SlTCP29 and SlTCP24 participate in the morphological development of tomato compound leaves by integrating multiple pathways
Abstract
Leaves are the primary vegetative organs of plants, and their morphology is an important trait affecting plant architecture, light energy utilization, environmental adaptation, and fruit quality and yield. Leaf development is highly flexible; however, understanding the regulatory mechanisms of factors coordinating leaf morphogenesis and differentiation remains limited. In this study, we obtained a double mutant for SlTCP29 and SlTCP24 genes from the CRISPR/Cas9 mutant population, both belonging to the CINCINNATA-like TCP (TEOSINTE BRANCHED, CYCLOIDEA and PCF1/2) transcription factor subfamily. Simultaneous mutations of SlTCP29 and SlTCP24 genes increase the complexity of tomato leaves, characterized by deeper leaf margin notches and increased number of leaflets. In conjunction with RNA-seq analysis, determination of plant hormone content, and molecular interaction assays, we identified the KNOXII gene SlTKNII5, SlMIR164a, and 1-aminocyclopropane-1-carboxylic acid synthase gene SlACS1A as direct downstream targets of SlTCP29 and SlTCP24, among which SlTKNII5 can physically interact with other KNOXII members to form heterodimers. Our study provides insight into the mechanisms by which SlTCP29 and SlTCP24 are involved in the morphological development of tomato compound leaves by integrating multiple pathways, including transcription factor, microRNA, and phytohormone.
1 INTRODUCTION
Leaves, the primary photosynthetic organs of plants, are important platforms for substance exchange and energy transformation between plants and their surroundings (Efroni et al. 2010; Su et al. 2022). Unlike simple-leaf plants, such as Arabidopsis, tomato has pinnately compound leaves, composed of multiple leaflets that gradually form during leaf development. The diverse morphology of tomato compound leaves makes it a model plant for studying organ development (Burko and Ori 2013).
Leaf formation is completed in three successive and overlapping stages: initiation, morphogenesis, and differentiation (Burko and Ori 2013; Su et al. 2022). In tomato, leaf primordia initiate from the peripheral zone (PZ) of the shoot apical meristem (SAM), and as the leaf primordia develop, leaflet primordia begin to initiate from the leaf primordia marginal meristem in a similar manner (Koenig et al. 2009). The auxin response factor SlMP/SlARF5 and the myeloblastosis oncoprotein (MYB) transcription factor LYRATE (LYR) promote leaflet initiation and growth in a parallel pathway (David-Schwartz et al. 2009; Israeli et al. 2019), The NO APICAL MERISTEM (NAM)/CUP-SHAPED COTYLEDON(CUC) transcription factor GOBLET (GOB) is specifically expressed at the boundaries between SAM and leaf primordia and in the distal side of the future leaflet primordia to correctly regularize the lateral organ boundaries at the SAM and the leaflet boundaries of the developing compound leaves(Thomas et al. 2008; Berger et al. 2009). AUX/IAA factor Entire(E)/SlIAA9 promotes leaflet proper separation in concert with GOB by inhibiting the auxin response between leaflets (Ben-Gera et al. 2012).
During leaf morphogenesis, the specific region of leaf primordia margins meristem, known as marginal blastozones (MBs), maintains a transient window of organogenesis activity (Hagemann and Gleissberg 1996; Burko and Ori 2013; Tsukaya 2021). The relative duration and spatial length of this morphogenetic window are the main contributors to the elaborate modifications of tomato compound leaves. Several transcription factors have been identified as important for maintaining homeostasis in the leaf morphogenetic window (Israeli et al. 2021). The Class I KNOTTED1-like homeobox (KNOXI) protein TKN2/LeT6 and MYB transcription factor Trifoliate (Tf) usually act as delayed differentiation factors with the function of inhibiting cell differentiation and maintaining the morphogenetic competence. In contrast, CINCINNATA-like TCP (CIN-TCP) transcription factor LANCEOLATE (LA) and MYB transcription factor CLAUSA (CLAU) usually act as maturation-promoting factors, accelerating cell differentiation and promoting determinate growth of tomato leaves (Koenig and Sinha 2010; Burko and Ori 2013; Naz et al. 2013; Bar et al. 2016).
The Class I KNOX genes are expressed predominantly in undifferentiated cells in the SAM, maintaining meristem activity. Misexpression of the maize Kn1 gene in tomato increases the ramification of leaflets in compound leaves. There are four KNOXI genes in tomato; among these, SlTKN1 and SlTKN2 are expressed in the developing leaf primordium (Hareven et al. 1996; Ezura et al. 2022). SlTKN3 affects cell expansion in tomato pericarp, and SlTKN4 plays a role in the formation and differentiation of meristems and vasculature (Yan et al. 2015; Sun et al. 2023). Expression of the SlTKN2 gene in leaflet primordia using a spatiotemporally specific promoter suggests that KNOXI proteins are more involved in delaying maturation and promoting leaflet formation at specific stages of compound leaf development than in a specific genetic pathway for compound leaf formation (Shani et al. 2009; Burko and Ori 2013).
The function of the CIN-TCP transcription factor subfamily in regulating leaf differentiation and maturation appears to be conserved across multiple species. Arabidopsis cin-tcp multiple mutants display wavy and wrinkled leaves due to excessive proliferation of leaf margin cells (Koyama et al. 2010). CIN-TCPs redundantly suppress leaflet emergence in monocotyledons along with KNOXII protein, and simultaneous down-regulation of both leads to the reactivation of KNOXI and CUC2, and triggers ectopic organogenesis to convert simple leaves into compound leaves (Challa et al. 2021). Additionally, CIN-TCPs can repress the expression of KNOXI genes BREVIPEDICELLUS(BP) and KNAT2 by interacting with the ASSYMETRIC LEAVES2 (AS2) protein (Li et al. 2012). Tomato La semi-dominant mutant with enhanced CIN-TCP gene function has simplified leaves (Ori et al. 2007; Shleizer-Burko et al. 2011). Even overexpression of KNOXI in La does not change simple leaves into compound leaves, suggesting that a reduced CIN-TCP threshold in compound leaves appears to be a prerequisite for KNOXI-induced leaflet elaboration (Hareven et al. 1996; Burko and Ori 2013). KNOXI and CIN-TCP transcription factors act on the undetermined and deterministic growth of leaves (Koenig and Sinha 2010; Burko and Ori 2013). Although the factors and genetic regulation that orchestrate the balance between compound leaf morphogenesis and differentiation have been reported, the specific transcriptional regulatory networks still need further exploration.
Plant hormones also play a crucial role in leaf morphology (Shwartz et al. 2016; Zhao et al. 2021; Navarro-Cartagena and Micol 2023). Related studies have demonstrated that cytokinin (CK) and gibberellin (GA) regulate the balance between morphogenesis and differentiation via negative reciprocal interactions (Fleishon et al. 2011; Bar and Ori 2015; Israeli et al. 2021). Tomato CIN-TCPs can positively regulate GA response and similarly promote the transcription of cytokinin degradation gene SlCKX2 to reduce the cytokinin content in leaves, thus pushing the development of leaves to differentiation (Yanai et al. 2011; Hu et al. 2023). KNOXI protein inhibits the expression of GA synthesis gene GA 20-oxidase1(GA20ox1) and activates the expression of the GA degradation gene GA 2-oxidase1(GA2ox1) and cytokinin synthesis gene ISOPRENYLTRANSFERASE 7(IPT7), thereby inhibiting GA activity and increasing cytokinin levels to prolong leaf morphogenetic activity (Hay and Tsiantis 2010; Bar and Ori 2015). Additionally, ethylene and KNOX protein KNAT2 have an antagonistic effect on the meristem. The 1-aminocyclopropane-1-carboxylic acid (ACC) treatment partially reversed lobed leaf morphology and restricted the expression domain of KNAT2 in SAM of KNAT2-GR plant induced by DEX, implying that ethylene signaling may also play a role in modulating meristem activity (Olivier et al. 2002).
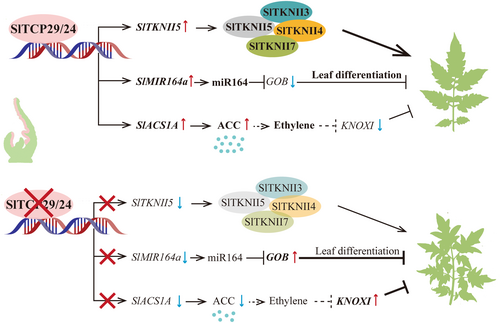
Here, we present the downstream gene networks implicated in the regulation of leaf development by SlTCP29 and SlTCP24, belonging to the CIN-TCP transcription factor family. Simultaneous mutations of the SlTCP29 and SlTCP24 genes significantly increased the complexity of leaves. We show that SlTCP29 and SlTCP24 directly activate the differentiation-associated Class II KNOX gene SlTKNII5, thereby promoting the formation of protein complexes among the KNOXII members. Meanwhile, SlTCP29 and SlTCP24 indirectly inhibit the expression of GOB gene and KNOXI genes. On the one hand, SlTCP29/24 can directly bind to the promoter of SlMIR164a, which encodes a mature microRNA, sly-miR164, which has been reported as a negative regulator of GOB(Berger et al. 2009; Hendelman et al. 2013). On the other hand, SlTCP29/24 directly activate the transcription of the ethylene biosynthesis-related gene SlACS1A, thereby potentially indirectly inhibiting the expression of leaf primordial KNOXI genes via the ethylene pathway. In summary, our findings provide new insights into the regulatory mechanisms of SlTCP29 and SlTCP24 during tomato compound leaf development.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
The sltcp29/24 double mutant with the background of M82 (Solanum lycopersicum) was screened from the previously constructed CRISPR/Cas9 mutant population (Bi et al. 2023). The T2 generation homozygous sltcp29/24 mutants were used in this study. Wild-type (WT) tomato plants M82 and sltcp29/24 mutants were grown in temperature-controlled incubators at 25°C ± 1°C during the day and 18°C ± 1°C at night, with a photoperiod containing 16 h of light and 8 h of darkness.
2.2 Leaf phenotyping
The leaf phenotypes of WT and sltcp29/24 mutants were observed 45 days after germination (DAG). The numbers of primary, secondary and intercalary leaflets in successive leaves were counted, then the leaf morphology was recorded using a scanner. At least 7 plants were used for each genotype. The terminal and lateral leaflets of the fifth leaf from WT and mutant plants were scanned, and leaf area and leaf dissection index were calculated using ImageJ software. The method refers to previous research (He et al. 2020). At least 10 leaves from different plants were used to observe and record each genotype. The leaf margin epidermal cell morphology of WT and mutant terminal leaflets was observed under a laser microscope; then, the cell counts and areas were analyzed using Photoshop and ImageJ. At least 5 leaves per genotype were observed and three different fields of vision per leaf were photographed.
2.3 In situ hybridization
The unique sequence of SlTCP29 and SlTCP24 were amplified from the WT cDNA and ligated into pSPT18 and pSPT19 vectors. The constructs were linearized, and T7 or SP6 RNA polymerase was used for the RNA labeling reaction according to the protocol of manufacturer (Roche). Leaf primordia from the m-P6 stage were selected for section preparation, and RNA in situ hybridization was performed as previously described (Nakayama et al. 2021).
2.4 Leaf primordium imaging
The fifth leaf primordium of WT and mutant plants was used for observation. Excess leaf primordia of the plants were trimmed off using a surgical blade to preserve the fifth leaf primordium. Changes in the morphology of the fifth leaf primordium of WT and mutant plants were regularly observed and photographed under a Zeiss digital microscope. At least 6 leaf primordia per genotype were observed at a time.
2.5 Collection of leaf primordia and RNA-seq analysis
The leaf primordia of the WT and mutant seedlings grown in an incubator for approximately 20 days were sampled using a ZEISS digital microscope, and the leaf primordium of m-P6 stage containing meristem was collected as the RNA sequencing sample. At least 0.3 g of leaf primordia were collected for each sample, and three independent samples from each group were collected for sequencing. The RNA-seq was conducted in SeqHealth (Wuhan, China) using the Illumina HiSeq X Ten sequencing platform. The raw data were qualified using Base Calling and Trimmatic software. The clean data were compared to the tomato genome in the Solanaceae Genomics Network (http://solgenomics.net/) to obtain comprehensive transcriptional information. Finally, the absolute value of logFC >1 and P-value <0.05 were used as criteria to screen differentially expressed genes (DEGs).
2.6 RT-qPCR analysis
Total RNA was extracted from different tomato tissues using an RNA pure plant kit (CWBIO). RNA samples of 1 μg were reverse-transcribed into cDNA using the Prime Script™ RT Master Mix (Takara). The tomato ACTIN gene (NCBI: NM_001330119.1) was used as an internal control. Primers are listed in Table S1. RT-qPCR analysis was performed using SYBR Green PCR Master Mix (Takara) on qTOWER3 G (Analytik Jena AG) following the manufacturer's protocol. Relative expressions were analyzed by the 2-ΔΔCt method (Livak and Schmittgen 2002). Three biological and technical replicates were performed for each assay.
2.7 Yeast one-hybrid assays
The promoter fragments of SlTKNII5, SlMIR164a, and SlACS1A, containing TCP transcription factor-binding motifs were cloned into the pAbAi vector as bait. After digestion with BstBI, the linearized construct was transformed into the Y1H Gold yeast strain using the Yeastmaker Yeast Transformation System 2 (Clontech). The full-length coding sequences (CDS) of SlTCP29 and SlTCP24 were cloned into the pGADT7 vector. Primers are listed in Table S1. The recombinant pGADT7 vector was transformed into Y1H Gold(bait/AbAi) strains and spread on SD/−Leu medium. Subsequently, positive DNA-protein interaction factors were screened based on the protocol of the manufacturer (Clontech).
2.8 Dual-luciferase assays
The full-length CDS of SlTCP29 and SlTCP24 were constructed into the pCAMBIA1300 vector driven by the CaMV 35S promoter as the effector. The SlTKNII5, SlMIR164a and SlACS1A promoter fragments were respectively inserted into the pGreen II 0800-LUC vector. Primers are listed in Table S1. The pCAMBIA1300 vector was used as a negative control. The recombinant plasmids were transformed into Agrobacterium tumefaciens GV3101 competent cells. The effector and reporter were mixed at a ratio of 1:1; then the mixture was infiltrated into tobacco leaves using a syringe. After 72 h incubation, the activities of firefly luciferase and renilla luciferase were measured using the dual-luciferase reporter assay system (Promega) on an Infinite 200 pro luminometer (TECAN). For firefly luciferase imaging, 1 mM D-Luciferin was sprayed on the abaxial sides of tobacco leaves. The leaves were kept in the dark for 10 min, and then luciferase images were captured using the CCD imaging system.
2.9 Electrophoretic mobility shift assay (EMSA)
The full-length CDS of SlTCP29 and SlTCP24 were cloned into the pGEX 6P-1 vector containing a GST tag and the pET-32a vector containing a His tag, respectively. Primers are listed in Table S1. Escherichia coli strain Rosetta2 cells were used to express the fusion proteins. The recombinant protein was incubated at 16°C for 16 h with 0.25 mM IPTG, and then purified using a GST-labeled purification kit (Beyotime). The probes of the SlTKNII5, SlMIR164a and SlACS1A promoters were synthesized and the binding site was labeled with biotin. The unlabeled probes were used as the cold competitors. The mutated biotin probes were used to detect binding specificity. GST protein and His protein were used as the negative controls. The EMSA reactions were conducted according to the instructions of the Chemiluminescent EMSA Kit (Beyotime).
2.10 Yeast two-hybrid assays
To test the interaction of KNOXII members at the protein level, the full-length CDS of SlTKNII5 was constructed into the pGBKT7 vector as bait, and the full-length CDS of SlTKNII3/4/7 were each constructed into the pGADT7 vector as prey. Primers are listed in Table S1. The recombinant plasmids were co-transformed into Y2H Gold yeast strain according to Yeastmaker Yeast Transformation System 2 (Clontech), and the transformants were cultured on SD/−Leu/−Trp (DDO) medium for 3–5 days. Positive transformants were then screened on SD/−Leu/−Trp/−Ade/-His (QDO) medium containing X-α-gal and AbA.
2.11 Firefly luciferase complementation imaging assays
The full-length CDS of SlTKNII5 with the stop codon removed was cloned into the pCAMBIA1300-nLUC vector, and the full-length CDS of SlTKNII3/4/7 were cloned into the pCAMBIA1300-cLUC vector. Primers are listed in Table S1. Then, the recombinant plasmids were transformed into Agrobacterium tumefaciens GV3101. Different combinations were mixed in a ratio of 1:1; then, the mixture was infiltrated into the tobacco leaves using a syringe. The plants grew in the dark for one day and in normal light conditions for two days. Finally, the fluorescence signal was captured using the CCD imaging system. At least three replicates of each combination were conducted for fluorescence detection.
2.12 Accession numbers
Tomato genes were collected from the Solanaceae Genomics Database (http://solgenomics.net/) under the following accession numbers: SlTCP29, Solyc08g048370. 2; SlTCP24, Solyc08g048390.2; GOB, Solyc07g062840.2; SlTKN1, Solyc04g077210.2; SlTKN2, Solyc02g081120.2; SlTKN3, Solyc05g005090.2; SlTKN4, Solyc01g100510.2; SlTKNII3, Solyc07g007120.2; SlTKNII4, Solyc12g010410.1; SlTKNII5, Solyc08g041820.2; SlTKNII7, Solyc08g080120.2; SlACS1A, Solyc08g081550.2; and SlMIR164a, MI0027570.
3 RESULTS
3.1 Simultaneous mutations in SlTCP29 and SlTCP24 affect the morphology of tomato compound leaves
We obtained a double-gene mutant with a frameshift mutation in the SlTCP29 gene and a codon mutation in the SlTCP24 with one amino acid deletion in the TCP domain from our previously constructed tomato transcription factor CRISPR/Cas9 mutant population (Figure S1a). SlTCP29 and SlTCP24 belong to the CIN-like TCP transcription factor subfamily and have high genetic homology (Figure S1b). The compound leaf morphology was the most obvious change in plant architecture caused by the changes in SlTCP29 and SlTCP24 protein sequences. It is worth mentioning that the leaf phenotype of our double mutant is consistent with that of the sltcp29/24 double mutant in the background of different tomato varieties reported in previous studies (Hu et al. 2023). In order to further investigate the specific details of the effect of sltcp29/24 mutants on leaf morphology, we collected scanning observations of leaf morphology and statistics of leaflets per leaf unit for the first six units in WT and sltcp29/24.
Initially, we observed the additional leaf margin serrations in the fully expanded cotyledons of sltcp29/24. Statistics on all types of leaflets in each leaf unit displayed that the number of primary leaflets of WT and mutant remained essentially the same. However, with leaf development, the mutant developed more secondary leaflets on the primary leaflet petiolule and intercalary leaflets on the rachis, resulting in a significantly higher number of leaflets in each leaf order in the mutant than the WT, and a more complex morphology of the compound leaves (Figures 1a,b and S2). The result that simultaneous mutation of SlTCP29 and SlTCP24 increased the number of leaflets in tomato is consistent with previous reports (Hu et al. 2023).
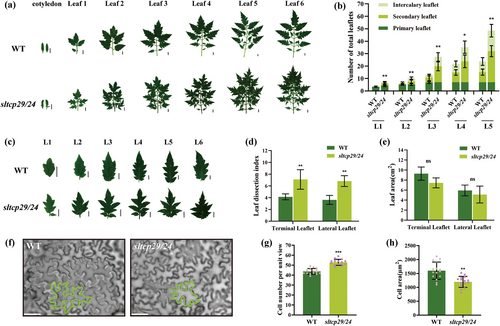
With the gradual differentiation of leaves, the leaflet margin was serrated more obviously in the mutant than in the WT, changing from lobed leaves into deeply serrated leaves (Figure 1c). Analyses of WT and mutant single-leaf complexity revealed that the leaf dissection index of both the terminal and lateral leaflets of the mutant was significantly higher than that of WT (Figure 1d). However, the leaf area of single leaflets of mutant primary leaflets was slightly reduced; but not significantly compared to the WT (Figure 1e). Observations of the epidermal cells of functional leaves that had completed differentiation revealed a significant reduction in the area of mutant leaf epidermal cells per unit field of view and a significant increase in their number compared to the WT (Figure 1f-h). The above results suggest that SlTCP29 and SlTCP24 synergistically maintain the deterministic growth of tomato leaflets and leaf margin normalization.
3.2 Temporal and spatial expression of SlTCP29 and SlTCP24 in the development of tomato compound leaves
We divided leaf development into m-P3 to P10 stages and then conducted RT-qPCR analysis to explore the expression patterns of SlTCP29 and SlTCP24 during compound leaf development (Figure S3). The results showed that SlTCP29 expression gradually increased with leaf primordium development, with the highest expression at the P7 stage, followed by a slight decrease in expression level, but continued to be expressed at the stage of P8-P10 (Figure 2a). In contrast, SlTCP24 was expressed at all stages of leaf development, and its expression level was higher in the m-P6 to P8 stages (Figure 2b).
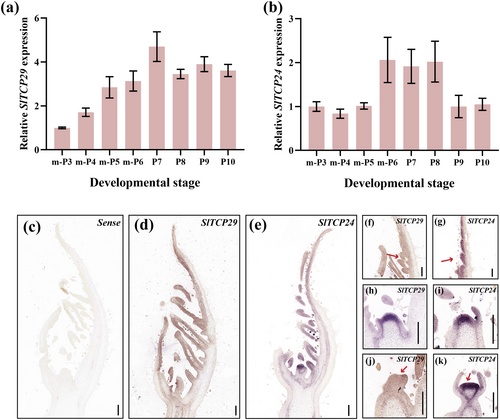
To further investigate the spatial expression of SlTCP29 and SlTCP24, we performed in situ hybridization analysis on the leaf primordium at the WT m-P6 stage. The results revealed that SlTCP29 and SlTCP24 were expressed in similar locations, with expression detected on the adaxial side of the leaf primordium and initiating leaflet primordium (Figure 2c, d, e), as well as in the leaf margin meristem in differentiation (Figure 2f, g). In addition, the expression of SlTCP29 and SlTCP24 was also detected in the SAM as well as in the floral meristem (Figure 2h-k), implying that SlTCP29 and SlTCP24 may play a role in the maintenance of meristematic tissues and floral bud differentiation. These above results indicate that SlTCP29 and SlTCP24 begin to play a role in the early stage of leaf primordium development and continue to accompany the various stages of leaf differentiation.
3.3 Simultaneous mutation of SlTCP29 and SlTCP24 delays the differentiation of tomato leaf primordium
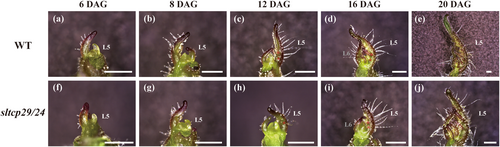
The elaboration of tomato compound leaf requires maintaining a transient indeterminate growth in the MBs of leaf primordium. To further investigate the impact of sltcp29/24 on leaf primordium development in tomato, we conducted morphological observations on the differentiation process of the fifth leaf (L5) primordium in both WT and sltcp29/24.
After 6 days of germination, the L5 primordium of WT and sltcp29/24 began to initiate (Figure 3a, f). After 8 days of germination, the growth of L5 primordium in sltcp29/24 was slower than in WT (Figure 3b, g). Twelve days after germination, the first leaflet primordium on L5 primordium in WT was differentiated, the second leaflet primordium began to initiate, and the leaf margins of the terminal leaflets also started to differentiate. In contrast, the first leaflet primordium on L5 primordium in sltcp29/24 began to initiate (Figure 3c, h). 16 days after germination, the differentiation of the L5 leaflet primordium was further evident in WT. Moreover, the leaflet primordium on L6 primordium was also initiated. Whereas the second leaflet primordium on L5 primordium in sltcp29/24 began to initiate, the leaflet primordium on the L6 primordium has not yet differentiated (Figure 3d, i). After 20 days of germination, the terminal leaflet of L5 in WT was distinctly differentiated and began to gradually enter the secondary morphogenesis stage. In contrast, the L5 primordium in sltcp29/24 remained in the morphogenetic stage (Figure 3e, j). These results suggest that simultaneous editing of SlTCP29 and SlTCP24 prolongs the duration of leaf primordium differentiation and decelerates the transition of leaf development from the primary to the secondary morphogenetic stage. This prolongation of transition time may be responsible for the increased indeterminate growth of compound leaves in the sltcp29/24 mutant.
3.4 Differential gene expression analysis in the leaf primordia between sltcp29/24 and WT
We analyzed RNA-seq in the leaf primordium containing the apical meristem from sltcp29/24 mutant and WT to investigate the transcriptional regulatory pathways of SlTCP29 and SlTCP24 in regulating leaf development. A total of 2,185 genes were differentially expressed; 763 up-regulated and 1,422 down-regulated (Figure 4a, b). Genes associated with the cell cycle and proliferation were up-regulated in mutants compared to WT. In contrast, genes associated with cell cycle inhibition and promotion of cell expansion were down-regulated in mutants (Figure 4c). The differential expression of some transcription factors associated with leaf margin establishment, such as GOB, and leaf elaboration, such as KNOXI genes, suggests that SlTCP29 and SlTCP24 may recruit multiple factors regulating tomato compound leaf development.
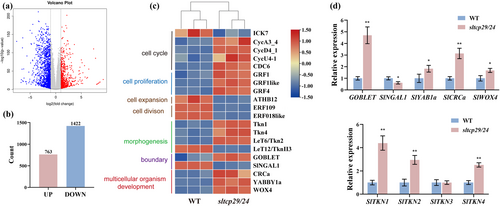
We observed that members of the Class I KNOX associated with leaf morphogenesis were generally up-regulated in the mutant, whereas the expression of the Class II KNOX gene LeT12/SlTKNII3, functionally antagonistic to Class I KNOX, was down-regulated (Figure 4c). To verify the accuracy of the RNA sequencing, we performed RT-qPCR analysis of several leaf development-related genes, specifically members of the KNOXI genes, and the results were consistent with the trend of the transcriptomic data (Figure 4d). These results imply that the increased leaf complexity caused by sltcp29/24 mutations may be due to the up-regulation of morphogenesis-related KNOXI members and the leaflet boundary gene GOB during early leaf primordium development, as well as the down-regulation of KNOXII genes which may promote organ differentiation.
3.5 SlTCP29 and SlTCP24 can promote SlTKNII5 transcription by binding to its promoter
The KNOXII genes are expressed in differentiated organs and exert an inhibitory effect on leaf complexity (Furumizu et al. 2015; Wang et al. 2023). Our RT-qPCR analysis found that four KNOXII members were significantly down-regulated in the sltcp29/24 mutant compared to WT (Figure 5a). Differential expression of other members, except for the SlTKNII3 gene, was undetected in the transcriptome, possibly due to their low abundance of transcripts.
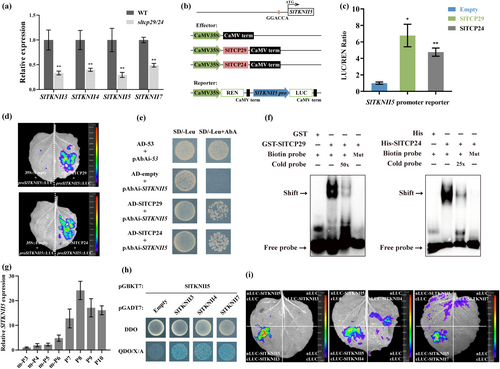
We analyzed the promoter sequences of the KNOXII members to clarify whether SlTCP29 and SlTCP24 promote leaf differentiation by regulating the KNOXII genes' transcription. We found that only the SlTKNII5 promoter contained a classical CIN-TCP transcription factor binding element (Figure 5b). To verify whether SlTCP29 and SlTCP24 can bind to the SlTKNII5 promoter, we performed dual luciferase assays and firefly luciferase imaging. The results demonstrated that SlTCP29 and SlTCP24 could transcriptionally activate SlTKNII5 expression in vivo (Figure 5c, d). Yeast one-hybrid and EMSA assays further demonstrated that SlTCP29 and SlTCP24 bind directly to the ‘GGACCA’ motif in the SlTKNII5 promoter (Figure 5e, f). RT-qPCR analysis indicated that SlTKNII5 expression gradually increased with the development of leaf primordia and peaked at the P8 leaflet stage, with a similar expression trend as that of SlTCP29, suggesting that SlTCP29 is co-expressed with SlTKNII5(Figure 5g).
To further validate the function of SlTKNII5 in leaf development, we obtained sltknII5 mutants using CRISPR/Cas9. However, we did not observe significant leaf morphology changes in the single-gene mutant (Figure S4). Previous studies have shown that heterodimers can be formed among KNOX family members to function (Zeng et al. 2021; Chen et al. 2023). The results of our yeast two-hybrid and firefly luciferase complementation imaging assays also indicate that SlTKNII5 could physically interact with SlTKNII3/4/7 (Figure 5h, i). Thus, these results suggest that SlTCP29 and SlTCP24 can directly activate the transcription of SlTKNII5, which may be genetically redundant with other KNOXII members, and that mutations in SlTKNII5 may affect only a portion of these protein complexes, and that there is a potential for the formation of homo- or heterodimeric combinations between other KNOXII members as well.
3.6 SlTCP29 and SlTCP24 promote the expression of tomato SlMIR164a by directly binding to its promoter
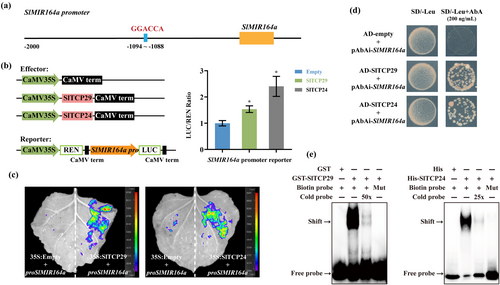
The tomato GOB gene is expressed at the initiating leaflet boundary and plays a role in the specification of developing leaflet separation and boundary (Berger et al. 2009). Our RNA-seq data revealed up-regulation of GOB gene expression in the sltcp29/24 mutant (Figure 4d), whereas the promoter sequence of GOB gene does not contain the classical motif bound by CIN-TCPs. Previous studies have shown that TCP3 in Arabidopsis can activate miR164a expression, and that miR164a is a negative regulator of the boundary gene CUC2(Koyama et al. 2010). Moreover, miR164 has been reported to be a negative regulator of GOB in tomato (Arazi et al. 2005; Berger et al. 2009). We speculated that SlTCP29 and SlTCP24 may indirectly regulate the expression of GOB by regulating the miR164 level, and consequently affect leaf margin serration morphology.
To confirm this hypothesis, we analyzed the upstream DNA regions of two precursor genes of tomato miR164 and identified a CIN-TCPs binding site in the promoter of SlMIR164a gene (Figure 6a). Subsequently, dual luciferase reporter assays and firefly luciferase imaging confirmed that SlTCP29 and SlTCP24 promoted SlMIR164a transcription (Figure 6b, c). Yeast one-hybrid and EMSA assays further confirmed that SlTCP29 and SlTCP24 could bind to the ‘GGACCA’ motif in the SlMIR164a promoter (Figure 6d, e). These results suggest that SlTCP29 and SlTCP24 may indirectly repress GOB gene expression at the leaf margin through direct transcriptional activation of SlMIR164a expression to adjust miR164 level, thus affecting the depth of the leaf margin tooth.
3.7 SlTCP29 and SlTCP24 may indirectly inhibit the expression of KNOXI genes by affecting ethylene biosynthesis
The balance between morphogenesis and differentiation of compound leaves has been shown to involve the engagement of several phytohormones (Ali et al. 2020; Israeli et al. 2021). In our RNA-seq data, several ethylene biosynthesis-related genes 1-AMINOCYCLO-PROPANE-1-CARBOXYLIC ACID (ACC) SYNTHASES (ACSs) and ACC-oxidase 1(ACO1) were significantly down-regulated in sltcp29/24 mutant (Figure 7a). Hormone content measurements at the shoot apical leaflets, showing that the content of ACC, the precursor of ethylene biosynthesis, was significantly down-regulated in the sltcp29/24 mutant (Figure 7b). The hormone level was consistent with gene expression.
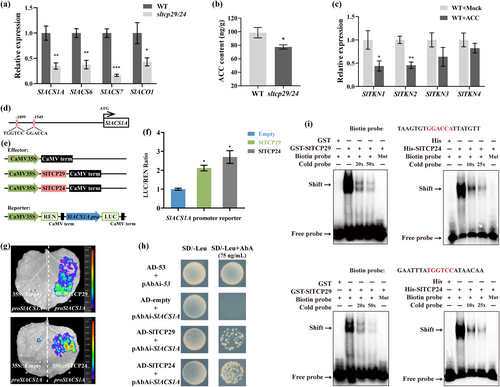
Previous studies have shown an antagonistic effect between ethylene signaling and KNAT2 protein in SAM, and ethylene may regulate meristem activity (Olivier et al. 2002). We further found that exogenous ACC treatment could decrease the expression of SlTKN1 and SlTKN2 in tomato stem tips (Figure 7c). We hypothesized that SlTCP29 and SlTCP24 may indirectly inhibit the expression of KNOXI genes during leaf primordium development by affecting ethylene biosynthesis. To confirm this hypothesis, we analyzed the promoter sequences 2000 bp upstream of the start codon of the differentially expressed SlACSs and SlACO1. Two potential CIN-TCP binding cis-elements were identified in the SlACS1A promoter (Figure 7d). Dual luciferase reporter analysis and firefly luciferase imaging demonstrated that SlTCP29 and SlTCP24 can transcriptionally activate SlACS1A expression in vivo (Figure 7e, f, g). Yeast one-hybrid and EMSA assays indicated that SlTCP29 and SlTCP24 can bind directly to ‘GGACCA’ and ‘TGGTCC’ motifs on the SlACS1A promoter (Figure 7h, i). These results suggest that SlTCP29 and SlTCP24 directly transcriptionally activate the expression of SlACS1A, further increasing the accumulation of ACC in shoot apical leaflets. SlTCP29 and SlTCP24 may indirectly affect the expression of KNOXI genes in leaf primordium through the ethylene biosynthesis pathway.
4 DISCUSSION
As the pivotal vegetative organ in plants, leaves necessitate the concerted action of diverse regulatory factors during their developmental process to influence various aspects of leaf morphology, thereby ensuring the establishment of appropriate patterning. CIN-TCP transcription factors play pivotal roles in various aspects of plant growth and development, and the effect of CIN-TCPs on leaf development seems to be conservative in different plant species (Wang et al. 2015; Koyama et al. 2017; Li et al. 2019; Vadde et al. 2019; Zheng et al. 2022; Lan et al. 2023).
In simple-leaf plants such as Arabidopsis and Antirrhinum majus, CIN-TCPs promote cell transformation from proliferation to differentiation, and actively suppress leaflet emergence (Das Gupta et al. 2014; Challa et al. 2019; Challa et al. 2021). Different from simple leaves, the elaboration of compound leaves in tomato depends on the activity of a brief morphogenesis window in leaf primordium margin meristem (Bar and Ori 2015). In this study, we found that the sltcp29/24 double mutant prolonged the duration of leaf primordium morphogenesis, exhibiting a more complex compound leaf morphology (Figures 1 and 3). Meanwhile, a related report also indicated that the phenotype of increased leaflet number was more obvious in the sltcp29/24 double mutant than in the SlTCP29 and SlTCP24 single mutants (Hu et al. 2023). In addition, the tissue expression patterns of SlTCP29 and SlTCP24 in leaf primordium were similar to those of tomato LA/SlTCP2 gene (Figure 2), and la-6 mutant also exhibited deepened leaf serrations, suggesting that CIN-TCPs may be involved in the regulation of primary morphogenetic window activity in a redundant and partially independent way in tomato compound leaf (Ori et al. 2007; Shleizer-Burko et al. 2011).
Delayed leaf primordium development of sltcp29/24 significantly changed the expression of leaf pattern-related genes, such as Class I and Class II KNOX members and GOB, compared to WT (Figure 4). Related studies have shown that the tomato KNOXI gene SlTKN2 is reactivated in the initiated leaf primordium to promote morphogenesis and inhibit tissue differentiation, while GOB is more involved in promoting proper separation of leaflets and outgrowth of leaf margin serrations (Berger et al. 2009; Kang and Sinha 2010). The changes in gene expression correlated with the increased leaf complexity of the mutant, suggesting that SlTCP29 and SlTCP24 may accelerate leaf primordium differentiation and appropriately regulate organ boundaries by coordinating multiple factors.
KNOXI proteins play an important role in meristem maintenance and regulation of leaf morphogenesis, whereas KNOXII proteins appear to be more involved in plant organ differentiation. KNOXII exhibits an antagonistic function to KNOXI. However, whether there is dominance or mutual inhibition between KNOXI and KNOXII has not been fully reported (Hay and Tsiantis 2010; Furumizu et al. 2015; Wang et al. 2023). Our study found that SlTCP29 and SlTCP24 have a direct positive regulatory effect on SlTKNII5, and that SlTKNII5 can form a dimer with other KNOXII members at the protein level (Figure 5). Previous studies have shown that besides redundantly regulating the spatial–temporal pattern of fruit ripening in tomato, plants with co-silencing of KNOXII genes also exhibit more complex leaf phenotypes than sltknII3 and sltknII7 single mutants, indicating that KNOXII genes may also play a redundant role in tomato compound leaf development (Keren-Keiserman et al. 2022), which may explain the lack of significant changes in leaf phenotype in our sltknII5 single-gene mutant (Figure S4). In Arabidopsis, TCP5 inhibits leaf margin development by regulating the KNOXII gene KNAT3 and BEL-like gene SAW1(Yu et al. 2021). These results suggest that the regulation of CIN-TCPs to KNOXII genes seems to have a conserved role in leaf development in different plant species.
Previous studies have shown that transcriptional regulation of KNOXI genes by CIN-TCPs is dependent on physical interaction with AS2 protein, or that CIN-TCP and KNOXII proteins co-actively inhibit KNOXI genes expression (Li et al. 2012; Challa et al. 2021). In this study, we found that SlTCP29 and SlTCP24 may indirectly inhibit SlTKN1 and SlTKN2 expression through the ethylene pathway. SlTCP29 and SlTCP24 have direct positive regulation on ethylene biosynthesis-related gene SlACS1A, and ACC content, the precursor for ethylene biosynthesis, was reduced in shoot apical leaflets in the sltcp29/24 mutant (Figure 7). Previous studies have shown that ethylene acts antagonistically with KNAT2 in SAM and exogenous ACC treatment suppresses the lobed leaf phenotype of DEX-induced KNAT2-GR plants in Arabidopsis (Olivier et al. 2002). Our results similarly demonstrated that ACC treatment could inhibit the expression of SlTKN1 and SlTKN2 in leaf primordium, suggesting that ethylene may play a role in leaf development. Arabidopsis TCP5 negatively regulates AtACS2 to inhibit ethylene biosynthesis, thereby affecting petal cell elongation (van Es et al. 2018), while our study shows that SlTCP29 and SlTCP24 positively regulate SlACS1A, thereby participating in the ethylene-mediated inhibition of KNOXI genes expression. These suggest that TCP transcription factors may exhibit different regulatory patterns in different species or organs, such as the opposite regulatory roles of AP2-like transcription factors on BP and RPL genes in the development of lettuce achenes and Arabidopsis siliques, which are two fruits with different structures (José et al. 2011; Luo et al. 2021).
Ethylene levels are increased in plants overexpressing the citrus ACS4 gene. CiACS4 promotes an increase in endogenous ethylene, which induces the expression of CiERF3 to form the CiACS4-CiERF3 complex, participating in plant height regulation and flowering inhibition (Chu et al. 2023; Chu et al. 2024). An ethylene-induced ERF transcription factor in sweet orange can bind to the promoter of KNAT-like gene CsKN1 and inhibit its transcription, participating in spring bud tip abscission. While in litchi, LcKNAT1 inhibited the expression of ethylene biosynthesis genes LcACS1, LcACS7 and LcACO2 in fruit abscission, and ethylene also inhibited the expression of LcKNAT1 via a feedback mechanism (Costa et al. 2020; Zeng et al. 2021). These indicate that there is a correlation between ethylene signaling and KNOXI protein in multiple aspects of plant growth. Our study revealed that SlTCP29 and SlTCP24 in tomato may mediate ethylene biosynthesis, indirectly inhibiting the expression of KNOXI genes in leaf primordium. Further investigation is needed to reveal the more detailed regulatory mechanism between ethylene signaling and KNOXI in leaf development.
The boundary-specific gene CUC2 is implicated in the regulation of plant leaf margins, and its expression is repressed by NGATHA-Like 1(NGAL1) and miR164 (Shao et al. 2020; Liu et al. 2022). The deeper serrated leaf blade phenotype of the sltcp29/24 double mutant was in accordance with the differential expression of organ boundary-related genes GOB(Arabidopsis CUC2 homolog gene) and SlNGAL1 shown in the RNA-seq data (Figures 2c and 4d). Although CIN-TCP in Arabidopsis has been reported to directly activate NGATHA3 expression (Patricia et al. 2015), our study found that even though there is a TCP-binding element in the SlNGAL1 promoter, both EMSA assays and yeast one-hybrid analysis showed that SlTCP29 and SlTCP24 do not have a direct regulatory effect on SlNGAL1(Figure S5). Our study revealed that SlTCP29 and SlTCP24 indirectly inhibit GOB expression by targeting SlMIR164a(Figure 6). Meanwhile, as a positive regulator of leaf margin development, the spatial expression of miR164 in the leaf primordium was similar to that of SlTCP29 and SlTCP24, both expressed on the adaxial side of the leaf primordium (Berger et al. 2009; Bar et al. 2015). Additionally, AtTCP4 has been reported to interfere with CUC protein dimerization in an age-dependent manner, thereby affecting leaf complexity (Rubio-Somoza et al. 2014). However, our yeast two-hybrid assays presented that SlTCP29 and SlTCP24 do not interact with GOB, suggesting that SlTCP29 and SlTCP24 may indirectly affect GOB expression in a miR164-dependent manner.
In summary, we investigated the role of SlTCP29 and SlTCP24 in tomato compound leaf morphogenesis. Our study proposes that these two transcription factors contribute to compound leaf development through transcriptional regulation of multiple common downstream target genes (Figure 8), thereby expanding upon previous reports on the involvement of CIN-TCPs in regulatory networks governing compound leaf formation in tomato (Burko et al. 2013; Hu et al. 2023). These findings offer a potential genetic tool for enhancing tomato traits by utilizing SlTCP29 and SlTCP24 to modulate leaf morphology, maintain the appropriate growth of leaves, and maximize light energy utilization.
AUTHOR CONTRIBUTIONS
M.B. and M.Q. conceived and designed the experiments. M.B., Z.W. and K.C. performed the experimental work and analyzed the data. M.B. wrote the manuscript. M.B., Z.W., S.M., and M.Q. edited the manuscript. All authors read and approved the manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant Nos. 31991184, 32172554), the Agricultural Major Project of Liaoning Province(2023JH1/10200010) and Liaoning Revitalization Talents Program.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data supporting the results of this article can be found within the manuscript and the supporting materials provided.




