CsLCD and CsDCD are involved in nitric oxide-enhanced salt tolerance in cucumber seedlings
Abstract
Nitric oxide (NO) and hydrogen sulphide (H2S) play important roles in plant growth, development and environmental adaptation. Currently, there is few information on the mechanism by which H2S may be involved in NO-induced salt tolerance. In this study, cucumber was used to investigate the role of NO and H2S and their relationship under salt stress. The results showed that the H2S donor sodium hydrosulfide (NaHS) and the NO donor sodium nitroprusside (SNP) significantly promoted the growth of cucumber seedlings under salt stress. However, hypotaurine (HT, an H2S scavenger) inhibited the positive effects of SNP under salt stress. Meanwhile, SNP treatment significantly increased the content of endogenous H2S, the activity of H2S synthesis-related enzymes LCD, DCD, and CAS, and the expression of H2S synthesis-related genes (CsLCD and CsDCD). In addition, the salt tolerance of cucumber seedlings was largely eliminated after silencing the H2S synthesis-related genes CsLCD/CsDCD, while overexpression of CsLCD/CsDCD significantly enhanced the salt tolerance. It was further found that SNP enhanced the salt tolerance of CsLCD/CsDCD overexpression lines, but it had no positive effect on the tolerance of CsLCD/CsDCD silencing lines. Therefore, NO might enhance salt tolerance in cucumber seedlings by upregulating the expression of H2S synthesis-related gene CsLCD/CsDCD.
1 INTRODUCTION
Salt stress is an important environmental stresses having a significant negative impact on plant growth and development (Zhao et al., 2021). However, plants can respond to salt stress signals through a variety of mechanisms, including regulating ion homeostasis and activating osmotic regulation pathways (Singh et al., 2017). Yu et al. (2021) found that hydrogen-rich water significantly alleviated the inhibition of salt stress on fresh and dry weights, root length, lateral roots and root-to-stem ratio of cucumber seedlings. Salt stress always induces the overproduction and accumulation of reactive oxygen species (ROS), causing oxidative stress. Plants have an active oxygen scavenging system, including various antioxidant enzymes, including catalase (CAT), superoxide dismutase (SOD), peroxidase (POD), and non-enzyme antioxidants, including glutathione (GSH), ascorbic acid (ASA), α-tocopherol (vitamin E), polyphenols, flavonoids, and carotenoids (Dvorák et al., 2021; Li et al., 2022a). Recently, several gasotransmitters have been identified to help mitigate salt stress in plants, such as nitric oxide (NO; Wang et al., 2022), hydrogen sulfide (H2S; Li et al., 2022b), carbon monoxide (CO; Feng et al., 2023), and hydrogen gas (H2; Yan et al., 2022).
H2S, a gas-signaling molecule, has been found to not only regulate plant growth, development and physiological metabolism but also enhance plant resistance to stresses such as salt stress, drought stress, and high osmotic pressure (Feng et al., 2023). Jiang et al. (2019) found that H2S could protect cucumber seedlings from salt stress by stabilizing Na+/K+ balance, increasing endogenous H2S and enhancing the antioxidant system. In wheat seedlings, H2S enhanced the antioxidant defense system and influenced transcription levels and stress-related signaling pathways to alleviate salt stress (Ding et al., 2019). H2S reinforced salt tolerance in eggplant seedlings through the modulation of enzyme activity and mineral absorption, reduction of ABA levels and mitigation of ROS damage (Ekinci et al., 2021). A study also showed that appropriate levels of H2S could stimulate seed germination (Fang et al., 2022). Yang et al. (2023) found that H2S mitigated salt stress in Arabidopsis thaliana through regulating auxin signalling. H2S also enhanced salt tolerance in Arabidopsis thaliana via persulfidation (Ma et al., 2023). The combination of NO and H2S enhanced salt tolerance in wheat by enhancing the ascorbate-glutathione (AsA-GSH) cycle (Kaya et al., 2023).
NO is another important gas-molecule playing a central role in plant growth, development and adaptive responses to environmental stresses (Simontacchi et al., 2013; Kaya et al., 2024). In lettuce plants, exogenous NO improved the energy use efficiency of the photosystem and increased proline (Pro) content to stimulate plant defence, thereby enhancing salt tolerance (Marques et al., 2024). NO also positively regulated polyamine (PA) homeostasis by increasing the accumulation of PA biosynthetic enzymes and decreasing the PA catabolic rate, thus helping Helianthus annuus L. seedlings to adapt to salt stress (Tailor et al., 2019). In Cicer arietinum L., NO could mitigate the adverse effects of high salinity by improving leaf relative water content, photosynthetic pigment biosynthesis, osmolyte accumulation and antioxidant defence system (Ahmad et al., 2016). Husain et al. (2019) found that NO could alleviate the membrane lipid peroxidation damage caused by salt stress in wheat and maize leaves, while increasing the activity of SOD and CAT, and reducing leaf membrane relative permeability, malondialdehyde (MDA) content, and hydrogen peroxide (H2O2) accumulation rate.
Cucumber (Cucumis sativus L.), belonging to the Cucurbitaceae family and the Cucumis genus, is an annual herbaceous crop (Sarwar et al., 2021) and a globally cultivated vegetable crop. The growth and development of this vegetable crop are susceptible to adverse environmental conditions (Kaur and Sharma, 2022). As mentioned above, H2S and NO played important roles in promoting plant growth and alleviating stress. Under heat stress, NO and H2S synergistically enhanced photosynthesis and reduced H2O2-induced oxidative stress and excess glucose (Glc)-mediated inhibition of photosynthesis in Triticum aestivum L. (Iqbal et al., 2021). Rather et al. (2020) demonstrated that NO and H2S could mutually cooperate to mitigate phytotoxicity under metal stress. Under arsenate (As) stress, the interaction of NO and H2S played an important role in alleviating the toxicity of Oryza sativa L. (rice) seedlings (Rather et al., 2020). However, the crosstalk between H2S and NO under salt stress remained unclear. Therefore, we investigated the roles of H2S and NO and their relationship in cucumber under salt stress.
2 MATERIALS AND METHODS
2.1 Plant materials
Cucumber (Cucumis sativus L.cv. Xinchun No.4) was used as the plant material for this study.
Cucumber seeds were supplied by Korun Seeds Ltd. (Shandong, China). Cucumber seeds were disinfected in a 0.03% potassium permanganate solution for 5 min, and then the sterile seeds were washed multiple times with distilled water and soaked for 5 h. They were sown on germination trays lined with two layers of moist filter paper covered with plastic wrap under dark conditions at 25°C to maintain moisture. Once the seeds germinated, the cultivation temperature was adjusted to 28°C/18°C with a 12 h light cycle and an irradiance of 250 μmol·m−2 s−1 photons. On the 7th day after germination, the cucumber seedlings were transplanted into 250 mL black hydroponic boxes. The nutrient solution was 1/3 modified Hoagland solution, starting with a 1/8 concentration of Hoagland solution, changing the nutrient solution every 2 days, and gradually increasing the concentration to 1/3. The seedlings were cultivated in a growth chamber with a temperature of 28°C/18°C, an irradiance of 375 μmol·m−2 s−1 photons and a 12 h light cycle.
2.2 Treatments
Cucumber at 20-day-old seedlings of uniform growth were selected for the experiment and were transferred to a 250 mL black hydroponic tank for cultivation. 50 mM NaCl (Luo et al., 2023) was used as salt stress, 200 μM NaHS (Luo et al., 2023) was used as an H2S donor, 100 μM HT (Qi et al., 2019) was used as a H2S scavenger and 100 μM SNP (Arasimowicz-Jelonek et al., 2009) was used as a NO donor. There were 5 treatments: (1) Hoagland nutrient solution (Hoagland and Arnon, 1950) as control; (2) 50 mM NaCl; (3) 50 mM NaCl +200 μM NaHS; (4) 50 mM NaCl +100 μM SNP; (5) 50 mM NaCl +100 μM SNP +100 μM HT. Each treatment was done with 6 replicates, with 15 seedlings per replicate. After 7 d of treatments, the plant height, stem diameter, fresh weight, dry weight and leaf area of cucumber seedlings were measured, and the plant phenotype was photographed. The nutrient solution was changed every 48 h during the treatment period. After 7 d of treatment, the second true leaf was taken, cut into pieces, mixed well, and stored at −80°C for subsequent experimental analysis.
2.3 Construction of CsLCD/CsDCD silencing and overexpression vector
The total RNA in cucumber leaves was extracted by Trizol method (Huang et al., 2021) and then the quality of RNA extraction was detected by agarose gel electrophoresis (Figure S1). High-quality RNA was reverse-transcribed into cDNA. cDNA was used as a PCR amplification template for the amplification of the target fragment (TRV-CsLCD-F: gtgagtaaggttaccgaattcATGGCTTCTCACTGTGAAAAAAACC, TRV-CsLCD-R: cgtgagctcggtaccggatccGGTTCTCGATTCAAGAATCCCC; TRV-CsDCD-F: gtgagtaaggttaccgaattcAAAATAAGCAAAGTTATGTTCTCAGCTA, TRV-CsDCD-R: cgtgagctcggtaccggatccTGCACATAAAACAGCAGTAGCAGC; pSUPER1300-CsLCD-F: ttacaattaccatggggcgcgccATGGCTTCTCACTGTGAAAAAAACC, pSUPER1300-CsLCD-R: aacatcgtatgggtaggtaccTTTGGAGAGTTGAGTGCAGGTG; pSUPER1300-CsDCD-F: ttacaattaccatggggcgcgccATGGGTGTCCGATTCGGTG, pSUPER1300-CsDCD-R: aacatcgtatgggtaggtaccGGGAACAAAATTTTTCAGATCATTAA). The successfully amplified products were recovered by using the TIANGEN universal purification and recovery kit for PCR product recovery.
The TRV vector (used for silencing) and the pSUPER1300 vector (used for overexpression; 35S promoter, GFP fusion in C terminal) were digested with EcoRI/BamHI and AscI/KpnI, respectively, and the target genes CsLCD and CsDCD fragments were inserted, and then the ligated plasmids were transformed into Escherichia coli. The extracted plasmid of a positive clone colony was sequenced to check for sequence correctness.
2.4 Infection methods: transient expression
Agrobacterium tumefaciens were supplied by Qingke Biotechnology Co., Ltd.. Single colonies of A. tumefaciens were selected and cultured in Luria-Bertani (LB) medium (with corresponding antibiotics added) on a shaker at 28°C and 220 rpm min− 1 for 12–16 h. 500 μL bacterial solution was then added to a new 5 mL LB liquid culture medium [with the addition of kanamycin, 100 mM MES, and 20 μL acetosyringone (AS)], and cultured at 28°C for 10–12 h. The bacterial solution was centrifuged at 5000 g for 5 min, and the supernatant was discarded and replaced with the infection solution [98 mL of sterilized water with 1 mL MgC12 (1 M); 1 mL MES (10 mM); 400 μ L AS (50 mM), pH 5.6]; the OD600 was adjusted to 1.2–2.0. The TRV1 suspension solution was mixed with TRV2, TRV2-CsLCD and TRV2-CsDCD suspension solution by equal volume. The pSUPER1300 plasmids expressing 35S::GFP (empty vector), 35S::CsLCD-GFP or -35S::CsDCD-GFP were transfected into Agrobacteriu tumefaciens GV3101 and separately incubated at 28°C for 3 h and then injected into the cotyledons of cucumber seedlings using a headless sterile syringe. The injected seedlings were cultivated for 7 d and then were transferred to a conical flask for cultivation using Hoagland nutrient solution. Six groups of wild-type (WT), TRV, and TRV:CsLCD and TRV:CsDCD and 35S::CsLCD-GFP and 35S::CsDCD-GFP were assigned. The silencing/overexpression efficiency of CsLCD and CsDCD in cucumber was detected using qRT-PCR fluorescence quantification (see Table S1 for primer sequences; Actin DQ115883 was used as internal reference gene; see 2.8 for more infrórmation about qRT-PCR). CsLCD and CsDCD expression was 28% and 27% of the non-transformed plants in the silenced plants, respectively, and 20 times more expressed in the transiently overexpressed plants (Figure S2).
2.5 Measurement of morphological parameters
The plant height and stem diameter were measured using a vernier caliper. Six cucumber seedlings were randomly selected for each treatment to weigh their fresh weight. Cucumber seedlings that had been measured for fresh weight were placed in an 80°C oven for 48 h before weighing their dry weight. The leaf area of cucumber seedlings was measured using a root scanner (STD4800, Regent Instrument Inc.) and leaf analysis software Win RHIZO 5.0 (Regent Instrument, Inc.). Each treatment was set to 6 replicates.
2.6 Measurement of relative electrical conductivity, and malondialdehyde and proline content
Malondialdehyde (MDA) content: 1 g cucumber leaves was cut into pieces, and ground with 2 mL of 100 g·L−1 trichloroacetic acid (TCA) and a small amount of quartz sand. An additional 8 mL of TCA was added and the mixture was ground thoroughly. The homogenate was then centrifuged at 1800 x g for 10 min. The supernatant collected served as the extract for testing. Two milliliters of this extract were combined with 2 mL of 6 g·L−1 TBA solution, mixed well, and heated in a boiling water bath for 15 min. After cooling, the mixture was centrifuged. The absorbance values were measured at wavelengths of 450 nm, 532 nm and 600 nm. Distilled water was substituted for the extract as a control (Heath and Packer, 2022).
Proline (Pro) content: cucumber leaves (0.5 g) were ground in a mortar containing 5 mL of 3% sulfosalicylic acid solution, and the homogenate was transferred to a tube. The centrifuge tubes were then placed in a boiling water bath for 10 min, followed by cooling and centrifugation at 1000 x g for 10 min. Then, the supernatant was collected and 2 mL were pipetted into a test tube with stopper; then, 2 mL of ice acetic acid, 4 mL of acid ninhydrin reagent, and 2 mL of 3% sulfosalicylic acid solution were added. The mixture was shaken well, heated in a boiling water bath for 1 h, and cooled to room temperature, and then 4 mL of toluene was added. After vigorous shaking, the red product was extracted and allowed to settle into layers. The red toluene solution was placed in a colorimetric dish and measured the absorbance at 520 nm (Abdelhameed et al., 2021).
Relative electrical conductivity (REC): the leaves of the treated group were rinsed with deionized water and the residual surface moisture was dried using filter paper. The cucumber leaves were chopped by a No. 4 punch to take samples. Ten pieces were placed in each test tube, and the process was repeated three times. Next, 15 mL of deionized water was added to each test tube. The test tubes were placed on a shaker for agitation. The conductivity (S1) and the blank conductivity (S0) were measured using a conductivity meter. The samples were then heated in a boiling water bath for 30 min, and the conductivity (S2) was measured after cooling to room temperature. The REC was calculated using the formula ((S1-S0) / (S2-S1)) × 100% (Yu et al., 2006).
2.7 Assay of H2S content
The quantification of H2S was performed using the methodology described by Muñoz-Vargas et al. (2018). The procedure entails the synthesis of methylene blue through the reaction of dimethyl-phenylenediamine in an HCl solution, which was then used to quantify H2S concentration.
2.8 Determination of H2S biosynthesis-related enzyme activity and related gene expression
Endogenous H2S biosynthesis-related enzyme activity was assayed according to Riemenschneider et al. (2005).
Enzyme solution extraction: the sample (0.5 g) was ground in a mortar with liquid nitrogen using a pestle. Soluble proteins were extracted using 1.5 mL of pre-chilled extraction buffer, which contains 20 mmol·L−1 Tris–HCl (pH 8.0), 0.1% (w/v) DTT, and 0.2% (w/v) sodium ascorbate. The mixture was homogenized and then centrifuged at 13,000 x g for 15 min at temperatures −4°C. The supernatant was collected for enzyme activity measurement.
LCD and DCD enzyme activity measurement: the 100 μL supernatant was added to 1 mL of reaction solution [LCD: 0.8 mL 100 mM Tris–HCl (pH 9.0, containing 2.5 mM DTT), 0.1 mL 0.8 mM L-cysteine; DCD: 0.8 mL 100 mM Tris–HCl (pH 8.0, containing 2.5 mM DTT), 0.1 mL 0.8 mM L-cysteine]. The mixture was kept at 37°C for 30 min, and then 100 μL FeCl3 solution (30 mM) and 100 μL N, N-dimethyl-p-phenylenediamine dihydrochloride (20 mM) were added to terminate the reaction. The absorbance of the solution was measured at 670 nm (Sun et al., 2021).
Cysteine synthase (CAS) activity measurement: the 100 μL supernatant was added to 1 mL of reaction solution, which contained 0.1 mL L-cysteine (10 mmol·L−1), 0.7 mL Tris–HCl (100 mmol·L−1, pH 9.0, containing 2.5 mM DTT), 0.1 mL KCN (7.5 mmol·L−1). The mixture was kept at 37°C for 30 min, and then 100 μL FeCl3 (30 mM) and 100 μL N, N-dimethyl-p-phenylenediamine dihydrochloride (20 mM) were added to terminate the reaction. The absorbance of the solution was measured at 560 nm (Riemenschneider et al., 2005).
Total RNA was extracted from 0.5 g cucumber seedling leaves using the Trizol method. RNA (500 ng) was synthesized using the Evo M-MLV Reverse Transcription Kit (Accurate) to synthesize cDNA (total reactant 10 μL, including 0.4 μL upper and lower primers, 7.2 μL sterilized water, and 2 μL RNA). cDNA (250 ng) was subjected to real-time quantitative RT-PCR (qRT-PCR) analysis using the SYBR Green pro Taq HS premixed qPCR kit (Accurate Biology) and Roche Light Cycle fluorescence quantitative analyzer. Total reactants (20 μL) containing 10 μL enzyme, 2 μL cDNA, 0.4 μL upper and lower primers, 7.2 μL sterilized water. The qRT-PCR reaction conditions are 95°C, 30 s, 1 cycle; 95°C for 5 s; 60°C, 30 s, 40 cycles; 4°C, ∞. The primers used for qRT-PCR analysis are shown in Table S1.
2.9 Determination of antioxidant enzyme activity and related gene expression
The samples (0.5 g) were ground to a powder in a mortar and then mixed with 5 mL of pH 7.8 PBS buffer to achieve a homogeneous extraction. The mixture was transferred to a centrifuge tube and made up to 5 mL. The homogenate was centrifuged at 4°C, 16000 x g for 15 min. The extracted supernatant was then used for subsequent enzyme activity assays.
SOD activity (Mittova et al., 2002): 500 μL supernatant was added to the reaction solution, which contained the PBS (pH 7.8) buffer, methionine (Met), nitroblue tetrazolium (NBT), ethylenediaminetetraacetic acid (EDTA), and riboflavin solution. After thorough mixing, the solution was irradiated under 500 μmol·m−2 s−1 photons light for 30 min. Subsequently, two control tubes were placed in the dark: one was shielded with foil, while the other remained exposed to 500 μmol·m−2 s−1 photons light for an additional 30 min. After post-reaction, the absorbance of the tube covered with foil was zeroed. The absorbance was measured at 560 nm.
CAT activity (Mittova et al., 2002): CAT was determined using the UV absorption method. The 100 μL supernatant was added to a reaction mixture containing 2.9 mL of 50 mM potassium phosphate buffer (pH 7.0) and 20 mM H2O2. A colorimetric measurement was performed at 240 nm and the absorbance change was recorded within 1 min.
POD activity (Metwally and Soliman, 2023): POD was determined using the guaiacol method. Guaiacol was used as a substrate. The 40 μL of supernatant was added to a substrate mixture containing 3 mL of 0.05 M potassium phosphate buffer, 50 μL of 0.02 M guaiacol and 19 μL of 30% H2O2. The absorbance was zeroed with PBS (pH 6.5) buffer and measured at 470 nm. The absorbance change was recorded.
Ascorbate peroxidase (APX) activity (Metwally and Soliman, 2023): APX was determined using the UV absorption method. 100 μL of supernatant was added to the mixed solution containing 1 mL of PBS (pH 7.0) buffer, 1 mL of 5 mM ascorbic acid and 1 mL of 1 mM hydrogen peroxide (H2O2). The absorbance was zeroed with PBS and the colourimetric measurement was carried out at 290 nm. The absorbance change was recorded within 1 min.
The expression of stress-related genes was determined as described in 2.8. The primers used for qRT-PCR analysis are shown in Table S1.
2.10 Statistical analysis
Each experiment had 6 replicates, and all data were represented as mean ± standard deviation (SD). All statistical analyzes were performed using SPSS 22.0 (SPPS Inc.). Duncan's multiple range test (p < 0.05) was chosen for statistical analyze. GraphPad Software 9.0.0 (GraphPad Software) was used to build graphics.
3 RESULTS
3.1 H2S was involved in NO-alleviated salt stress
Compared to the control, salt stress significantly slowed down cucumber seedling growth (Figure 1a). NaHS (an H2S donor) and SNP (a NO donor) treatments remarkably restored the phenotype of seedlings under salt stress. Additionally, NaCl + SNP + HT treatment inhibited seedling growth compared with NaCl + SNP treatment (Figure 1a).
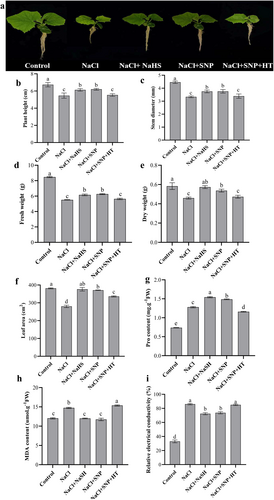
Moreover, compared with the control, NaCl treatment significantly decreased plant height, stem diameter, dry weight, fresh weight and leaf area (Figure 1b-f). However, a noticeable increase in these parameters was observed in NaCl + NaHS and NaCl + SNP treatments compared with NaCl treatment. In contrast, in comparison with NaCl + SNP treatment, NaCl+SNP + HT treatment reduced these parameters (Figure 1b-f).
Concerning Pro and MDA content and REC, they increased by 72.97%, 22.44% and 160.61%, respectively, upon NaCl treatment compared to control (Figure 1g-i). However, compared with NaCl treatment, NaCl+NaHS and NaCl+SNP treatments increased Pro content, while they decreased MDA content and REC. Compared with NaCl+SNP treatment, NaCl+SNP + HT treatment increased MDA content and REC, but decreased Pro content (Figure 1g-i). The results suggest that H2S and NO could alleviate salt stress in cucumber seedlings and H2S might play an important role in NO-alleviated salt stress.
3.2 NO increased H2S content, L-cysteine (LCD), D-cysteine (DCD) and CAS activity, and CsLCD and CsDCD relative expression
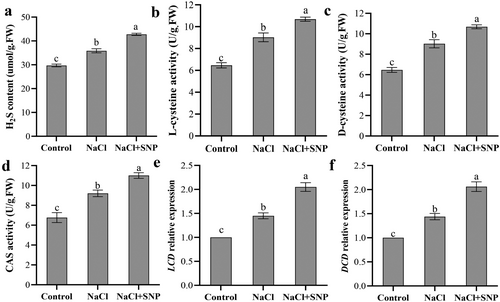
Compared with the control, salt stress significantly increased endogenous H2S content (Figure 2a). Additionally, the endogenous H2S content in NaCl+SNP treatment was significantly higher than that in NaCl treatment. Compared to the control, NaCl treatment increased the activity of LCD, DCD and CAS by 39.41%, 39.47% and 36.08%, respectively. Compared with salt stress, NaCl+SNP treatment resulted in higher activity of LCD, DCD and CAS (Figure 2b-d). As shown in Figure 2e-f, compared with the control, NaCl treatment upregulated the expression levels of CsLCD and CsDCD by 44.98% and 43.93%, respectively. The expression levels of CsLCD and CsDCD were significantly higher in NaCl+SNP treatment than in NaCl treatment. These suggest that exogenous NO may increase endogenous H2S content by enhancing its syntheses enzyme (LCD, DCD and CAS) activity as well as the related gene (LCD and DCD) expression.
3.3 Silencing/overexpression of CsLCD and CsDCD decreased/increased salt stress tolerance
Under control conditions, there were no significant differences in the phenotype among WT, TRV:00, TRV:CsLCD, TRV:CsDCD, 35S::CsLCD-GFP and 35S::CsDCD-GFP plants. Under salt stress, although all plants developed a dwarf phenotype, this was more pronounced in TRV:CsLCD and TRV:CsDCD plants (Figure 3a).

In contrary, the plant height, stem diameter, dry weight, fresh weight and leaf area were significantly higher in salt-stressed 35S::CsLCD-GFP and 35S::CsDCD-GFP plants than in salt-stressed WT and TRV:00 plants (Figure 3b-f). Thus, the overexpression of CsLCD and CsDCD genes might enhance cucumber seedling growth under salt stress, while silencing of CsLCD and CsDCD genes might decrease salt tolerance.
Under control conditions, there was no significant difference in MDA content and REC among all lines. Under salt stress, compared to that in WT plants, the Pro content significantly increased in 35S::CsLCD (+9.13%) and 35S::CsDCD (+9.85%) plants, while it significantly decreased in TRV:CsLCD (−35.75%) and TRV:CsDCD (−37.41%) plants. Moreover, compared with those in WT plants, MDA content and REC were significantly decreased in 35S::CsLCD (−38.25% and − 40.76%) and 35S::CsDCD (−37.38% and − 41.13%) plants under salt stress, while those significantly increased in TRV:CsLCD (+27.07% and + 23.02%) and TRV:CsDCD (+26.85% and + 22.27%) plants (Figure 3g-i). The results indicate that CsLCD and CsDCD could improve cell membrane stability and osmoregulation ability under salt stress.
To further confirm the role of CsLCD and CsDCD in the antioxidant system, the antioxidant enzyme activity was measured. Under control conditions, there were no significant differences in POD, SOD, CAT, and APX activity among all lines (Figure S3), while their activities increased remarkably under salt stress. Notably, 35S::CsLCD-GFP and 35S::CsDCD-GFP plants exhibited a more significant increase in POD, SOD, CAT, and APX activity compared to WT and TRV:00 plants and vice versa for the TRV:CsLCD and TRV:CsDCD plants (Figure S3). For example, under NaCl treatment, compared with that in WT plants, POD activity was decreased in TRV:CsLCD and TRV:CsDCD plants by 29.26% and 29.58%, respectively; SOD activity was decreased by 18.34% and 18.48%, respectively; APX activity was decreased by 28.07% and 28.72%, respectively; CAT activity was decreased by 23.38% and 25.48%, respectively. Thus, CsLCD and CsDCD might enhance antioxidant ability under salt stress.
3.4 Silencing/overexpression of CsLCD and CsDCD decreased/increased the relative expression of stress-related genes
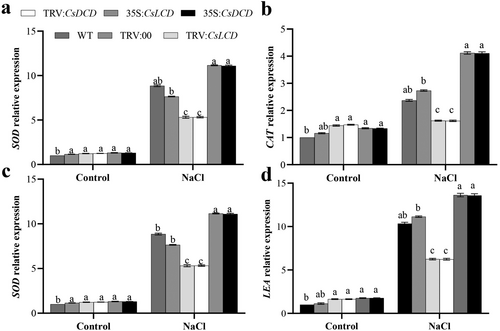
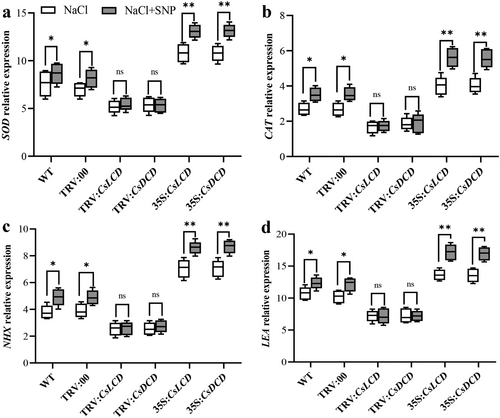
Under control conditions, plants transiently expressing TRV:CsLCD, TRV:CsDCD, 35S::CsLCD-GFP and 35S::CsDCD-GFP showed no significant differences in the expression of the stress-related genes SOD, CAT, NHX and LEA (Figure 4a-d). However, under NaCl stress, their expression was significantly upregulated by 25.98%, 73.84%, 127.74% and 31.69%, respectively, in 35S::CsLCD-GFP plants compared with that in WT plants, and by 25.42%, 73.24%, 126.19% and 31.21 in 35S::CsDCD-GFP plants In contrast, the expression of SOD, CAT, NHX and LEA genes in TRV:CsLCD and TRV:CsDCD plants was significantly lower than that in WT plants under NaCl stress. Thus, CsLCD and CsDCD might upregulate the expression of stress-related genes under salt stress.
3.5 CsLCD and CsDCD participate in NO-enhanced salt tolerance
When SNP was added with NaCL, WT, TRV:00, 35S::CsLCD-GFP and 35S::CsDCD-GFP plants showed a more robust phenotype than TRV:CsLCD and TRV:CsDCD plants.
Under NaCl+SNP treatment, the plant height, stem diameter, dry weight, fresh weight, and leaf area were significantly increased in WT (+7.94%, +15.07%, +12.09%, +15.50% and + 10.74%), TRV:00 (+10.77%, +11.19%, +11.50%, +14.96% and + 10.79%), 35S::CsLCD (+10.01%, +17.89%, +23.61%, +15.16% and + 24.30%) and 35S::CsDCD (+8.72%, +23.04%, +17.19%, +20.96% and + 26.17%) plants compared to those under salt stress. However, those parameters in TRV:CsLCD and TRV:CsDCD plants showed no differences bewteen NaCl and NaCl+SNP treatments (Figure 5b-f). This indicates that CsLCD and CsDCD might be involved in NO-alleviated salt stress.
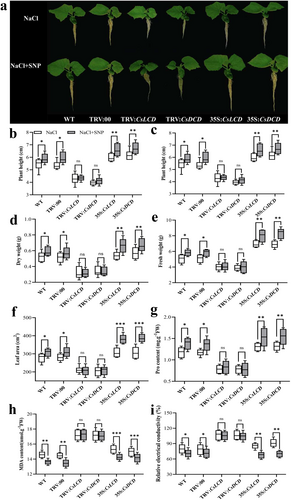
Moreover, the Pro content was significantly higher in NaCl+SNP-treated WT (+17.96%), TRV:00 (+15.81%), 35S::CsLCD (+16.92%) and 35S::CsDCD (+16.87%) plants than in NaCl-treated ones, while the Pro content in TRV:CsLCD and TRV:CsDCD plants had no significant difference between the two treatments.
Concerning the MDA content and REC, they significantly decreased in NaCl+SNP-treated WT, TRV:00, 35S::CsLCD-GFP and 35S::CsDCD-GFP plants compared to salt-stressed ones, while there were no significant differences in TRV:CsLCD and TRV:CsDCD plants between NaCl and NaCl+SNP treatments (Figure 5g-i). Therefore, NO significantly promoted the stability of the cell membrane and osmotic ability by upregulating CsLCD and CsDCD.
When looking at the activity of POD, SOD, CAT, and APX, adding SNP (NaCl + SNP) significantly increased their activity, compared to only salt treatment, in WT, TRV:00, 35S::CsLCD-GFP and 35S::CsDCD-GFP plants, but there was no significant difference in TRV:CsLCD and TRV:CsDCD plants between the two treatments (Figure S4). Importantly, upon NaCl+SNP treatment, the activity of POD, SOD, CAT, and APX in 35S::CsLCD-GFP and 35S::CsDCD-GFP plants was significantly higher than that in WT plants.
Last, we checked the expression of stress-related genes SOD, CAT, NHX and LEA in WT, silenced and overexpressed plants subjected to either NaCl treatment or NaCl+SNP treatment. All those genes were upregulated by the NaCl+SNP treatment in WT, TRV:00, 35S::CsLCD-GFP and 35S::CsDCD-GFP but had no significant difference the expression in TRV:CsLCD and TRV:CsDCD plants between the two treatments (Figure 6a-d). Importantly, SOD, CAT, NHX and LEA expression was higher in 35S::CsLCD-GFP and 35S::CsDCD-GFP plants than in WT plants upon NaCl + SNP treatment, while it was lower in TRV:CsLCD plants than in WT plants (Figure 6a-d). Therefore, NO could upregulate CsLCD and CsDCD genes to enhance the expression of stress-related genes, thereby enhancing salt tolerance in cucumber seedlings.
4 DISCUSSION
Currently, the phenomenon of soil salinization is gradually intensifying and salt damage is becoming a key restricting factor for plant growth (Hannachi and Van Labeke, 2018). NO and H2S serve as gaseous signalling molecules that regulate growth and development and respond to abiotic stresses (Hasanuzzaman et al., 2018; Zhang et al., 2021). For example, NO and H2S could enhance the antioxidant system of cucumber seedlings and reduce the accumulation of ROS, thereby regulating disease resistance in cucumber seedlings (Mishra et al., 2021). Luo et al. (2023) also demonstrated that H2S facilitated the growth and photosynthetic efficiency of cucumber seedlings under salt stress, augmented the ascorbate-glutathione cycle, and enhanced the uptake of mineral ions. These beneficial outcomes underscore the pivotal role of H2S in mitigating salt-induced stress. In the present study, exogenous H2S and NO could alleviate the negative impacts of salt stress by promoting cell membrane stability and osmoregulation ability in cucumber seedlings (Figure 1). Another study reported NO also significantly enhanced resistance to salt stress in wheat seedlings by increasing photosynthetic efficiency, stomatal conductance, chlorophyll content, and antioxidant enzyme activity (Zheng et al., 2009). Wang et al. (2021) also demonstrated that NaHS alleviated the inhibitory effect of salt stress on rice. A previous study showed that H2S, in synergy with NO, could enhance nutrient and water absorption in cucumber seedlings under salt stress by promoting root tip growth and lateral root formation (Sun et al., 2021). In this study, based on the fact that hypotaurine (HT, a H2S scavenger) reduced the alleviating effect of NO on salt stress, we suggest that H2S might be involved in NO-alleviated salt stress in cucumber seedling growth (Figure 1). Kapoor et al. (2023) demonstrated that the application of NO and H2S alone or in combination increased salt tolerance and inhibited NaCl-induced biochemical alterations and oxidative damage in Coriandrum sativum L. However, the relationship between NO and H2S in salt stress regulation has not been elucidated. Thus, our study provides new evidence of the involvement of H2S in NO-mitigated salt stress.
Under salt stress, NaSH treatment could increase endogenous H2S content in Malus hupehensis seedlings, enhance antioxidant enzyme activity, and maintain root vitality and structure (Li et al., 2020a). Li et al. (2020b) demonstrated that salinity triggered LCD activity, which in turn induced H2S production in Spartina alterniflora. During the process, H2S upregulated the activity of antioxidant enzymes such as ascorbate peroxidase, superoxide dismutase and S-nitrosoglutathione reductase, thus mitigating the damage caused by reactive nitrogen species. In the present study, salt stress significantly enhanced LCD/DCD and the production of H2S (Figure 2). Siddiqui et al. (2021) also found that salt stress induced H2S generation in tomatoes through the activation of the LCD/DCD pathway. In addition, H2S and NO have a close correlation in enhancing stress tolerance in plants. NO and H2S alleviated chromium stress in Zea mays L. by synergistically affecting the metabolic interactions (Kharbech et al., 2022). Khan et al. (2020) reported that exogenous calcium alleviated cadmium stress in Vigna radiata seedlings by enhancing the activity of nitrate reductase and LCD/DCD, which increased the synthesis of NO and H2S. In our study, the addition of NO further increased the synthesis enzyme activity of H2S and related gene expression and H2S level in cucumber seedlings under salt stress (Figure 2). Thus, our results provide new evidence that NO may induce endogenous H2S under stress conditions. However, in barley seedling roots, H2S could induce NO production to reinforce salt tolerance (Chen et al., 2015). H2S also could protect common bean plants from salt stress by enhancing the levels of endogenous H2S and NO (Dawood et al., 2022). Chen et al. (2021) also found that H2S increased the levels of endogenous NO and the activity of nitrate reductase, thereby effectively mitigating Cyclocarya paliurus salt stress. By combining these results and our results, we could conclude that H2S and NO may mutually regulate their endogenous levels under stress, suggesting an extremely complex relationship between NO and H2S.
CsLCD and CsDCD are key genes involved in the biosynthesis pathway of H2S, which has been proven to play a significant role in enhancing plant stress tolerance (Arif et al., 2021). In Arabidopsis, overexpression of AtDCDes could make transgenic plants (the H2S synthetase mutant Atd-cdes) more sensitive to ethylene and drought (Hou et al., 2016). Du et al. (2019) found that MAPK gene could reinforce the drought tolerance in lcd/des1 (a double mutant of H2S synthesis). Under manganese stress, AtLCD gene overexpression lines (OE5 and OE32) showed significant resistance, but the deletion mutant (lcd) was more sensitive (Hou et al., 2022). In our study, 35S::CsLCD-GFP and 35S::CsDCD-GFP plants exhibited stronger growth capability and cell membrane stability and osmotic regulation capacity under salt stress, while TRV:CsLCD and TRV:CsDCD plants were less resistant to salt stress (Figure 3). It was evident that LCD and DCD genes might play an important role in plant stress tolerance. Shen et al. (2012) found that AtLCD and AtDCD showed significant resistance to cadmium (Cd)-induced toxicity and ameliorated Cd-induced oxidative stress in Escherichia coli. Meanwhile, CYSTEINE SYNTHETASE 1 (OsCS1), with high homology to Arabidopsis LCD,has been cloned in rice and OsCS1 could enhance tolerance to Cd stress in Arabidopsis (Shen et al., 2019). Jin et al. (2011) demonstrated that AtLCD and AtDCD improved drought tolerance in Arabidopsis. In the current study, overexpression of CsLCD and CsDCD increased antioxidant enzyme activity and the expression of stress-related genes in cucumber seedlings under salt stress (Figures S3 and 4). The results of the present study provide new evidence that CsLCD and CsDCD may be involved in enhancing salt tolerance.
Previous research has shown that LCD and DCD, as important genes in the H2S synthesis pathway, enhanced plant resistance to stress (Liu et al., 2015; Muñoz-Vargas et al., 2023). In Brassica rapa, eugenol increased the resistance of seedlings under Cd stress by upregulating the expression of BrLCD and BrDCD (Hu et al., 2018). Exogenous phytosphingosine α (PSKα) could upregulate the expression of LCD and DCD genes to delay broccoli yellowing under cold conditions (Aghdam et al., 2021). Khan et al. (2021) found that NO may directly act on LCD and DCD in wheat seedlings, regulating their activity to affect the biosynthesis of H2S. In our study, NO could enhance the growth capability of 35S::CsLCD-GFP and 35S::CsDCD-GFP plants under salt stress, but didn't work on TRV:CsLCD and TRV:CsDCD plants (Figure 5). This suggested that NO could modulate CsLCD and CsDCD to affect H2S synthesis, thereby increasing plant salinity tolerance. Sun et al. (2022) found that NO activated the expression of H2S synthesis genes (LCD1 and OAS-TL) to enhance thermotolerance in maize seedlings. Interestingly, exogenous EBL also promoted LCD and DCD production to enhance tolerance to boron (B) toxicity in Capsicum annuum L., and this process was accompanied by increasing the content of MDA, H2O2 and free Pro, and electrolyte leakage (Kaya, 2020). In the current study, we found that NO could promote antioxidant enzyme activity by regulating CsLCD and CsDCD (Figure S4). Additionally, NO could increase the expression levels of resistance-related genes in 35S::CsLCD-GFP and 35S::CsDCD-GFP plants, while NO didn't increase the expression levels of resistance-related genes in TRV:CsLCD and TRV:CsDCD plants (Figure 6). Xiang et al. (2023) also found that mannitol-induced DCD and H2S in Arabidopsis root system promoted auxin homeostasis and alleviated the inhibition of root growth under osmotic stress. Therefore, combining the results of previous research and the current study, we conclude that CsLCD and CsDCD participate in NO-enhanced salt tolerance. NO also increases antioxidant defense mechanisms and osmotic balance under salt stress by regulating CsLCD and CsDCD.
5 CONCLUSION
In summary, both H2S and NO could effectively alleviate salt stress in cucumber seedlings. H2S might participate in NO-alleviated salt stress by increasing Pro content and reducing REC and MDA content. Furthermore, NO increased endogenous H2S content by enhancing H2S synthesis-related enzyme activity and upregulating LCD and DCD expression under salt stress. In addition, overexpression of CsLCD and CsDCD enhanced salt tolerance, whereas silencing CsLCD and CsDCD largely weakened the alleviation of salt stress. Therefore, NO may upregulate the expression of H2S synthesis genes CsLCD and CsDCD, and further increase the activity of H2S synthesis-related enzymes, resulting in enhanced salt tolerance in cucumber seedlings.
AUTHOR CONTRIBUTIONS
Weibiao Liao: Conceptualization, writing, review and editing. Ailing Li: Writing original draft, Writing - Review & Editing, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation. Ailing Li and Xuelian Li: preparing material and collecting data. Xuejuan Pan and Xuetong Wu: involving in the experiments and data analysis.
ACKNOWLEDGMENTS
We gratefully acknowledge the College of Horticulture, Gansu Agricultural University for providing experimental instruments and materials.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (Nos. 32072559, 32160705, 32360743); the Key Research and Development Program of Gansu Province, China (No. 21YF5WA096). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
All data generated during this study are included in this article and its Supplementary Figure files.




