Gibberellic acid and light effects on seed germination in the seagrass Zostera marina
Abstract
Seagrass meadows have been heavily affected by human activities, with Zostera marina L. (Zosteraceae) being one of the most impacted species. Seed-based methods are currently the preferred approach for their restoration, yet low germination rates and poor seedling establishment remain significant challenges. This study explored the combined effects of light spectra (white, red, and darkness), photoperiod, and gibberellic acid (GA3−0, 50, 500, and 1000 mg L−1) on Z. marina seed germination using a fully crossed incubation experiment. Penalised logistic regression and Cox proportional hazards analysis were chosen to account for low germination events and to analyse the temporal dynamics of germination. We found that light conditions, particularly red light and darkness, when combined with GA3, significantly enhanced germination probability. Furthermore, mid (50 mg L−1) and high (500 mg L−1) GA3 concentrations reduced time-to-germination. Morphometric analysis of the cotyledonary and leaf tissue development indicates no adverse effects of the treatments on seedling development. Our findings suggest that light and GA3 treatments effectively improve germination success and reduce dormancy in Z. marina seeds. Seed treatments can mitigate stress- or manipulation-induced dormancy and can represent a viable strategy for on-demand germination, such as in the context of seed-based restoration efforts.
1 INTRODUCTION
Seagrasses are marine flowering plants widely distributed along the world's coastal areas (Short et al., 2007a). Representing approximately 72 species across five families, seagrasses thrive in both tropical and temperate regions (Short et al., 2011) and occupy roughly 0.2% of the ocean surface (Duarte & Chiscano, 1999; Orth & Heck Jr., 2023). Seagrass meadows provide valuable ecosystem services to coastal communities, including carbon sequestration (Serrano et al., 2021), providing nursery and foraging habitats for marine organisms (McDevitt-Irwin et al., 2016), thus supporting global fisheries (Unsworth et al., 2019a), and contributing to coastal resilience through sediment stabilisation that mitigates erosion (Duarte, 2002). Unfortunately, seagrass ecosystems face increasing pressure from human activities and climate-change effects, with annual global loss rates increasing from 0.9% in 1940 to 7% in recent decades (Short et al., 2007; Waycott et al., 2009; Unsworth et al., 2019b).
Restoration efforts are being carried out worldwide to rehabilitate these valuable ecosystems and offset the biodiversity loss of the habitat associated with seagrass meadows (van Katwijk et al., 2016; Jacob et al., 2018; Valdez et al., 2020). Seagrass conservation and restoration efforts are increasingly prioritised by international bodies such as the United Nations (Environment, U.N., 2020), the European Union (Council of the European Union & European Parliament, 2024), as well as state authorities, emphasising the urgent need to protect and restore these vital marine habitats.
Zostera marina L. (Zosteraceae), the most wide-ranging seagrass species, has been the subject of numerous restoration attempts in the last decades (Infantes et al., 2016; Yang et al., 2016). Seed-based methods are currently among the favoured restoration techniques (Unsworth et al., 2019), as they offer several advantages, including minimal disruption to donor meadows (Zhang et al., 2022), increased genetic diversity (Sinclair et al., 2013), and reduced logistic constraints, as observed for wild-sourced sod transplantation (Unsworth et al., 2023).
Seed broadcasting holds the potential to facilitate a rapid expansion of seagrass meadows, with successful examples reported in the Northern Hemisphere. For instance, previous restoration efforts conducted along the East Coast of the United States have achieved very high growth rates, with increases exceeding 16 times the original meadow extent (from 213 to 3612 ha of newly vegetated habitat) (Orth et al., 2020). Additionally, advancements in seed broadcasting techniques, such as seed injection (Gräfnings et al., 2023) and seagrass seed line methods (Unsworth et al., 2019a), have significantly enhanced the effectiveness and scalability of seagrass restoration efforts over the past decade. These technical innovations are complemented by conceptual approaches, such as rewilding, which focus on restoring ecological balance and promoting self-sustaining ecosystems (van Katwijk et al., 2021).
The growing number of restoration projects aimed at mitigating the degradation of Z. marina meadows has led to an exponential rise in seed demand (Unsworth et al., 2023). To meet this demand, numerous new Z. marina nurseries have been established globally; however, seed production remains constrained (Global Seagrass Nursery Network, personal communication). Thus far, most seagrass restoration efforts rely on wild-collected seeds (Unsworth et al., 2023). Optimising and maximising the use of available seed stocks is crucial to sustaining and scaling restoration initiatives.
Z. marina populations invest substantial energy in seed production, with yields varying widely from a few thousand to up to 10 million seeds per hectare (Statton et al., 2017a). This variation depends on factors such as plant density and individual seed productivity (Kendrick et al., 2023). After release, seeds exhibit high viability, with approximately 77% remaining viable one year after collection (Cumming et al., 2017). However, global studies consistently report poor regeneration outcomes in seed-based restoration efforts (Statton et al., 2017a; Unsworth et al., 2023). Despite high initial viability, the majority of seeds fail to germinate or establish successfully (over 90% fail to germinate or establish successfully) (Marion and Orth, 2009; Orth et al., 2007, 2003; Unsworth et al., 2023). Seed characteristics, including size and mass, can significantly influence seed quality (e.g. germination and establishment success); nevertheless, collection, handling, and storage practices associated with restoration can induce stress and extend seed dormancy, thereby significantly impacting seed batch quality and overall restoration outcomes (Orth et al., 2020; Unsworth et al., 2019b) (Dooley et al., 2013; Orth et al., 2020, 2000; Probert and Brenchley, 1999; Turner et al., 2013; Unsworth et al., 2019b). These factors collectively play a crucial role in determining the viability and effectiveness of seagrass restoration efforts.
Previous studies on the germination and dormancy of Z. marina seeds have predominantly examined the effects of temperature and salinity. The relative importance of these factors varies depending on the latitudinal population (Probert & Brenchley, 1999). Low-latitude populations (regardless of hemisphere) often respond to seawater temperature, while intertidal populations also appear to be influenced by reduced salinity (Phillips et al., 1983). However, the role of light, a potentially critical factor in regulating seed dormancy, has been largely overlooked, particularly in Z. marina populations where seeds frequently exhibit dormancy.
Dormancy is an evolutionary adaptation that enables seeds to reach maturity, delaying germination under adverse conditions (Finch-Savage & Leubner-Metzger, 2006; Yan & Chen, 2020). Abscisic acid (ABA) plays a key role in maintaining seed dormancy (Koornneef et al., 2002), whereas gibberellins (GAs) counteract dormancy by promoting its release and stimulating germination. Although dormancy is a natural mechanism aligning seed maturity and development with seasonal shifts (Zhu et al., 2023), it can also be induced as a plant response to stress (Zhang et al., 2020a).
In most angiosperms, light and photoperiod are critical factors for dormancy release and germination stimulation (Imaizumi et al., 2017; Klupczyńska & Pawłowski, 2021; Wang et al., 2021). Light exposure can cause elevation of GA levels within the seed embryo, initiating enzymatic breakdown of the endosperm (Kahn et al., 1957; Oh et al., 2006; Toyomasu et al., 1998), facilitating embryo expansion and cotyledon emergence (Finch-Savage & Leubner-Metzger, 2006; Yan & Chen, 2020).
The role of light in dormancy regulation has also been recognised as essential for marine plants (De Los Santos et al., 2010; Strydom et al., 2017; Davey et al., 2018; Zhang et al., 2020b; Waite et al., 2021). Some seagrass species, such as Halophila ovalis (R. Br.) Hook.f. and Thalassia hemprichii (Ehrenb.) Aschers., show increased germination rates upon red light exposure (Strydom et al., 2017; Waite et al., 2021; Soong et al., 2013). There is evidence that the light response of Z. marina is altered compared to terrestrial plants, potentially due to modifications in its photo-hormonal pathways (Olsen et al., 2016). These alterations may influence its responsiveness to light stimuli, impacting key physiological processes such as germination and growth. Plants detect light through photoreceptors (Gyula et al., 2003; Li et al., 2011), and the Z. marina genome retains genes for six of these photoreceptors (Olsen et al., 2016). Light activation of photoreceptors initiates physiological processes that regulate hormonal pathways, particularly those involved in dormancy and germination (Jhanji et al., 2024; Panda et al., 2022).
Dormancy in terrestrial plants is better documented. In agricultural and restoration practices, seeds are often exposed to stimuli, such as light and hormonal pre-treatments, to break dormancy and enhance germination success (Dutta, 2018; Chua et al., 2020; Kettenring & Tarsa, 2020). Applying these techniques to seagrasses could address significant restoration challenges, as seed dormancy and inconsistent germination hinder success. Preliminary treatments for seeds, including scarification, temperature fluctuations, and freshwater pulses, have been explored for seagrasses (Kaldy, 2014; Statton et al., 2017b; Liu et al., 2023; Wang et al., 2017). However, variable responses have hindered the development of a reliable protocol.
Light and gibberellic acid (GA3) treatments, which are known to stimulate dormancy release and enhance germination in diverse angiosperms, may similarly benefit seagrasses by enabling more synchronised and reliable germination outcomes. Despite their established efficacy in other plant species, light and hormonal pre-treatments remain underexplored in seed-based seagrass restoration, particularly for Z. marina. Few studies have examined the effect of light on Z. marina seed germination (Moore et al., 1993; Wang et al., 2017), and none, to our knowledge, have investigated the combined effect of light and hormone treatments on dormancy and germination.
In this study, we investigate the effects of distinct light spectra, photoperiods, and GA3 concentrations on germination in a North Atlantic population of Z. marina, aiming to identify optimal treatments for dormancy release. We assess germination success and explore the potential for light and hormone treatments to stimulate on-demand germination, thereby supporting restoration efforts through improved seedling establishment. Additionally, we conduct morphometric analyses of post-germination seedlings to evaluate any growth effects associated with the treatments.
2 MATERIALS AND METHODS
2.1 Seed collection and storage
Seed-bearing shoots of Zostera marina were collected from a donor meadow at Hamburger Hallig (54.5996° N, 8.8184° E, Germany) during low tide in early September 2021, coinciding with peak seed maturity. A collection permit was released by Landesbetrieb für Küstenschutz, Nationalpark und Meeresschutz Schleswig-Holstein (Germany), on June 25th, 2021 (n. 3141–537.46). Seed-bearing shoots were transported within 28 h to the lab facility at Ghent University in refrigerated boxes (8 ± 1°C), where the experiment was performed.
Seed release from the reproductive shoots was facilitated by gentle seawater agitation provided through aeration with an air bubbler in buckets (30 L, 30 psu). Seed ripening was ensured by maintaining salinity and temperature conditions representative of in-situ conditions (30 psu, 10 ± 0.5°C) for a period of 42 days until natural temperature drop occurred.
Following seed release, seeds were hand-sorted, rinsed with natural seawater, and subjected to cold stratification in complete darkness at 3 ± 0.5°C for 120 days to mimic winter dormancy.
Sterilised natural seawater (autoclaved natural seawater, 121°C, 15 PSI, 20 min) with adjusted salinity (28 psu) was used as storage media (referred to as SSW). To prevent oomycete infections, 0.2 mg L−1 copper sulphate (CuSO₄) was added to the storage solution, following the protocol of Govers et al. (2017). To maintain a consistent CuSO₄ concentration and ensure its biocidal efficacy, the storage solution was refreshed weekly. SSW salinity was specifically adapted to 28 psu to correspond with natural field salinity during the period of seed germination.
Field-specific temperature, salinity, and irradiance data used during storage and experiment were retrieved directly from the Federal Maritime and Hydrographic Agency of Germany (BSH, https://www.bsh.de/EN/DATA/data_node.html) and the National Oceanic and Atmospheric Administration (Mishonov et al., 2024) for the site (54.5996° N, 8.8184° E). These data were used to match seasonal averages during storage, cold stratification, and the germination period, ensuring that experimental conditions closely reflected those experienced by the seeds in their natural habitat.
Seeds were subjected to a viability test and visually inspected for maturity based on their colouration. Seeds ranging from cyan to black were selected, as also described by Xu et al. (2016). To ensure viability and, due to the lack of a rapid, non-destructive method for assessing seed viability, seed quality was evaluated manually. Seeds were first visually inspected using a microscope (Leica, MZ 16), and only seeds with an intact (i.e. no damages) and firm coat (after gentle pressure with tweezers) were shortlisted. A sinking test in natural seawater (28 psu) was adopted to further consolidate our selection. Seeds sinking quickly in natural seawater were selected and considered viable for the experiments. This non-destructive method was selected based on the experience of Marion & Orth (2010). Selected seeds were surface-sterilised with ethanol (EtOH 70% v/v) for 2 min and subsequently with a solution of 1% sodium hypochlorite (NaOCl) for 5 min. Seeds were then rinsed five times in SSW.
2.2 Experimental design, culture media, and culture condition
A fully crossed experimental design was adopted to assess the effect of light spectra, photoperiod, and hormone priming on the germination of Z. marina seeds (Figure 1). To ensure experimental feasibility and facilitate daily monitoring operation in full axenic conditions of the in vitro replicate and subsequent follow-up of each seedling, a reduced number of seeds was employed for this study.
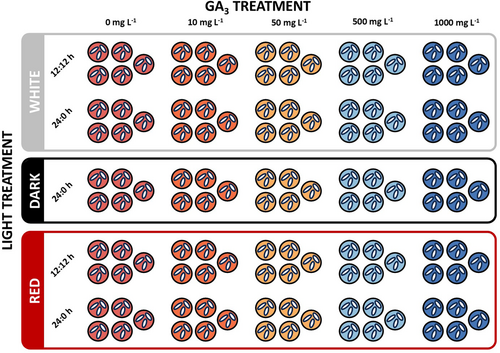
Three light conditions were selected for incubation: white light (400–720 nm, 5000 K, 158 μmol m−2 s−1, BlueView), darkness (no light), and red light (620–750 nm, intensity 144 μmol m−2 s−1 BlueView). The white and red light were tested with two photoperiods of 12:12 h and 24:0 h (light:dark regime), in addition to a darkness incubation. Photosynthetically active radiation (PAR) was measured by means of a handheld full-spectrum quantum meter (MQ-200, Apogee).
Five hormone solutions (soaking media) were prepared by dissolving different concentrations of gibberellic acid (GA3, Sigma-Aldrich), a major bioactive form of gibberellic acid, in SSW: 10, 50, 500, and 1000 mg L−1, alongside a negative control with 0 mg L−1. These concentrations were chosen based on studies indicating an effective GA₃ range for seed germination in various plant species, including marine plants (e.g. Corns, 1960; Thompson, 1969; Conacher et al., 1994; Ameen & Al-Imam, 2007; Mello et al., 2009; Martínez et al., 2016; Abdullah & Abdulrahman, 2017). Hence, a broader range of GA3 concentrations was chosen due to the lack of prior experiments on Z. marina seeds. GA3 solutions were mechanically filtered under laminar flow using sterile 0.2 μm syringe filters (Pall, Acrodisc). Excess solutions were stored at −20 ± 0.5°C and used for the weekly refreshment of the experimental units.
Each treatment, defined by the combination of light spectrum, photoperiod, and phytohormone, was replicated five times, resulting in a total of 15 seeds per treatment. Independent replication was achieved by applying each treatment across five separate Petri dishes, ensuring variability between units was accounted for. Within each Petri dish, three seeds were included to account for within-unit variability, resulting in a total of 375 seeds across all treatments (Figure 1).
Experimental units (Petri dishes, 35 mm, 5 mL, Avantor), each representing a unique combination of treatments as described in the previous section, were incubated at 10 ± 0.5°C for the entire duration of the experiment, a total of 42 days unless seeds germinated earlier.
The soaking media (SSW and GA₃ + SSW) were refreshed weekly to maintain stable hormone concentrations and, overcome hormone degradation and to prevent nutrient depletion, metabolic byproduct accumulation, and microbial growth. For each refreshment, in full aseptic condition under the laminar flow, the old media were carefully removed with a sterile pipette to avoid disturbing the seeds, and freshly prepared GA₃ + SSW (or SSW alone) solution was added to each Petri dish. This process ensured consistent, controlled conditions for evaluating treatment effects on germination.
2.3 Germination assessment and seedling development measurement
Germination was assessed three times a week over a period of 42 days. Germination was defined as the emergence of the cotyledon from the seed coat (Liu et al., 2023). Germinated seeds (hereafter referred to as seedlings) were transferred to a new experimental unit (Petri dish, 50 mm, 10 mL, Avantor) and supplemented with SSW (GA3-free media). Seedlings were incubated at 10 ± 0.5°C under white light at a mean intensity of 158 μmol m−2 s−1 with a 12:12 h photoperiod in a phytohormone-free medium containing SSW. SSW was refreshed weekly to maintain the same nutrient composition. A photograph of each seedling was taken twice a week using a microscope (Leica MZ16) coupled with a Canon EOS 600D camera. Pictures were analysed using the ImageJ version 1.52d software for morphometric measurement of the seedlings (Schneider et al., 2012). A certified micrometre graticule (Pyser-SGI, serial n. SC1539) was used to calibrate the software for pixel-μm conversion. Morphometric measurements were recorded for each seedling, cotyledon, number of roots, and true leaves, with each of their lengths measured two times a week. True leaves were defined as the leaves capable of performing photosynthesis, as described by Sugiura et al. (2009) and Taylor (2011). The length of the cotyledon was defined as the distance from the basal hypocotyl to the tip of the cotyledonary blade, and the length of true leaves was defined as the distance from the base of the cotyledonary sheath to the tip of the leaf. Maximum growth was calculated as the sum of the linear measurements of cotyledon and leaf length.
2.4 Statistical analysis
All analyses were performed using R statistical software (v.4.0.2; R Core Team, 2020). Seed germination results are presented as sum and binomial confidence intervals. Mean germination time, cotyledon, and leaf length results are presented as arithmetic mean ± standard error, unless stated otherwise.
The effects of the light spectrum, photoperiod, and hormone concentration on germination were analysed using a penalised logistic regression model implemented through the R package logistf (Firth, 1993; Heinze & Puhr, 2010; Puhr et al., 2017). Firth's penalisation approach was selected to account for low event rates and mitigate bias in coefficient estimates. Backward stepwise selection between models was carried out using the Akaike Information Criterion (AIC). ANOVAs with post-hoc Tukey HSD tests were performed via the multcomp R package (Herberich et al., 2010) to compare nested models and determine the exclusion of variables. The outcome variable, germination probability (GB), represents the likelihood of seed germination under varying conditions of light, photoperiod, and phytohormone concentration. Significance was consistently interpreted at p < 0.05.
The effects of light spectrum, photoperiod, and hormone concentration on the time to germination were analysed using a time-to-event (survival) approach with the R packages survival and survminer (Therneau & Grambsch, 2000). Survival analysis considers both the occurrence of an event, such as germination (binary response), and the time it takes for the event to occur (continuous response). As a survival model, we selected a Cox proportional hazards method to account for both categorical and continuous predictors (McNair et al., 2012), with predictors being light spectrum, photoperiod, and hormone and the response variable being time to germination (days). To ensure an accurate comparison between the statistical analyses (penalised logistic regression and Cox proportional hazard model), the selection of variables was based on the outcomes of the logistic model's ANOVA. To visualise the effect of predictors on the response variable, survival curves were computed via the Kaplan–Meier method through the R package survival.
Linear models were used to assess the potential backdrop effects of light spectrum, photoperiod, and hormone concentration on cotyledon and leaf growth after 60 days. The normality of residuals was evaluated using the Shapiro–Wilk test (stats R package), while the homogeneity of variances was tested using Levene's test (car package). To meet the assumptions of the analysis, leaf length measurements were square root-transformed prior to model fitting. Models were fitted using the lm function (Zuur et al., 2009) and estimated marginal means (EMMs) were calculated using the emmeans R package. Pairwise comparisons of EMMs were conducted with Tukey-adjusted p-values to account for multiple testing. All statistical analyses were performed in R, and results are presented as means ± standard error unless otherwise stated.
3 RESULTS
3.1 Influence of light and hormone treatment on seed germination
Overall, total seed germination reached 7.57% (95% CI 4.1–13.6), with an average daily germination rate of 0.80 ± 0.40% seeds. Seeds incubated in darkness showed the highest germination (10.67%, 95% CI 5.5–19.7%) compared to those in red light (8.33%, 95% CI 5.1–13.3%), and white light (3.70%, 95% CI 1.7–7.8%), although these differences were not statistically supported (p = 0.1). Control groups (0 mg L−1 GA3) had consistently low germination, peaking at 11.12% (95% CI 3.1–32.8%) under red light with a 12:12 h photoperiod.
Following variable selection, the final logistic model included the factors of light spectra, hormone, and the interactions of light and hormone and hormone and day, while photoperiod was not included. Germination probability (GB) was significantly enhanced by GA3 treatment alone (χ2(4) 42.55, p = 1.28 e−08) and by the combination of light and GA3 (χ2(8) 114.66, p < 0.001). Darkness positively influenced seed germination, though a negative dose-effect trend emerged as GA3 concentrations increased (Figure 2b), except in the control group (0 mg L−1 GA3). Under this light condition, the highest mean germination was observed in seeds exposed to 10 mg L−1 GA3, 26.7%, 95% CI 10.8–51.9%, and this result was supported statistically (z = 2.82, p = 2.21 e−04). Seeds incubated in darkness and in the absence of GA3 (control) showed no germination, consistent with natural dormancy behaviours in many plant species. Although germination generally declined with higher GA3 levels, a notable exception was observed with seeds exposed to 50 mg L−1 GA3 in darkness.
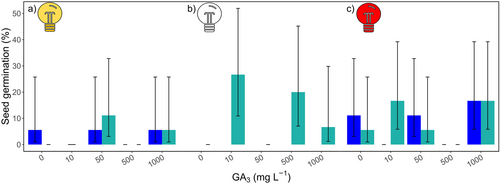
Similarly, there was no germination for seeds exposed to red light at 500 mg L−1 GA3 and under white light at both 10 or 500 mg L−1. These findings suggest that specific light-GA₃ combinations can inhibit germination, possibly through synergistic interactions that counteract GA₃'s germination-promoting effects.
GA3 treatment alone increased germination probability, with significant effects observed at 10 mg L−1 GA₃ (z = 3.33, p = 1.29e−06), 50 mg L−1 GA₃ (z = 1.70, p = 0.063), and 1000 mg L−1 GA₃ (z = 2.15, p = 0.011). 500 mg L−1 GA₃ resulted in a modest increase in germination probability; however, the effect was not statistically significant (z = 0.95, p = 0.376). These results generally indicate a dose-dependent response, with higher GA₃ concentrations associated with enhanced germination. These findings suggest that optimised GA₃ levels could be beneficial for seed germination, offering potential applications in restoration efforts.
3.2 Temporal patterns of seed germination: a time-to-event approach
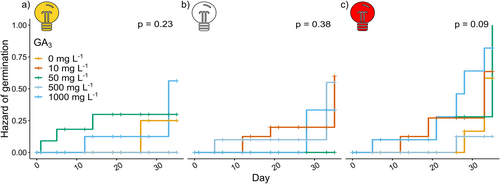
To better understand the temporal dynamics of seed germination, we analysed how different GA₃ concentrations and light conditions influenced the rate and likelihood of germination over time. This approach allowed us to assess not only which treatments enhance germination rates but also how quickly seeds respond under varying light and hormone conditions.
For seeds exposed to white light, the likelihood of germination increased by 2.72 ± 0.61 times at 1000 mg L−1 GA3, 1.96 ± 0.64 times at 50 mg L−1 GA3, and 1.74 ± 0.63 times at 10 mg L−1 GA3. Seeds exposed to white light and 50 mg L−1 GA3 exhibited the earliest germination response, with an average time to germination of 6.67 ± 1.26 days (Figure 3a). Notably, only the combination of the red light and 500 mg L−1 GA3 significantly reduced the time to germination (p = 0.033, Figure 3c). According to the Cox proportional hazard model, seeds exposed to red light germinated 1.83 ± 0.48 times faster than those exposed to white light; however, this difference was not statistically significant (p = 0.09).
3.3 Seedling development
The a posteriori effect of seed treatments on seedling development was monitored to assess potential side effects on seedling growth. After 60 days, cotyledons reached a mean maximum length of 18.96 ± 5.06 mm and grew at a mean rate of 0.75 ± 0.21 mm day−1, while the mean maximum length was 16.78 ± 9.21 mm with a growth rate of 0.84 ± 0.39 mm day−1. The first true leaf appeared on average 10.9 ± 2.80 days after germination.
The post-germination development of Zostera marina seedlings was assessed to evaluate the potential effects of light, photoperiod, and hormone treatments on cotyledon and leaf length responses. After a 60-day seedling follow-up, the maximum cotyledon length (28.23 mm) was observed in seeds exposed to red light and 1000 mg L−1 GA₃ under a 24-hour photoperiod (Figure 4c, b, o) with the second-highest growth recorded in darkness with 10 mg L−1 GA₃ (21.65 mm). Leaf development followed a similar trend, with the longest leaf length recorded under red light, a 24-hour photoperiod, and 10 mg L−1 GA₃ (34.06 mm).
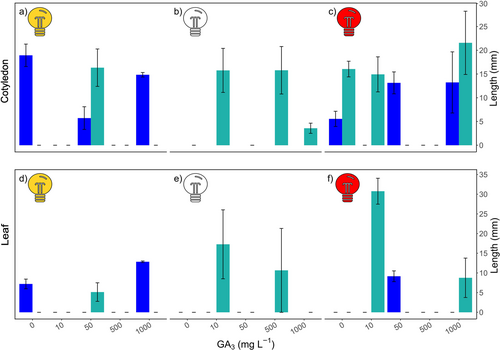
However, the linear model analysis indicated that the interaction between light, photoperiod, and hormone had non-significant effects on both cotyledon (F(12, 10) = 1.12, p = 0.43) and leaf lengths (F(8, 6) = 0.99, p = 0.51). Nonetheless, certain individual treatments exhibited notable growth. For instance, under darkness (24-hour photoperiod) and 500 mg L−1 GA₃, substantial cotyledon (20.79 mm) and leaf (21.31 mm) development was observed, suggesting a potential influence of darkness-incubated treatments (with 500 mg L−1 GA₃) on early seedling growth.
Conversely, the shortest cotyledon lengths were observed in some high-concentration treatments, such as darkness with 1000 mg L−1 GA₃, where cotyledon length was as low as 2.50 mm. Our results highlight variability in post-germination responses. While certain light and hormone combinations were associated with significant growth, the overall interactions were not statistically significant, suggesting that light and hormone treatments may have a limited or negligible impact on early seedling development.
4 DISCUSSION
Our study provides new experimental evidence that light conditions and GA3 treatments modulate germination and subsequent seedling development in the seagrass Zostera marina, highlighting the interplay between light- and hormone-mediated seed responses. This work aimed to determine whether targeted treatments could effectively break seed dormancy and enhance germination without impairing the subsequent stages of seedling development. Our findings demonstrate that these treatments not only promote germination but also reduce the time to germination, advancing our understanding of germination regulation and offering practical applications for seagrass restoration initiatives.
4.1 Effects of light on germination
The germination response observed in Z. marina seeds upon light exposure underscores an active involvement of plant photoreceptors in germination physiology. This seagrass species harbours six characterised photoreceptors, including phytochromes and phototropins. These photoreceptors likely drive light-dependent signalling pathways that activate enzymatic processes, facilitating seed wall weakening and endosperm degradation, which are crucial steps in promoting germination (Olsen et al., 2016).
Our findings demonstrate consistent germination of Z. marina seeds in the absence of GA3 when exposed to white or red light but not in darkness, confirming the photoblastic nature of Z. marina seeds.
Seeds exposed to white light, encompassing both red and blue wavelengths, exhibited reduced germination compared to those exposed to red light alone. This response aligns with adaptations of Z. marina seeds in intertidal meadows, where seeds are often buried under thin sediment layers yet exposed to filtered daylight, with shifted red-to-far-red ratios (Probert & Brenchley, 1999; Strydom et al., 2017). Such conditions, combined with attenuated light intensity, likely mimic the germination-inhibiting effects of white light observed in our study.
During low tide, seeds are exposed to light enriched with blue and green wavelengths (Morel & Maritorena, 2001), potentially mimicking the germination-inhibiting effects of white light observed in this and other studies (Strydom et al., 2017; Waite et al., 2021). Similar photoinhibition mechanisms have been observed in terrestrial angiosperms, where it functions as an adaptive strategy to delay germination under unfavourable environmental conditions, such as high light intensity combined with water scarcity, which are commonly encountered in desert ecosystems or specific microhabitats in coastal regions (e.g. coastal dunes) (Mérai et al., 2023). A comparable strategy may exist in dynamic marine environments, where light, temperature, salinity, and irradiance fluctuate rapidly.
While our data did not indicate a significant effect of photoperiod under white light, the inhibition observed under a 24 h-photoperiod suggests the involvement of complex regulatory mechanisms that optimise germination timing and embryo development. The retention of key photoreceptor genes, such as CRY1, PHOT1, PHOT2, PHYA, and PHYB, in Z. marina, alongside the evolutionary loss of others like CRY2, CRY5, CRYDASH, and PHYC, highlights specialised adaptation for light perception in marine environments (Mawphlang and Kharshiing, 2017; Nautiyal et al., 2023).
Red light, known to promote germination in many terrestrial and aquatic species (Mathews, 2006; Ziegler et al., 2023), acts through the conversion of the inactive Pr form of phytochrome to the active Pfr state, triggering downstream germination-promoting pathways (Furuya and Schäfer, 1996). Notably, our study observed enhanced germination under red light, contrasting findings from Lidao Bay (China), where red light had no impact on Z. marina germination (Wang et al., 2017). This discrepancy may reflect population-specific adaptations or ecological responses to seasonal turbidity shifts, which increase red wavelengths in water (Strydom et al., 2017, Statton et al., 2017b). The positive effect of red light on germination has been similarly documented in other seagrasses, including Halophila ovalis (Waite et al., 2021), Thalassia hemprichii (Ehrenb.) Aschers. (Soong et al., 2013), and Zostera japonica Asch. & Graebn. (Kaldy et al., 2014), suggesting a shared evolutionary strategy among seagrass species.
The absence of germination in darkness (in the absence of GA3), consistent with patterns observed in many terrestrial plant species (Penfield and King, 2009), suggests a shared light-dependent germination mechanism that may also operate in seagrasses. In terrestrial plants, this dependency is often linked to phytochrome-mediated activation, which prevents premature germination when seeds are buried too deeply in the soil. Although Z. marina germinated to varying degrees under all light treatments, the complete lack of germination in darkness highlights the essential role of light in breaking dormancy and triggering germination. The underlying mechanisms for this light dependency in seagrasses remain an intriguing subject for further research, particularly given their unique aquatic environment and evolutionary context.
4.2 Effects of GA3 treatment on germination
Treating seeds with GA3 is known to initiate a pre-germinative metabolic state that enhances germination and seed vigour in many terrestrial plants of both agricultural (Kaur et al., 2006; Azab, 2018; Budzeń et al., 2018; Lee et al., 2018) and conservation interest (Yang et al., 2005; Barden et al., 2017). Furthermore, GA3 is widely employed in horticulture to stimulate germination and improve, for instance, the size and quality of fruits (Rademacher, 2015). Our findings provide the first insights into the use of GA3 as an exogenous treatment to stimulate germination in Zostera marina, addressing a notable gap in current research.
We observed that seeds exposed to GA3 showed increased germination compared to non-GA3-treated controls, suggesting that GA3 plays a role in overcoming dormancy. These results align with findings in terrestrial plants, where GA3 has been widely employed to stimulate germination (Rademacher, 2015). However, prior studies on other seagrass species have yielded mixed results; for instance, Glasby et al. (2015) reported no effect of GA3 (on concentrations ranging from 0.1 to 20 mg L−1) on germination in Posidonia australis Hook.f., while Loques et al. (1990) found that 1 mg L−1 GA7 did not enhance germination in Zostera noltii Hornem. These inconsistencies may stem from differences in media composition or experimental conditions that could suppress germination responses (Hootsmans et al., 1987). In contrast, our study found that the earliest germination occurred at 10 mg L−1 GA3 under white light, underscoring the critical interplay between GA3 concentration and light conditions in driving germination.
The interaction between light and GA3 appears to be nuanced and complex. Notably, Conacher et al. (1994) reported that 50 mg L−1 GA3 promoted germination in Zostera capricorni Asch. while higher concentrations (500 mg L−1) did not. Similarly, our observation in Z. marina reveals a comparable pattern, particularly under white and red light conditions. The absence of germination at 0 mg L−1 GA3 under complete darkness, contrasted with successful germination at higher GA3 concentrations under identical light conditions, reflects findings in terrestrial plants where exogenous GA₃ treatments mimic red light-induced signalling cascades to promote germination (Toyomasu et al., 1998). In Lactuca sativa L., for instance, germination is regulated by phytochrome, and the application of gibberellin (GA) can bypass the red-light requirement by inducing GA biosynthesis pathways (Toyomasu et al., 1998).
The varied responses to GA3 treatments in Z. marina, Z. capricorni, and Z. noltii suggest potential species-specific mechanisms, likely influenced by differences in the genetic and molecular regulation of germination (Xu et al., 2021; Zhang et al., 2022). Adaptations to the marine environment, including changes in hormone signalling and biosynthesis, have been identified at the genomic level (Chen et al., 2021). For instance, modifications in GA signalling pathways in Zostera muelleri Irmisch ex Asch. (Lee et al., 2016) and the loss of ethylene biosynthesis genes in Z. marina (Golicz et al., 2015) reflect evolutionary changes that could impact germination responses. Nevertheless, the specific roles of individual genes in GA pathways in seagrasses remain largely unexplored (Chen & Qiu, 2022).
While our study underscores the potential of light and GA3 treatments to modulate germination in Z. marina, it also uncovers complexities, such as the non-linear dose response at specific GA3 levels. The inhibitory effects observed at certain concentrations, such as 500 mg L−1, challenge conventional dose–response assumptions and suggest a non-monotonic relationship. Possible explanations include complex hormonal interactions (Vanstraelen and Benková, 2012), physiological thresholds (Bradford & Trewavas, 1994), seed sensitivity variations (Jalal et al., 2021), and potential antagonistic interactions between light and hormone treatments (Seo et al., 2009).
These findings highlight the need for further investigation into the optimal concentrations and conditions for GA3 to support seed germination and the release of dormancy. Future research should incorporate intermediate GA3 concentrations, gene expression analyses, and hormonal profiling to better understand the underlying mechanisms. Such an effort would enhance our understanding of hormonal control in seagrasses and support the development of targeted seed treatment strategies for ecological restoration and conservation initiatives.
4.3 Impact of light and GA3 treatments on early-stage seedling development
Although this study did not evaluate the effects of the light and hormone treatments on Zostera marina seedling development, the potential carryover effects of seed treatments should not be overlooked. In terrestrial plants, GA3 and light treatments are widely employed to promote and support seedling development (Rademacher, 2015; Waqas et al., 2019). For example, GA3 has been shown to enhance primary and secondary growth in adult plants such as sugar beet and korarima (Eyob, 2009; Azab, 2018). A similar approach has been explored in some marine plant species, with GA3 concentrations ranging from 0.01 to 35 mg L−1 promoting leaf and rhizome growth in Cymodocea nodosa (Ucria) Asch (Zarranz et al., 2010). However, no such effects were reported for Posidonia australis (Glasby et al., 2015). In contrast, Zostera noltii seedlings exposed to 1 mg L−1 GA7 exhibited reduced growth (Loques et al., 1990), and in Halophila ovalis, short-wavelength were found to significantly reduce above- and below-ground biomass (Strydom et al., 2017).
Overall, our study found that none of the seed treatments negatively impacted the growth, morphology, or survival of cotyledons and leaves in Z. marina seedlings. Subsequent seedling growth rates were comparable to previously reported values from studies without treatments (Taylor, 1957; van Lent & Verschnure, 1995; Niu et al., 2012). While these results suggest that seed treatments do not adversely affect Z. marina seedling development, it is important to note that our findings may provide only a partial understanding of the broader effects of seed treatments, particularly given that some GA3 concentrations did not yield germinative outcomes.
To further elucidate the impact of seed treatments on seedling development, we recommend that future studies incorporate morphometric analyses, assessments of photosynthetic performance, leaf pigment composition, and the expression of genes encoding key enzymes involved in developmental and metabolic pathways. This comprehensive approach would offer deeper insights into the physiological and developmental effects of seed treatments on Zostera marina.
4.4 Implications for restoration
Seagrass conservation and restoration have been identified as global priorities by the United Nations (Environment, U.N., 2020) and the European Union (Council of the European Union & European Parliament, 2024). In response to the growing impacts of climate change on marine ecosystems, seed-based restoration of Zostera marina habitats is increasingly employed to rehabilitate this widely distributed yet declining seagrass species. Efforts within the seagrass research and restoration community are intensifying to advance scientific methods and logistic standards, aiming to scale up restoration initiatives. Enhancing germination success and maximising the utilisation of seed stocks are critical for bolstering restoration outcomes and strengthening ecosystem resilience (Nordlund et al., 2024).
To ensure the affordability and scalability of restoration projects, it is important to develop cost-effective methods that enhance the efficiency of restoration efforts. Over the past decades, researchers have described and improved seed-based restoration techniques (Unsworth et al., 2019a; Tan et al., 2020; Gräfnings et al., 2023), methods to support germination (Waite et al., 2021; Xu et al., 2021), and seedling establishment (Verduin et al., 2013). However, existing techniques, such as seed scarification and freshwater pulse, have yielded inconsistent and unpredictable results. Addressing current limitations in seed availability by improving germination rates and optimising nursery practices is essential to meeting the increasing demand for large-scale seagrass restoration.
Our study demonstrates that alleviating potential stress-induced dormancy in Z. marina seeds through targeted manipulation, such as treatments with light and phytohormones, offers a promising strategy to enhance germination potential prior to sowing. While our findings provide valuable insights into the potential of light and hormone seed pre-treatments to promote germination and overcome dormancy, further studies are needed to evaluate the consistency and predictability of these effects across a broader range of conditions, including different hormone concentrations. This research provides an important step toward evaluating the feasibility of these techniques for applications in nurseries and restoration projects. Future investigations should focus on determining the optimal concentrations and incubation durations needed to induce early germination processes (pre-germinative stages) without triggering sprouting before sowing. This approach has significant potential to enhance germination rates, reduce germination timelines, and achieve synchronised germination, thereby supporting large-scale restoration initiatives.
Moreover, as this study was conducted in a controlled laboratory setting, we acknowledge potential limitations that may arise from overlooking environmental variables or interactions present under natural conditions. These factors could influence the practical applicability of our findings in real-world scenarios. We, therefore emphasise the importance of translating this laboratory-based knowledge to field settings to gain a more comprehensive understanding of the efficacy of seed pre-treatment methods under natural environmental conditions. Such efforts are crucial for advancing the success of seagrass restoration projects and ensuring the practical utility of these techniques in large-scale applications.
AUTHOR CONTRIBUTIONS
RP planned and designed the research. TD requested the sampling permit from the competent authority. RP and LW performed experiments and analysed the data. RP and TVdS prepared the figures. RP led the writing of the manuscript, with extensive input from TVdS, NK and AV.
ACKNOWLEDGEMENTS
This research was supported by Flanders Innovation & Entrepreneurship (VLAIO) and the Research Foundation Flanders (FWO). The research leading to the results presented in this publication was carried out with infrastructure funded by EMBRC Belgium-FWO international research infrastructure I001621N.
We gratefully thank the Landesamt für Umwelt des Landes Schleswig-Holstein (LfU), and Landesbetrieb für Küstenschutz, Nationalpark und Meeresschutz Schleswig-Holstein, Nationalparkverwaltung, for financing the seagrass monitoring, through which field knowledge was acquired, and for granting the permit to sample seagrass seeds.
CONFLICT OF INTEREST STATEMENT
None.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon request.




