecarboxylic acid (ACC), the gibberellin (GA) intermediate product GA8 and GA29, and their secondary metabolites were conducted using high-performance liquid chromatography. During the extraction process, the leaf samples were spiked with an isotope standard to ensure accuracy. The quantification was then performed using an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies) equipped with an electrospray ionization source.
2.7 Antioxidant enzyme activity detection
The Superoxide dismutase (SOD) activity has been measured by (Zhang et al., 2021), One unit of SOD is defined as the enzyme amount needed to inhibit 50% of the photochemical reduction of NBT at 560 nm and is expressed as U g−1 of protein.
The Peroxidase (POD) activity has been measured by (Zhao et al., 2009), with one unit defined as an increase in absorbance of 0.01 per minute at 460 nm, expressed as U g−1 of protein.
Catalase (CAT) activity has been measured following the method described by (Zhang et al., 2021), One unit of CAT was defined as the decomposition of 1 μM H2O2 per milligram of protein per second. Absorbance was measured at 405 nm and expressed as μmol min−1 g−1 of protein.
Ascorbic acid peroxidase (APX) activity has been measured following the method of (Q. Tang et al., 2020). One unit of activity was defined as a 0.01 decrease in absorbance at 290 nm per minute and expressed as nmol min−1 g−1 of protein.
2.8 MDA content detection
2.9 Chlorophyll content detection
The levels of photosynthetic pigments, chlorophyll a, and chlorophyll b, in S. lycopersicum leaves were measured by (Arnon, 1949), as previously detailed by (C. Tang et al., 2021).
2.10 Statistical analysis
Statistical data are expressed as means ± standard errors (SE) from three repetitions. Bar charts represent mean values with standard deviations, using the t-test to examine if differences between groups of samples were significant at a P-value of <0.01.
3 RESULTS
3.1 Green light inhibits B. cinerea infection of S. lycopersicum
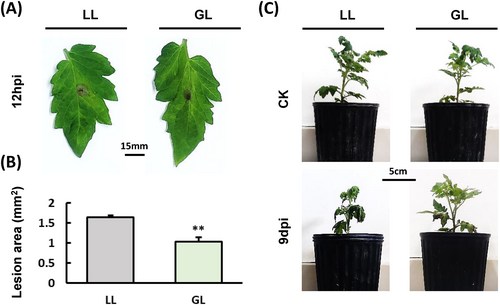
To confirm if exposure to green light not only enhances the resistance of S. lycopersicum to P. cichorii but also to B. cinerea, Initially, the B. cinerea conidia suspension was inoculated onto S. lycopersicum leaves and incubated under both white light (WL) and green light (GL) conditions for 48 hours post-inoculation (hpi) (Figure 1A). The results demonstrated that the lesion area of B. cinerea under GL was significantly smaller compared to that under WL (Figure 1B). The growth, development, and resistance of live S. lycopersicum leaves are affected by varying light qualities during in vitro inoculation. Subsequently, B. cinerea was inoculated on S. lycopersicum plants under both WL and GL conditions (Figure 1C). The results are consistent with the results of inoculating S. lycopersicum leaves with B. cinerea, indicating that B. cinerea infection was inhibited under GL.
3.2 RNA-seq statistical analysis
To verify the inhibition of B. cinerea infection in S. lycopersicum under GL conditions, B. cinerea was inoculated into the leaves of S. lycopersicum plants under both GL and WL conditions for 48 hpi, using three biological replicates for RNA-seq analysis (Figure S1). We first performed a principal coordinate analysis (PCA), the S. lycopersicum principal components 1 and 2 explained 99.1 and 0.5% of the total observed variance (Figure 2A), respectively, and the quality of the sequencing data was sufficient for further study. PCA results of S. lycopersicum showed very high differences between WL and GL. We obtained 2317 DEGs (Different Expression Genes) in GL vs. WL, in which 1076 genes were significantly up-regulated, and 1241 genes were dramatically down-regulated in B. cinerea infected S. lycopersicum leaves under GL as compared to WL condition (Figure 2B).
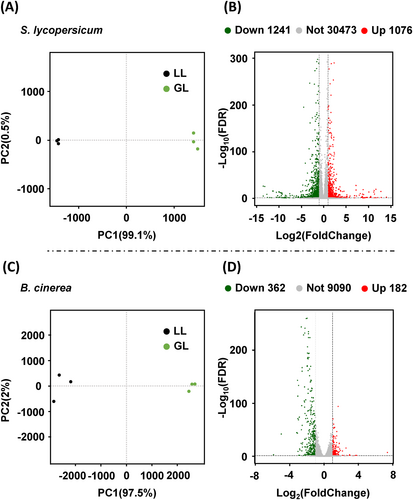
The B. cinerea principal components 1 and 2 explained 97.5 and 2% of the total observed variance (Figure 2C). PCA also shows significant differences in B. cinerea between WL and GL conditions. There are 544 DEGs between WL and GL of B. cinerea, and 182 genes were significantly up-regulated and 362 genes down-regulated in B. cinerea under GL as compared to the WL condition (Figure 2D).
3.3 GO and KEGG function analysis of DEGs
To evaluate DEG functional characteristics, we performed GO and KEGG enrichment analyses. Among them, the S. lycopersicum plenty of DEGs were mainly enriched in Cellular Component (CC) and Biological Process (BP) rather than Molecular Function (MF) (Figure S2 A, C, E), the B. cinerea plenty of DEGs were mainly enriched in CC and MF rather than BP (Figure S2B, D F). In S. lycopersicum infected by B. cinerea in GL vs. WL, DEGs were linked to the chloroplast thylakoid membrane, chloroplast stroma, and chloroplast envelope (Figure S2C). In B. cinerea, which is infecting S. lycopersicum under GL vs. WL, DEGs were linked to the nucleoplasm and extracellular region (Figure S2D).
KEGG enrichment analysis of S. lycopersicum infected by B. cinerea in GL vs. WL highlighted enrichment in photosynthesis, glyoxylate, and dicarboxylate metabolism, and carbon fixation in photosynthetic organisms (Figure S3A). In B. cinerea that is infecting S. lycopersicum under GL vs. WL, the KEGG are highlighted enrichment in ribosome biogenesis in eukaryotes (Figure S3B).
3.4 Green light can induce JA synthesis of S. lycopersicum
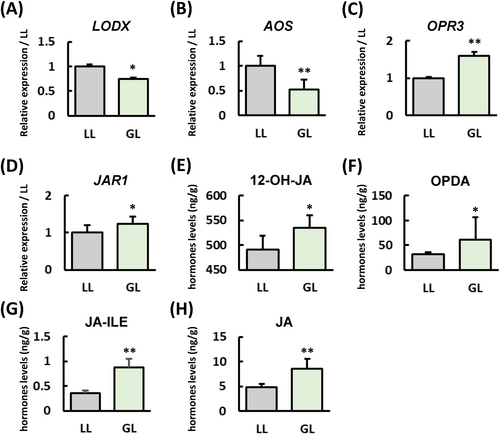
B. cinerea, a typical necrotrophic fungus, is generally resisted by plants through the JA signalling pathway. To understand the response of S. lycopersicum to this pathogen, we analyzed the differential expression of genes (DEGs) in the GL versus WL conditions, focusing on various hormone pathways (Table S1). Our analysis revealed a significant number of genes associated with the ethylene signalling pathway. In contrast, there were relatively few genes linked to the SA and JA pathways, which are typically involved in resistance mechanisms. To confirm whether the JA synthesis pathway is affected by GL, we analyzed the S. lycopersicum's JA synthesis-related pathway genes expression (Figure 3A-D) and content of JA-related metabolites (Figure 3E-H), the results showed that the expression levels of OPR3 and JAR1 under GL increased in S. lycopersicum compared to WL. Compared with WL, the JA-related secondary metabolites under GL have all increased. The above results indicate that GL can enhance its resistance to B. cinerea by inducing JA synthesis in S. lycopersicum.
3.5 The impact of green light on the levels of other various plant hormones in S. lycopersicum
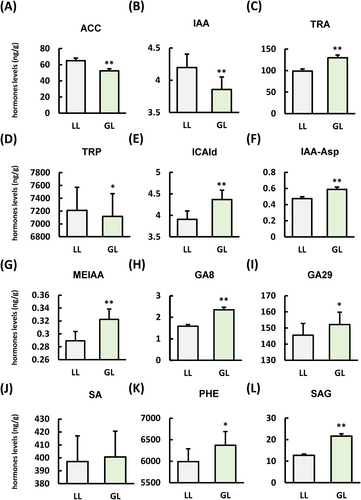
During disease resistance, plants often undergo hormonal crosstalk to combat pathogen invasion. Our research indicates that green light inhibits the synthesis of ACC (Figure 4A), a key intermediate in ethylene production compared with WL. The GL inhibits the synthesis of IAA (Figure 4B) and its precursor TRP (Figure 4D) compared with WL, while significantly increasing the content of the IAA precursor TRA (Figure 4C) and its related metabolites ICAld (Figure 4E), IAA-Asp (Figure 4F), and MEIAA (Figure 4G). In addition, GL significantly induces the intermediate products GA8 (Figure 4H) and GA29 (Figure 4I) in GA compared to the WL condition.
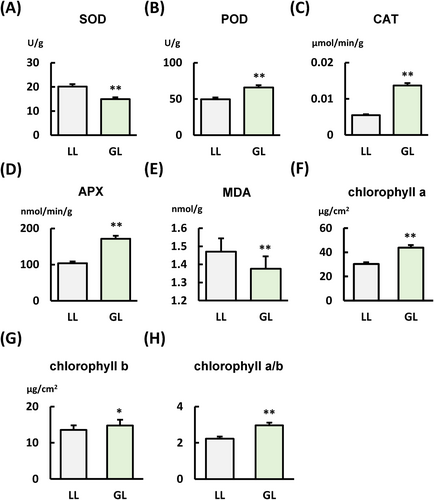
3.6 Green light can boost antioxidant enzyme activity and promote chlorophyll synthesis in S. lycopersicum
Antioxidant enzymes are essential for plant disease resistance. They aid in resisting pathogen invasion by eliminating reactive oxygen species (ROS), maintaining redox balance, and strengthening plant defence mechanisms. Therefore, we detected the activity of several key antioxidant enzymes associated with disease resistance (Figure 5). The results indicated that, compared to WL, GL inhibited SOD activity and MDA (Figure 5A and E) synthesis but enhanced the activity of POD, CAT, and APX (Figure 5B-D). Chlorophyll is crucial in plant disease resistance, producing reactive oxygen species and anti-disease hormones while coordinating nuclear defence responses as a signalling center. Our findings indicate that compared to the WL condition, GL could promote the synthesis of chlorophyll a and b and increase the ratio of chlorophyll a to b (Figure 5F-H).
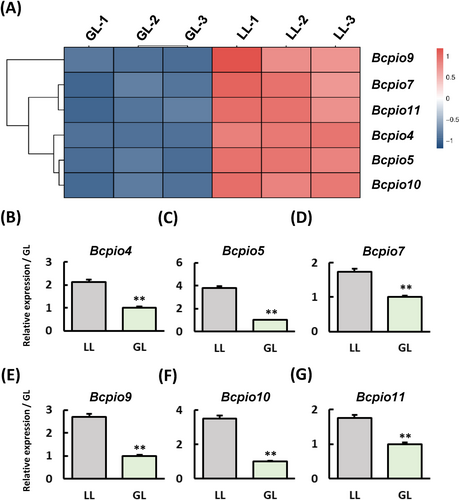
3.7 The B. cinerea plant-induced colonization outset genes expression has been inhibition under green light condition
The analysis of S. lycopersicum's DEGs in GL vs. WL revealed that genes linked to grey mould resistance were not significantly affected, likely due to GL's strong inhibitory effect on B. cinerea. In the analysis of B. cinerea DEGs that are infecting S. lycopersicum in GL vs. WL, we observed that a type of PIO gene expression (Gioti et al., 2006) was significantly downregulated in GL vs. WL (Figure 6A). We detected the expression of PIO in B. cinerea under WL and GL (Figure 6B-G), and the results showed that GL can indeed inhibit the expression of PIO in B. cinerea, which suggests that GL may reduce B. cinerea infection of S. lycopersicum by inhibiting B. cinerea colonization.
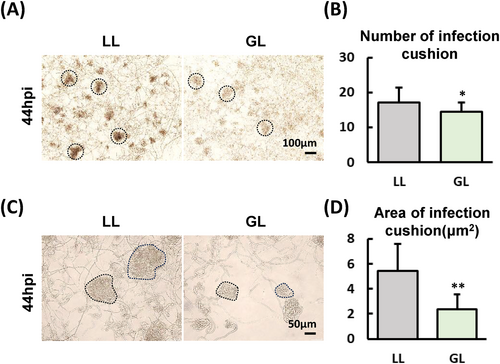
3.8 The B. cinerea IC formation has been inhibition under green light condition
Among S. lycopersicum and B. cinerea interaction system DEGs, the results of PIO gene expression in B. cinerea under GL and WL conditions suggest that GL could inhibit the colonization of B. cinerea on S. lycopersicum leaves. To verify this hypothesis, we observed the IC of B. cinerea under WL and GL conditions (Figure 7A), the results showed that the number of ICs in B. cinerea under GL was significantly lower than that under WL (Figure 7B), and the colour of ICs under WL was significantly black than under GL. This result implied that the IC development of B. cinerea under GL is delayed compared to WL. In addition, we also measured the size of IC, and the results showed that the size of IC under GL was significantly smaller than that under WL (Figure 7C and D), the above results indicating that GL does indeed have an inhibitory effect on the development of IC in B. cinerea compared to WL conditions.
3.9 The HOG pathway expression of B. cinerea is inhibited by green light
The HOG pathway components histidine kinase (HK) Bos1 and the mitogen-activated protein kinase (MAPK) BcSak1 are important for the development, pathogenicity, and adaptation to hyper-osmotic and oxidative stress in B. cinerea (Liu et al., 2008). The DEGs of B. cinerea in GL vs. WL indicate that GL can inhibit the expression of the bos1, Bcsak1, and downstream of BcSak1 transcription factors ccdr48 and bop1 compared to WL (Figure 8A), and RT-qPCR results also verified this point (Figure 8B-E).

At the same time, we also tested the changes in the germination of B. cinerea's conidia and the growth rate of hyphae under WL and GL conditions (Figure 8F and G), The results showed that GL inhibited the B. cinera's conidia germination (Figure 8H), but had no significant effect on the mycelial growth of B. cinerea (Figure 8I). The above results indicate that the GL regulates the conidia germination process.
4 DISCUSSION
Plants can absorb green light and regulate different physiological characteristics to affect their defence mechanisms (Shah et al., 2021). When exposed to GL, wheat, and radishes accumulate higher biomass compared to BL (Wu & Spalding, 2007). This observation implies that GL and BL may operate through distinct signalling pathways that regulate plant growth, development, and disease resistance processes. Phytohormones, especially SA and JA, are crucial in regulating plants' defence mechanisms against phytopathogenic fungi except for photosynthesis and photomorphogenesis (Du et al., 2017; Huché Thélier et al., 2016). JA and MeJA are crucial against necrotrophic fungi pathogens (Reyes-Díaz et al., 2016). Research on S. lycopersicum indicates that OPR3 plays a role in its defence of B. cinerea (Scalschi et al., 2015), JAR1 has been confirmed to play a role against B. cinerea in A. thaliana (Ferrari et al., 2003). Specifically, GL was found to enhance the expression of OPR3 and JAR1 significantly, and in S. lycopersicum transcriptome data, we observed and verify GL could down-regulate the expression levels of the COP1-interactive proteins CIP1 and CIP7. Previous studies have reported that CIP1 plays a role in regulating ABA in A. thaliana (Ren et al., 2016), COP1 enhances defence against turnip crinkle virus (TCV) and avrRPM1 bacteria by stabilizing the resistance (R) proteins HRT and RPM1, respectively (Lim et al., 2018). This suggests that GL may influence the light response in S. lycopersicum by inhibiting the expression of CIP1 and CIP7, potentially boosting the S. lycopersicum's resistance to B. cinerea. Moreover, the inhibition of AOS by GL, in contrast to OPR3 and JAR1, highlights a distinct regulatory mechanism in JA synthesis (Figure 3). Using PlantCARE to predict the promoters of key genes involved in JA synthesis revealed that the promoter of AOS contains significantly fewer light-responsive regulatory elements compared to other genes (Table S2). This finding suggests that the promoter regions of OPR3 and JAR1 may possess specific GL-responsive elements, which could account for their differential regulation by GL. Further research is needed to understand the regulatory mechanism of GL on JA synthesis in the future.
Ethylene also plays important roles in plant defence responses to pathogens except for SA and JA (Dı́az et al., 2002). The ethylene response factor SlERF.C1 acts as a key regulator to trigger the ethylene-mediated defence against B. cinerea in S. lycopersicum fruits without compromising ripening (Deng et al., 2024). Our study identifies changes in gene expression associated with multiple ethylene signalling pathways (Table S1). Furthermore, green light could suppress the expression of SlWRKY46 and SlWRKY81 in S. lycopersicum (Bian et al., 2021), and SlWRKY46 may play a negative regulatory role in the infection of B. cinerea by inhibiting the activity of antioxidants and disease-resistant enzymes, regulating SA and JA signalling pathways, and regulating ROS homeostasis (Shu et al., 2021). Unfortunately, we did not see significant expression changes of slwrky46 and slwrky81 in the transcriptome data of S. lycopersicum, suggesting that GL may not directly regulate the expression of slwrky46 and slwrky81 in S. lycopersicum. Moreover, the antioxidant enzyme activity, like POD, CAT, and APX in S. lycopersicum, is enhanced under GL conditions, which also helps combat tomato grey mould.
For B. cinerea, our results indicate that GL inhibited the expression of key genes in the HOG signalling pathway in B. cinerea (Figure 8). The HOG pathway plays a role in resisting hyper-osmotic conditions, responding to light signals, and regulating pathogenicity in phytopathogenic fungi (Kilani et al., 2020; Liu et al., 2008, 2011; Temme et al., 2012). After the mutation of B. cinerea HOG pathway components Bos1 and BcSak1, the pathogenicity of the mutant significantly decreases (Heller et al., 2012; Liu et al., 2008). The absence of BcSak1 also affects the penetration of hyphae into the host (Segmüller et al., 2007), and the HOG pathway plays a crucial role in regulating the synthesis of the cell wall and melanin (Liu et al., 2011), which are key factors in IC formation (Choquer et al., 2021). Transcriptome analysis of ∆sak1 reveals that BcSak1 influences the expression of multiple pathways, growth, development, secreted proteins, and cell wall degradation enzyme-related genes in B. cinerea (Kilani et al., 2020). In addition, the mutation of G protein Bcg3 upstream of MAPK affects the germination of conidia (Doehlemann et al., 2006). The HOG pathway may be involved in the initial colonization of B. cinerea on the host surface by regulating PIO-related genes during conidia germination and pathogenesis.
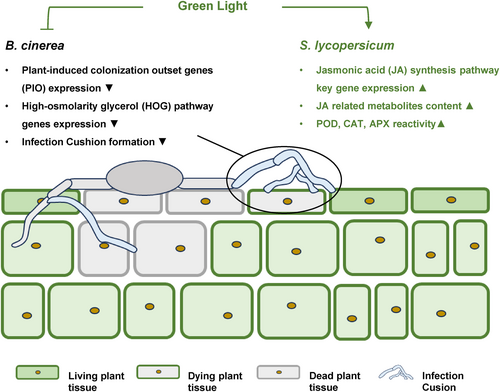
The influence of environmental light on the interaction between B. cinerea and S. lycopersicum is significant, affecting both the pathogen's infection process and the host's resistance mechanisms. Our study provides evidence that GL inhibits the expression of PIO-related genes, reduces the colonization of B. cinerea, and induces JA synthesis in S. lycopersicum (Figure 9). These findings suggest that GL may exert a more substantial effect on B. cinerea than S. lycopersicum. By elucidating these molecular interactions, our research offers valuable insights into managing B. cinerea infection in S. lycopersicum through the strategic use of environmental light.
AUTHOR CONTRIBUTIONS
Yunfei Cai: Formal Analysis, Investigation, Validation, Writing – Original Draft. Jiali Ying: Writing – Review & Editing. Youju Ye: Software, Writing – Original Draft. Shuangshuang Wen: Formal Analysis, Investigation. Renjuan Qian: Conceptualization, Funding acquisition, Methodology, Writing - Review & Editing, Supervision.
ACKNOWLEDGEMENTS
We would like to express our gratitude to Professor Xu Ling, Professor Jiang Yina, and Associate Professor Zhu Pinkuan for their assistance in the experiment.
FUNDING INFORMATION
This work was supported by the Zhejiang Provincial Natural Science Foundation (LQN25C010001). Wenzhou scientific research project (N20240006, X2023104). Zhejiang Leading Talents Program (2024C04006). Special funding project of Zhejiang Academy of Agricultural Sciences (2024XJXK03).
Open Research
DATA AVAILABILITY STATEMENT
All data that support the findings of this study are included in this manuscript.




