The dual role of methylglyoxal in plant stress response and regulation of DJ-1 protein
Abstract
Methylglyoxal (MG) is a highly reactive metabolic intermediate that plays important roles in plant salt stress response. This review explores the sources of MG in plants, how salt stress promotes MG production, and the dual role of MG under salt stress conditions. Both the positive role of low concentrations of MG as a signalling molecule and the toxic effects of high concentrations of MG in plant response to salt stress are discussed. The MG detoxification pathways, especially the glyoxalase system, are described in detail. Special attention is given to the novel role of the DJ-1 protein in the glyoxalase system as glyoxalase III to remove MG, and as a deglycase to decrease glycation damage caused by MG on DNA, proteins, and other biomolecules. This review aims to provide readers with comprehensive perspectives on the functions of MG in plant salt stress response, the roles of the DJ-1 protein in MG detoxification and repair of glycation-damaged molecules, as well as the broader functional implications of MG in plant salt stress tolerance. New perspectives on maintaining plant genome stability, breeding for salt-tolerant crop varieties, and improving crop quality are discussed.
1 INTRODUCTION
In nature, plants are sessile and thus are subject to signals and stresses from the ever-changing environment. The integrity and stability of plant genomes face multiple challenges from both external and internal factors. Environmental stresses can trigger a range of DNA alterations, including point mutations, deletions, translocations, telomere shortening, persistent single-strand and double-strand breaks, chromosomal rearrangements, and defects in nuclear structure, as well as gene disruptions caused by virus or transposon integration (López-Otín et al., 2023). These changes may have a profound impact on gene expression and function, further affecting plant growth, development, and adaptation. External stresses, in particular salt stress, pose a major challenge to plant survival and development (Ashraf et al., 2021). Globally, approximately 954 million hectares of land are affected by varying degrees of salinity (Zaman et al., 2018). During plant growth and development, high soil salinity reduces osmotic pressure in the rhizosphere, increases water loss and compromises the plant's water uptake capacity, increases ionic toxicity, and hinders intracellular metabolic balance by inducing osmotic and oxidative stress (Van Zelm et al., 2020).
During the initial stages of osmotic stress, plants maintain intracellular water balance by adjusting intracellular solute concentrations (e.g., organic solutes and electrolytes) to improve cellular osmoregulation (Flowers et al., 2015). Salt stress leads to the accumulation of ions, especially Na+, which interfere with the absorption of crucial mineral nutrients, such as K+, which are important for plant growth (Shi et al., 2000). Osmotic and ionic stresses, as primary factors, lead to an increase in the levels of reactive oxygen species (ROS) (Mittler, 2017) and reactive carbonyl species (RCS), which in turn trigger a series of secondary stresses and the accumulation of advanced glycation end products (AGEs) (Mostofa et al., 2018). These changes can directly affect the integrity of plant DNA, causing gene mutations or dysfunction, further affecting the processes of seed germination, plant growth and development, as well as flowering and fruit/seed development (Quan et al., 2007).
Carbonyl stress can be triggered by RCS, a family of molecules with highly reactive carbon atoms, which include a wide range of aldehydes and ketones such as formaldehyde, acetaldehyde, methylglyoxal (MG), malondialdehyde (MDA) and 4-hydroxy-2E-nonenal (HNE) (Wang et al., 2019). Among them, MG, a strong endogenous electrophilic reagent, can be formed through both enzymatic and non-enzymatic pathways during cellular metabolic processes (Lee & Park, 2017). Due to the carbonyl (C=O) and enal (=C−CHOH−) groups in its structure, MG readily reacts with intracellular nucleophilic compounds, including DNA and amino or thiol groups in proteins, to form stable covalent adducts. These adducts, known as AGEs, compromise the structure and function of proteins and DNA, causing gene mutations and genomic instability (Vistoli et al., 2013). Therefore, MG can be toxic in plant cells, and its role in plant salt stress response has started to receive widespread attention.
To counteract the toxicity of MG, organisms primarily detoxify MG through a glyoxalase (GLO) system, which consists of glutathione (GSH) mediated glyoxalase I (GLO I, S-D-lactoylglutathione lyase; EC 4.4.1.5) and glyoxalase II (GLO II, S-2-hydroxyacylglutathione hydrolase; EC 3.1.2.6), which together constitute the major metabolic pathway of MG (Ghosh et al., 2014; Kaur et al., 2017). Compared to this conventional pathway, GSH-independent glyoxalase III (GLO III, converting MG into lactate; EC 4.2.1.130) provides a more direct and efficient means of detoxification (Lee et al., 2012). Its human counterpart, DJ-1, is widely associated with Parkinson's disease and various types of cancer (Raninga et al., 2014). In human bodies, DJ-1 not only acts as GLO III to scavenge free MG but also functions as a deglycase to repair proteins and DNA damaged by glycation (Pfaff et al., 2017; Richarme et al., 2017). In fact, the role of MG is dual, depending on its concentration in the cell: at high concentrations, MG triggers cytotoxicity, while at low concentrations, it may act as a signalling molecule that regulates a complex network to mitigate the negative effects of stresses (Singla-Pareek et al., 2003; Zhu, 2016).
This review delves into the complex roles of MG in plant salt stress response and the regulatory function of the DJ-1 protein. We summarize the biosynthetic pathways of MG in plant cells in comparison with MG synthesis in humans and microorganisms. Additionally, we address how salt stress influences MG production and the dual role that MG may exhibit in high-salt environments, discussing its negative effects as a toxic metabolite and its potential protective role in signalling. Furthermore, we focus on the research hotspot of DJ-1 protein as a deglycase and GLO III and explore its response to salt stress by regulating the MG levels. The goal is to provide readers with a comprehensive view of the functions of MG in response to plant salt stress and to elucidate the role of DJ-1 protein in MG detoxification and repair of glycation-damaged molecules. This knowledge enables a deep understanding of the plant MG systems and their function in stress responses. It may help springboard the molecular breeding effort of crops for stress resilience and global food security.
2 SOURCES OF MG IN ORGANISMS
MG is a ubiquitous carbonyl compound also known as pyruvaldehyde or 2-oxopropanal (CH3−CO−CHO or C3H4O2) (Berdowska et al., 2023). Due to its high reactivity, it has garnered extensive attention in broad research areas of human, animal, microbial and plant biology. MG is mainly produced by cellular metabolic pathways in different organisms, such as glycolysis, amino acid metabolism, and lipid peroxidation as a by-product (Garai et al., 2023). The MG production pathways can be divided into non-enzymatic pathways and enzymatic pathways.
2.1 Non-enzymatic pathways for MG production
Among non-enzymatic pathways of MG production, leakage from the glycolysis pathway contributes to over 90% of MG primary sources. In glycolysis, glucose is converted to fructose-1,6-bisphosphate through a series of reactions. This six-carbon sugar compound is then cleaved into two three-carbon phosphate compounds, glyceraldehyde 3-phosphate (GAP) and its isomer dihydroxyacetone phosphate (DHAP) (Phillips&Thornalley, 1993) (Figure 1a). Due to the instability of these three-carbon phosphates, DHAP and GAP are susceptible to rapid loss of the α-carbonyl proton during the isomerization transition to form the enediolate phosphate intermediate (Richard, 1993). The C3-hydroxyl group in DHAP and its resulting enediolate phosphate 1 is stabilized, requiring internal proton transfer to convert to enediolate phosphate 2. Triose phosphate isomerase (TPI) catalyzes the isomerization reaction by eliminating the β-phosphate group rapidly, leading to the formation of the corresponding ketone and facilitating MG production (Sousa Silva et al., 2013). Therefore, the rate of conversion of GAP to MG is approximately eight times that of DHAP (Phillips & Thornalley 1993). It is worth noting that this process, despite the involvement of TPI, is regarded as non-enzymatic because the MG formation itself does not depend on direct enzyme catalysis. Under certain conditions, such as a strongly alkaline environment, DHAP and GAP can also be directly converted to MG without any enzymes. In humans, only about 0.09–0.4% of the glycolysis flux may be associated with MG production (Martins et al., 2001; Thornalley, 1988). This is mainly because, under normal physiological conditions, the non-enzymatic pathway for MG production exists only as a side reaction in the glycolysis process, and the spontaneous dehydration reaction rate of DHAP and GAP is relatively low. In plants, the glycolysis pathway is connected to photosynthesis, in which the Calvin Cycle reaction produces three-carbon sugars (3-phosphoglycerate (3-PGA)), which are then converted to glucose. The glucose can be used as a substrate for glycolysis to produce MG. MG production during photosynthesis increases with light intensity or CO2 concentration and correlates positively with O2 evolution rates (Takagi et al., 2014).
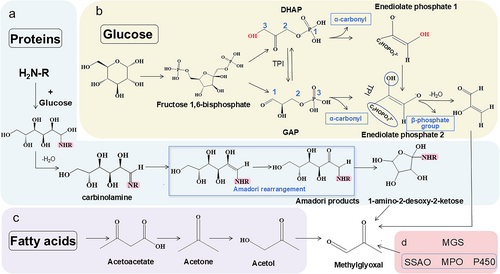
In addition, the process of forming advanced glycation end products (AGEs), a non-enzymatic glycation reaction, is another important non-enzymatic pathway of MG production. It is a slow process first described by the French chemist Louis-Camille Maillard in 1912 as the non-enzymatic browning of food during heating. Interestingly, the glycation reaction that generates MG in plants and other organisms shares similarities with the Maillard reaction in terms of chemical mechanisms. In this process, the aldehyde groups of reducing sugars (e.g., glucose and fructose) readily react with free amino acids or amino acid groups in proteins to produce carbinolamine intermediates, which, upon dehydration, form imine adducts. The imine adducts are converted to MG through spontaneous Amadori rearrangement as well as oxidative cleavage (Davídek et al., 2006) and retro-aldol degradation (Cämmerer et al., 1999; Vistoli et al., 2013) (Figure 1b).
Lipid peroxidation reactions mainly refer to a series of free radical reactions occurring in plant unsaturated fatty acids. This is one of the major damaging changes induced by oxygen radicals and also an important pathway for MG generation (Esterbauer et al., 1982). When leaves are exposed to intense light, double bonds in unsaturated fatty acids are susceptible to 1O2 attack to form lipid peroxides (Triantaphylides et al., 2008). In the presence of specific catalysts such as transition metal ions (Fe3+) or reducing agents, the lipid peroxidation reaction is enhanced. The intermediates like acetoacetate or acetone spontaneously decompose through the Fenton reaction to generate carbonyls, including MG (Grosch, 1987) (Figure 1c).
2.2 Enzymatic pathways for MG production
During the catabolism of glucose, amino acids, and fatty acids, the production of MG in bacteria and animals involves both enzymatic and non-enzymatic pathways. The enzymatic pathways have not been reported in plants. Methylglyoxal synthase (MGS) is the only enzyme known to catalyze the direct conversion of DHAP to MG during glycolysis (Cooper, 1984; Cooper & Anderson, 1970; Hopper & Cooper, 1972). MGS is widely found in microorganisms (e.g., E. coli and Pseudomonas aeruginosa), and the enzyme has been isolated from goat liver (Cooper & Anderson, 1970). Additionally, there are several other enzymes in microorganisms and animals that are involved in the production of MG. For example, during the catabolism of glycine and threonine to form aminoacetone intermediates, semicarbazide-sensitive amine oxidase (SSAO) catalyzes the production of MG from these intermediates (Lyles & Chalmers, 1992). Acetoacetate formed in the process of fatty acid catabolism can be oxidized by myeloperoxidases (MPO) to generate MG (Panasenko et al., 2022). Similarly, cytochrome P450 (CYP450) oxidizes acetone to MG using nicotinamide adenine dinucleotide phosphate (NADPH) as a cofactor (Koop & Casazza, 1985). During triacylglycerol hydrolysis in lipid metabolism, glycerol kinase also converts the resulting glycerol phosphate intermediates into MG (Jung et al., 2011) (Figure 1d). Clearly, the production of MG is an inevitable and multifaceted process. However, the equivalent enzymatic pathways have not yet been discovered in plants, presenting a great opportunity for investigating the mechanisms of MG production in the plant kingdom. The aforementioned discoveries made in microorganisms and animals can certainly inform plant research.
3 SALT STRESS PROMOTES MG PRODUCTION IN PLANTS
Under normal metabolic conditions, plants typically maintain low levels (30–75 μM) of MG (Yadav et al., 2005a). Under salt stress, metabolic perturbance leads to the accumulation of MG in various plants. For instance, Yadav et al. observed a 73% increase in MG content when tobacco (Nicotiana tabacum var. petit havana) seedlings were treated with 200 mM NaCl for 24 h (Yadav et al., 2005c). Mostofa et al. reported a 58% increase in MG content when rice (Oryza sativa L. cv. BRRI dhan52) seedlings were treated with 150 mM NaCl for 4 days (Mostofa et al., 2015). Nahar et al. observed increases of 74 and 102% in MG contents when mung bean (Vigna radiata cv. Binamoog-1) seedlings were treated with 200 mM NaCl for 24 and 48 h, respectively (Nahar et al., 2015). Kamran et al. found large increases of 171.6 and 189.7% in the MG content when Chinese flowering cabbage (Brassica parachinensis L.) was treated for 10 days with 150 and 200 mM NaCl, respectively (Kamran et al., 2020).
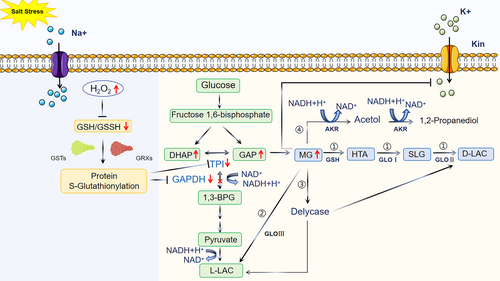
Salt stress is one of the major environmental factors limiting plant growth and agricultural production (Che-Othman et al., 2017). High salinity in the soil causes plant cells to experience severe osmotic, ionic and oxidative stresses (Gong, 2021). To adapt, plants must rapidly initiate a multitude of responses, including redox changes in response to accumulation of ROS, such as hydroxyl radicals (•OH), hydrogen peroxide (H2O2), and superoxide anion radicals (•O2−) (Zhu et al., 2007). These ROS can cause severe damage to cellular molecules and induce cell death (Mittler et al., 2004). Some plant cells have successfully adapted to oxidative stress by ROS scavenging through the antioxidant defence systems. GSH, a tripeptide composed of glutamate, cysteine and glycine, is the most abundant antioxidant in cells. GSH readily reacts with ROS and is converted to oxidized glutathione (GSSG). Changes in the ratio of GSH to GSSG can be used as an indicator of cellular redox state. Furthermore, the maintenance of GSH levels is essential for plant growth, development, and adaptation to oxidative stress (He et al., 2023; Zhu et al., 2021). Under salt stress, elevated levels of H2O2 promote oxidation of GSH to GSSG (i.e., lowering the GSH/GSSG ratio), which is sensed by glutathione-S-transferases (GSTs) and glutaredoxins (GRXs). This further induces S-glutathionylation (a reversible thiol modification) of key enzymes and regulatory proteins in metabolic and signalling pathways (Mailloux, 2020). S-glutathionylation of enzymes or proteins not only mitigates oxidative stress and its impact on glycolysis (Mailloux & Treberg, 2016), but also reduces the enzymatic activities of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and triose phosphate isomerase (TPI) in the glycolytic pathway (Fratelli et al., 2002; Michelet et al., 2006). When the glycolytic pathway is inhibited, the conversion of GAP and DHAP to MG is promoted (Thornalley & Bellavite, 1987). This was demonstrated in Arabidopsis thaliana under salt stress; the accumulated H2O2 could modify the –SH groups in the cysteine residues of TPI to sulfenic acids (–SOH), which directly inhibited the activity of TPI thus limiting the isomerization between DHAP and GAP and promoting MG accumulation (Figure 2). As mentioned in the previous section, the production of ROS themselves under salt stress can promote the generation of MG through non-enzymatic pathways.
In addition, type II acyl-CoA-binding proteins (ACBPs) in plants play an important signalling role by promoting the conversion of membrane phospholipids to phosphatidic acid (PA). Plant lipoxygenases (LOX) are responsible for catalyzing the deoxygenation reaction of linoleic acid (C18:2) and linolenic acid (C18:3) to generate hydro-peroxidized fatty acids (Fu et al., 2023). These reaction products are crucial for the synthesis of oxidized lipids, of which jasmonic acids (JAs) are the most extensively studied. Typically, ACBP inhibits LOX activity and restricts it to the endoplasmic reticulum, preventing lipid peroxidation of polyunsaturated fatty acids. Salt stress elicits specific PA signalling and the generation of ACBP splice variants, which deregulate the inhibition of LOX by ACBP. This activation of LOX exacerbates lipid peroxidation (Lung et al., 2022), as well as the accumulation of ROS, which may further promote MG production. These studies highlight potential molecular mechanisms of MG accumulation under salt stress, but they do not identify the specific enzymatic pathways in plants.
4 DUAL ROLE OF MG UNDER SALT STRESS
MG, like ROS, has a dual nature, being a toxic substance at high concentrations and acting as a signalling molecule in low amounts. During oxidative stress, the glyoxalase detoxification pathway is very efficient in alleviating its effects. GSH reduces MG to D-lactate, which can flow further to maintain homeostasis in the process of lipid peroxidation. Meanwhile, D-lactate can be taken up by mitochondria of both yeasts and mammals and oxidized in an energetically efficient manner by D-lactate dehydrogenase (D-LDH). The newly synthesized metabolites from the matrix further participate in the pentose phosphate pathway (PPP), constituting a major source of NADPH, which is essential for the maintenance of redox homeostasis and biosynthetic processes. In addition to this, the increased cytoplasmic level of S-lactoylglutathione (SLG) favours mitochondrial inputs and metabolism, thereby replenishing intra- and extracellular glutathione (GSH) pools and D-lactate formation. Therefore, it is important to study MG metabolism and its homeostasis in plants (de Bari et al., 2020).
4.1 MG in low concentration acts as a signalling molecule under salt stress
Signalling molecules are typically small molecules that respond rapidly to environmental changes (de Bari et al., 2020) and can be quickly produced inside the cell and trigger downstream signalling pathways. MG may be regarded as a signalling molecule potentially involved in a variety of plant physiological processes, including stomatal movement, plant growth and development, and stress response (Hossain et al., 2016).
Plants have evolved different mechanisms to sense and respond to environmental stresses, such as regulating gas exchange and water loss through stomatal pores on leaf surfaces (Jia&Zhang, 2008). Light-induced stomatal opening is mediated by K+ accumulation in guard cells, whereas stomatal closure is associated with an increase in cytosolic calcium concentration (Ca2+cyt). Ca2+cyt in guard cells oscillates in response to environmental stresses (Pei et al., 2000; Young et al., 2006). Hoque et al. found that MG inhibits photoinduced stomatal opening and induces stomatal closure but does not affect the guard cell viability. It decreases K+ influx by directly inhibiting inward-rectifying potassium channels (Kin) in the guard cells, thereby affecting plant water transpiration and gas exchange (Hoque et al., 2012a; Hoque et al., 2012b). Additionally, MG-induced stomatal closure involves extracellular peroxidase-mediated oxidative damage and (Ca2+cyt) oscillations. This process is completely inhibited by a peroxidase inhibitor salicylhydroxamic acid (SHAM) (Samuilov & Kiselevsky, 2016), but is not affected by the respiratory burst NADPH oxidase mutants atrbohD and atrbohF (Dietrich et al., 2001). Thus, RBOH-dependent ROS production does not appear to be involved in MG signalling.
Exogenously applied MG modulates other plant signalling molecules, such as phytohormones gibberellins and abscisic acid (ABA), which play key roles in the regulation of plant growth and development and response to environmental stresses. For instance, the addition of exogenous MG significantly inhibits seed germination and root elongation. This effect is closely related to the ABA signaling pathway, especially through the upregulation of stress-responsive genes, like RD29B and RAB18. These genes play pivotal roles in salt stress response, suggesting that MG exerts a regulatory role in this process by modulating the ABA-dependent signalling pathway (Hoque et al. 2012a). Li et al. found that exogenous MG can cross the plasma membrane into plant cells and activate GLO I and GLO II in the shoots and roots of wheat seedlings under salt stress (Li et al., 2017). This activation helps to mitigate the increase of MG levels and the accumulation of GSH. Moderate accumulation of MG activates specific signalling pathways, inducing the expression of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione reductase (GR). This process also enhances the levels of ascorbate (ASA), GSH, and osmolytes such as proline, glycine, betaine, and soluble sugars, thereby alleviating the increase in •O2−, H2O2, and MDA under oxidative stress. Roy et al. (2004) proposed that 0.5 mM MG can completely replace cytokinin to effectively promote the differentiation of healing tissues into plantlets (Roy et al., 2004). MG can also self-regulate its levels in cells through a feedback mechanism of activating TPI. In the isomerization reaction, the balance shifts towards the formation of GAP, thereby reducing the levels of MG precursor DHAP (Sharma et al., 2012). GAP, in turn, supplements the energy reserves via glycolysis, thereby helping plants to survive adverse conditions. Exogenously added MG may act as a stress signal, which has a positive effect on seed germination, growth and development, alleviation of oxidative stress, and even acquisition of stress resilience.
MG and its derivatives (e.g., AGEs) can affect cell signalling pathways through various mechanisms, inducing post-translational modifications (PTMs) (Donnellan et al., 2022; Galligan et al., 2018; Lu & Holmgren, 2014). In one study, Zheng et al. identified a mechanism by which MG regulated gene expression through methylglyoxalylation of histones under salt stress (Fu et al., 2021). Histones are prone to PTMs because of their high proportion of lysine and arginine residues. When histones undergo PTMs, they can affect gene expression by altering chromatin structure and recruiting various specific binding proteins. Thus, histone modifications play a key role in a variety of biological processes, such as cell differentiation, tissue development, cancer induction, and environmental response. Salt stress can lead to the accumulation of MG in plants, which can induce histone PTMs and improve the accessibility of plant chromatin, thus promoting the expression of salt stress-responsive genes, alleviating the damage to plants, especially in cells lacking GLO I. Modern proteomics, biochemistry and genetics tools will greatly facilitate the characterization of specific PTMs mediated by MG and its signalling role in plant cells.
4.2 High concentrations of MG are toxic to plants under salt stress
Not all PTMs of proteins induced by MG are beneficial. A typical example is non-enzymatic glycation (the Maillard reaction) in cells, leading to non-functional proteins that should be eliminated by proteolysis (Verzijl et al., 2000). Under salt stress, plants accumulate large amounts of MG (Kaur et al., 2016) that react easily with nitrogen, oxygen or sulfur atoms containing lone electron pairs, such as the amino or thiol groups in proteins (Figure 3), ultimately forming AGEs. Therefore, high concentrations of MG (e.g., > 75 μM) are toxic to plant cells. Glycated residues mainly accumulate on extracellular proteins, often making them inactive due to covalent modifications to the catalytic or binding sites and structural changes, resulting in irreversible damage (M Ciulla et al., 2011). Furthermore, glycation most notably targets the guanidino group of arginine residues, and condensation of MG with its Nη atom yields the corresponding carbinolamine amine (Henle et al., 1994), which is then cyclized to form a diol intermediate and dehydrated twice to produce hydroimidazolone derivatives (MG-H1, MG-H2, MG-H3) (Vistoli et al., 2013) (Figure 3. 3.1–3.3), which are in a dynamic equilibrium state because they can be ring-opened to form N7-carboxyethylarginine (CEA) (Figure 3. 3.4). This can, in turn, be recyclized, while the addition of MG continues to form argpyrimidine (Figure 3. 3.5) or Nδ-(4-carboxy-4,6-dimethyl-5,6-dihydroxy-1,4,5,6-tetrahydropyrimidine-2-yl)-L-ornithine (THP) (Figure 3. 3.6), with MG-H1 and argpyrimidine being the major glycation adducts. The reaction between MG and lysine residues produces Nε-(carboxyethyl) lysine (Figure 3. 3.7) and a cross-link between two lysines, 6–1-(5S)-5-ammonio-6-oxido-6-oxohexyl-4-methyl-imidazolium-3-yl-L-norleucine (MOLD, Figure 3. 3.8). Moreover, after MGO has reacted with lysine, it could further react with arginine residues to form a stable carbonyl cross-link, 2-ammonio-6-({2-[(4-ammonio-5-oxido-5-oxopentyl)amino]-4-methyl-4,5-dihydro-1H-imidazol-5-ylidene}amino) hexanoate (Lederer & Klaiber, 1999) (MODIC, Figure 3. 3.9). In addition, MG can also react with cysteine residues to produce reversible hemiacetal adducts (Figure 3. 3.10). These MG-derived AGEs are collectively referred to as MAGEs. They are thought to accumulate slowly throughout the life span, making their concentration represent a good biomarker of plant senescence, and may serve as a label for proteolytic degradation of dysfunctional proteins (Havé et al., 2015).
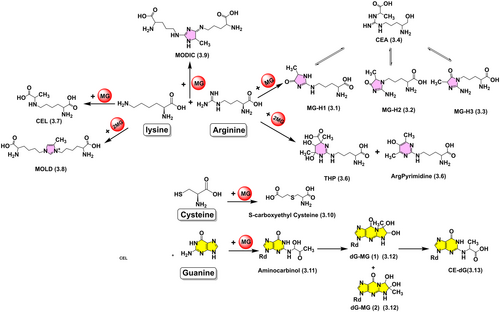
Nucleic acids can also be irreversibly modified by DNA glycation. Under physiological conditions, the most reactive nucleotide is deoxyguanosine (dG). In vivo, MG reacts with dG to generate nucleotide AGEs, mainly the imidazolopurinone derivative dG-MG (Thornalley et al., 2010) (Figure 3. 3.11), which is self-cyclized from the respective aminocarbinol (Figure 3. 3.12). In addition, stable adducts of N2-(1-carboxyethyl)-deoxyguanosine (CEdG) (Figure 3. 3.13) have also been reported (Papoulis et al., 1995; Thornalley, 2003), but their relative content was less than that of dG-MG. In cultured HL60 cells, the DNA glycation products were reported to be at the same level or even significantly more abundant than the major DNA oxidation product 8-oxoguanine (per 106 nucleotides: 8-oxo-dG, 1.48 ± 0.39; dG-MG, 18.2 ± 6.5 and CEdG, 1.33 ± 1.12), suggesting that MG glycosylation as DNA damage may be as important as oxidation (Thornalley et al., 2010). There is evidence that DNA glycosylation leads to a loss of genomic integrity. High concentrations of MG produce interstrand DNA cross-links (Rahman et al., 1990) and strand breaks (Pischetsrieder et al., 1999), and increase mutation frequency (Cajelli et al., 1987; Migliore et al., 1990), the majority of the mutations being G:C > C:G and G:C > T:A transversions (Murata-Kamiya et al., 1997, 2000).
5 MG DETOXIFICATION PATHWAYS
MG has been widely recognized for its high reactivity and toxicity in biological systems. In humans, MG is closely associated with a variety of diseases, such as diabetes, heart disease, and Alzheimer's disease. For example, the concentration of MG in the blood of patients with type 1 diabetes is 5.9-fold higher than in normal subjects, whereas the concentration in patients with type 2 diabetes is 3.6-fold higher (Zhu et al., 2021). In plants, rice, Indian mustard, tobacco, and barley accumulated 2–6 times more MG under drought, salt, and cold stresses than under normal growth conditions (Yadav et al., 2005b). It is noteworthy that the stems of the plants produce more MG than roots. Plants accumulate large amounts of sugars through photosynthesis (Qiu et al., 2008). In photosystem I, the photoreduction of oxygen competes with the photoreduction of NADP+, accompanied by the generation of RCS, including MG. Additionally, glycolysis uses sugars to produce the majority of MG. Hence, MG detoxification becomes crucial.
In humans, several enzymatic systems, such as aldo-keto reductase (AKR), aldehyde dehydrogenase (ALDH), and GLO, can reduce the toxicity of MG (Aldini et al., 2013) (Figure 2). However, the function of the ALDH family has not been clearly reported in plants. Saito et al. found a superfamily of AKR in A. thaliana, which consists of 14 subfamilies, of which the AKR4C subfamily is able to reduce the level of RCS using NADPH (Saito et al., 2013). The GLO system, consisting of three enzymes, GLO I, GLO II and GLO III (Benov et al., 2004), is the major pathway for MG detoxification in plants.
GLO I belongs to the metalloenzyme class known as adjacent oxygen chelatases, characterized by an ancient βαβββ motif necessary for binding metal ions (Deponte, 2013; He et al., 2020). Based on the metal ion specificity, GLO I can be categorized into Ni2+/Co2+-dependent and Zn2+-dependent groups (Kaur et al., 2013). In contrast, no such classification exists for GLO II, and the crystal structures of GLO II proteins from humans and A. thaliana indicate that they are monomeric, consisting of an N-terminal β-lactamase structural domain with conserved Fe(II) Zn(II) centers in the active site, and a C-terminal structural domain with five α-helices (Cameron et al., 1999; Deponte, 2013). These metals are important not only for regulating enzyme activity but also for substrate binding. GLO I and GLO II are dependent on GSH. The function of GSH depends on the reactivity of the cysteamine thiol group, which forms a complex with metals and can be alkylates to a thioether or oxidized to a disulfide to form GSSG. The detoxification system begins with spontaneous non-enzymatic reaction of GSH with MG to form a hemithioacetal (HTA) (Figure 2), which is irreversibly isomerized by GLO I to S-lactoylglutathione (SLG) further converted by GLO II (with a high degree of specificity for the GSH portion of the substrate) to lactic acid and GSSG (Thornalley, 1990). In Arabidopsis, overexpression of GLO I increases plant tolerance to salinity and helps maintain lower levels of MG (Dorion et al., 2021) compared to wild-type plants. In tobacco, ectopic expression of OsGLO II-2 increases plant tolerance to salinity and MG stress (Ghosh et al., 2014). Maintenance of MG and GSH levels, as well as higher photosynthetic rates and lower oxidative damage in transgenic plants, are mechanisms that promote plant tolerance to stresses. GLO III, an enzyme that converts MG to lactate without the need for GSH (Figure 2), has higher activity and is more efficient at detoxification of MG than GLO I and GLO II (Okado-Matsumoto & Fridovich, 2000), a topic further discussed below.
6 DJ-1 PROTEIN: A NOVEL PLAYER IN THE GLYOXALASE SYSTEM
DJ-1, an 189-amino acid protein produced by the PARK7 gene in humans, has been linked to familial Parkinson's disease. Homozygous or heterozygous mutations in this gene have been shown to lead to early onset of the disease. DJ-1 is widely expressed in the nucleus, cytoplasm, and mitochondria. In addition to the complex roles that DJ-1 plays in pathophysiology, it also functions at multiple molecular and cellular levels, including molecular chaperone function, transcriptional regulation, protection from oxidative stress, maintenance of mitochondrial function, and deglycation during glycolysis.
6.1 The structure and function of the DJ-1 superfamily
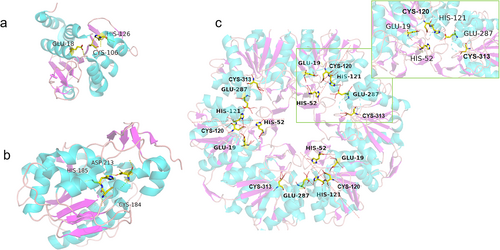
The DJ-1 superfamily is widely distributed in nature, with the core structure and specific active sites conserved in humans, bacteria, and plants. Except for the extended and terminal regions, the tertiary structures of the members of the DJ-1 superfamily are similar (Görner et al., 2007), and all of them share a core α/β sandwich fold (Bonifati et al., 2003), also known as the α/β hydrolase fold. This structure is highly conserved, with a motif called the “ribonuclease elbow”, which carries an invariant cysteine residue, Cys106, in the human protein (Holmquist, 2000). This residue plays a central role in protease/peptidase activity by acting as a nucleophile (Lee et al., 2003), and its oxidation is critical for the cytoprotective activity of DJ-1 (Canet-Avilés et al., 2004; Neumann et al., 2004). In the vicinity of Cys106 in the active site, a glutamate residue Glu18 (or Asp18 in some homologs) is responsible for lowering the pKa value of Cys106, facilitating its oxidation (Witt et al., 2008).
Despite sharing similar tertiary structures, DJ-1 superfamily members exhibit diverse biochemical activities. It has been shown that human DJ-1 has chaperone activity (Lee et al., 2003), whereas Hsp31 from E. coli has both peptidase activity and chaperone activity (Fu et al., 2023; Mujacic & Baneyx, 2007). Conversely, YhbO from E. coli and DR1199 from Deinococcus radiodurans lack both chaperone and peptidase activities, although they are closely related to human DJ-1 and E. coli Hsp31(Abdallah et al., 2006; Abdallah et al., 2007). The reason for this functional diversity is that they have different interfacial regions, which can form diverse dimerization/oligomerization patterns. Members of the DJ-1 superfamily can homo-oligomerize in at least four different binding orientations through interfacial regions I–IV, which directly affects the oligomerization state and function of the protein. As a result, DJ-1 proteins can be classified into four major types. The dimerization form generated from interface I is called the DJ-1 type, and that formed through interface II is called the YhbO type. Regions III and IV play unique roles in the dimerization pattern within the DJ-1 superfamily; in Hsp31 proteins, this N-terminal extension contains about 45 amino acid residues, a structural feature crucial for the formation of its dimerization contacts. Moreover, Hsp31 possesses an insert near regions I and II, which provides an additional structural platform for dimer formation and protein function. Together, these elements form a unique dimerization surface responsible for mediating specific protein–protein interactions. The form of dimer produced by region IV is referred to as the YDR type. The dimer interface of region IV is completely different from the other types in the DJ-1 superfamily. In the yeast YDR533Cp protein, this region consists of β7, β8 and β9 strands.
Therefore, DJ-1 superfamily members exhibit diverse functional capabilities, in part through variation of the stoichiometry of the complexes they form (Pei et al., 2000). DJ-1 proteins usually form dimers or hexamers in vivo, and this oligomerization state is critical for their stability and biochemical activity (Kim et al., 2005; Wei et al., 2007). It has been shown that dimerization of DJ-1 helps to maintain its active structure, thus ensuring the proper execution of its function. For example, the dimerized form of certain DJ-1 proteins is required for their antioxidant response and molecular chaperone functions. Altered oligomerization states (e.g., abnormal aggregation) have been associated with the development of neurodegenerative diseases, underscoring the importance of the correct DJ-1 oligomerization for the maintenance of its normal biological function. Except for interface III, which contains a long N-terminal extension, patches I, II, and IV are all contained within the core structure of the DJ-1 superfamily, suggesting the possibility of higher-order oligomerization through the combination of several interface types. It has been reported that dimerization contacts can form hexamers in YhbO-type proteins and present them in the solution (Jia & Zhang, 2008). Only hexamers exhibit protein hydrolysis activity, and rotational contacts between the two interfaces may be responsible for the assembly of functional hexamers.
6.2 The research focus on DJ-1 protein and MG metabolism
Recent studies on the function of DJ-1 proteins have focused on their ability to detoxify MG and revert MAGEs. The mechanism of action of DJ-1 on MG is complex, and it has triggered a wide range of discussions and controversies. DJ-1 was initially reported to act as a unique GLO III-like activity capable of removing MG and related compounds in a GSH-independent manner (Lee et al., 2012). This could hinder the glycation of important macromolecules by MG while sparing GSH for other reactions in response to oxidative stress. Additionally, there is evidence to support that DJ-1 has only glyoxalase activity, and some studies have shown that human DJ-1 indirectly reduces glycation damage mainly by consuming free MG but does not directly act on glycation adducts (Andreeva et al., 2019; Pfaff et al., 2017). Recombinant DJ-1 converts MG or the related α-dicarbonyl probes into the corresponding α-hydroxy acid products to prevent stable glycation (Coukos et al., 2023). This protective effect of DJ-1 does not require physical interaction with the target protein, providing direct evidence for a GSH-independent glyoxalase rather than a deglycase mechanism.
Subsequent studies raised controversy over the function of the DJ-1 protein. It was reported that DJ-1 removes MG adducts from arginine, lysine, and cysteine side chains and releases deglycated proteins and lactate, thereby directly repairing glycated amino acid residues (Richarme et al., 2015). In addition, Richarme et al. found that DJ-1 and its E. coli homologs can directly deglycate MG-derived nucleotide adducts in the same way(Richarme et al., 2017). Matsuda et al. also found that recombinant DJ-1 was able to protect CoA and β-alanine, its precursor in the CoA synthesis pathway, from MG. L-lactic acid was formed as a byproduct instead of D-lactic acid produced by traditional glyoxalase, supporting the direct deglycation activity of DJ-1 (Matsuda et al., 2017). Based on these findings, human DJ-1 is currently classified as a “deglycase” in several major databases such as UniProt and NCBI (Figure 4). X-ray crystallographic studies of DJ-1 with its inhibitors (Tashiro et al., 2018) map the active site pocket near the protein surface, a structural layout that would allow DJ-1 to exclusively recognize and interact with the glycation site. This specific binding mode is suitable for the selective removal of specific glycation modifications without interference from other parts of the substrate. Therefore, DJ-1 can efficiently recognize and remove specific glycation modifications caused by MG without broadly interacting with large portions of a substrate macromolecule. This structural property is the key functional basis of DJ-1 as a putative deglycase, enabling it to target modified proteins or other biomolecules for precise repair.
These studies collectively demonstrate that DJ-1 can protect against glycation of proteins and nucleic acids. However, most DNA repair pathways and enzymes are typically substrate-specific, and the finding that DJ-1 can repair a wide range of structurally altered substrates, including carbonylated nucleotides, DNA/RNA, amino acids, and even proteins, is striking. It is also noteworthy that glyoxalase directly converts free reactive carbonyl groups like MG via a chemical reaction rather than acting on already formed glycation modifications. In contrast, deglycases usually remove glycation modifications directly from proteins or other biomolecules, thereby reversing them to their unmodified state, such as proteins modified by MG. Therefore, the mechanism of DJ-1 function in repairing MG by mainly targeting glycated adducts or free small molecule aldehydes, has become an important point of debate. In other words, is DJ-1 a GLO III or a deglycase?
Although the debate is ongoing, this complexity highlights the potential versatility of DJ-1 in the cell through both direct repair of glycation adducts and an indirect effect on the glycation status by scavenging free MG. Further studies are needed to clarify these mechanisms and to explore the specific role of DJ-1 in different organisms, cells and their responses to stress and disease.
6.3 DJ-1 family in plants
Recently, it has been reported in plants that the DJ-1/PfpI superfamily in A. thaliana comprises six genes that share similarities with the recently discovered bacterial and animal glyoxalases, each possessing two tandem DJ-1/PARK7 domains in A. thaliana while only one DJ-1/PARK7 domain in bacterial and animal (Kwon et al., 2013). Among them, each homolog differs in function. In these Arabidopsis DJ-1 homologs, there are two complete DJ-1/PARK7 polypeptide chains, which all contain functionally conserved DJ-1/PARK7 amino acid residues, including specific residues associated with Parkinson's disease (PD) in humans. Comparative analysis of amino acid sequences has revealed the occurrence of a gene duplication event to create two tandem DJ-1/PARK7 domains in A. thaliana. During the process of evolution, such gene duplication events provide the opportunity for the two DJ-1/PARK7 domain copies to diverge functionally: one copy may retain its original function, while the other may evolve to acquire entirely new functions. AtDJ-1A is an oxidative response protein that improves plant tolerance to abiotic stress by activating SOD (Xu et al., 2010). AtDJ-1B is a bifunctional protein that is sensitive to H2O2 and has a glyoxalase enzyme (Lewandowska et al., 2019). AtDJ-1C is localized to chloroplasts and is essential for plant growth and development but lacks the conserved Cys residue, resulting in no glyoxalase activity (Lin et al., 2011). AtDJ-1D contains a hemi-sulfide acetal, which is typical of GLO III (Choi et al., 2014b), and is also a stable deglycase (Prasad et al., 2022). AtDJ-1D is widely expressed in seeds, roots, leaves, and pollen (Schmid et al., 2005). It was hypothesized that AtDJ-1D may be a major detoxification enzyme in A. thaliana (Kwon et al., 2013). Since AtDJ-1E and AtDJ-1F do not have catalytic residues in their C-terminal domains, they exhibit very low enzyme activities, so their exact functions are unknown. In addition to Arabidopsis, the DJ-1 protein in date palm has also been functionally validated for the detoxification of MG. Overexpression of PdDJ-1 in Arabidopsis may negatively affect MG homeostasis, inhibiting the photosynthesis machinery and plant functions (Jana et al., 2021).
It is worth noting that DJ-1 proteins may be involved in plant stress responses by affecting the stability, localization, or activity of Nonexpressor of Pathogenesis-Related 1 (NPR1) and dehydration-responsive element binding proteins (DREBs). NPR1 is a key regulator in plant defence responses, activating the expression of a series of defence genes mainly by regulating the signalling pathway mediated by salicylic acid (Zavaliev et al., 2020). DREBs play a central role in plant responses to abiotic stresses such as drought, low temperature, and high salinity, enhancing stress tolerance by specifically binding to dehydration-responsive elements (DREs) in target genes to regulate their expression levels (Lata & Prasad, 2011). This is similar to the mechanism by which DJ-1 in animal cells responds to oxidative stress by regulating nuclear factor erythroid 2-related factor 2 (Nrf2) activity (Zhao et al., 2021). DJ-1 may enhance plant tolerance to abiotic stress by regulating intranuclear translocation and/or stability of NPR1 or regulating the activity of DREBs, thereby modulating plant disease resistance responses. Such regulation may involve direct binding of DJ-1 to these transcription factors or PTMs, such as redox, phosphorylation or protein proteolysis. With the fast advancement of proteomics technologies (Couto-Rodríguez et al., 2023; Liu et al., 2022; Perron et al., 2022), addressing these overarching hypotheses will greatly advance the field of plant biology, and specifically the progress in plant DJ-1 and MG research.
7 SUMMARY AND PERSPECTIVES
MG functions as a signalling molecule or a toxicant, depending on its concentration and cellular context. It can be generated by glycolysis, protein Amadori rearrangement, and lipid peroxidation. Under physiological conditions, MG may function through other signalling pathways, e.g., hormones. It can readily glycate macromolecules such as proteins, nucleic acids, and phospholipids to produce AGEs, which are irreversible. AGEs reflect and exacerbate oxidative stress, inflammation, and disease state in cells. Cellular MG levels are influenced by a variety of factors, and salt stress causes an increase in MG levels. Accumulation of H2O2 in oxidative stress tends to decrease the GSH/GSSH ratio, which triggers S-glutathionylation and inhibition of GADPH activity, resulting in a further increase in MG levels. To respond to stress conditions, organisms have MG detoxification mechanisms, e.g., the glyoxalase systems that function in both GSH-dependent and -independent manner.
Human DJ-1 is closely associated with Parkinson's disease, and its homologs have multiple well-documented functions (oxidative stress response, transcriptional regulation, mitochondria maintenance, etc.) in animals and microorganisms. The structural complexity of DJ-1 supports its diverse functionality. In spite of the ongoing debate, DJ-1 appears to function as both a novel GLO III and a deglycase in the detoxification of MG. In plants, the study of MG and glyoxalase systems is still in its infancy. The potential interactions of DJ-1 with NPR1 and DREBs are very exciting. Research in this direction will not only advance a deep understanding of the role of DJ-1 in plant functions and stress responses but also provide a theoretical basis and approaches for enhancing crop resilience in the changing climate.
The dual role of MG suggests that it needs to be tightly regulated and compartmentalized in cells and organisms. Such regulation appears to involve multiple mechanisms and pathways, forming a molecular network. Elucidation of this sophisticated MG regulatory network involving key nodes of ROS, GSH and GLOs/DJ-1, and key edges of glycolysis, redox and PTMs is an exciting frontier in future biological and medical research.
AUTHOR CONTRIBUTIONS
Yutong Sun conceived the idea and contributed to writing the initial draft and preparing the figures. Haiying Li, Sixue Chen, Bing Yu, Inga R. Grin, and Dmitry O. Zharkov provided invaluable insights through their meticulous editing and comprehensive review of the draft. Each author made significant contributions and gave their approval to the final manuscript, ensuring its readiness for publication.
ACKNOWLEDGMENTS
This work was supported by Heilongjiang Provincial Key Laboratory of Plant Genetic Engineering and Biological Fermentation Engineering for Cold Region. The authors thank Daniel Chen from the MD program of the University of South Florida Morsani College of Medicine for critical reading and editing of the manuscript.
FUNDING INFORMATION
This research was funded by International Cooperation and Exchange of the National Science. Foundation of China Project (32261133530) and the National Science Foundation of China Project (32072122) and the Russian Science Foundation (23–44-00050).




