Physiological and transcriptomic characterization of cold acclimation in endodormant grapevine under different temperature regimes
Abstract
It is essential for the survival of grapevines in cool climate viticultural regions where vines properly acclimate in late fall and early winter and develop freezing tolerance. Climate change-associated abnormities in temperature during the dormant season, including oscillations between prolonged warmth in late fall and extreme cold in midwinter, impact cold acclimation and threaten the sustainability of the grape and wine industry. We conducted two experiments in controlled environment to investigate the impacts of different temperature regimes on cold acclimation ability in endodormant grapevine buds through a combination of freezing tolerance-based physiological and RNA-seq-based transcriptomic monitoring. Results show that exposure to a constant temperature, whether warm (22 and 11°C), moderate (7°C), or cool (4 and 2°C) was insufficient for triggering cold acclimation and increasing freezing tolerance in dormant buds. However, when the same buds were exposed to temperature cycling (7±5°C), acclimation occurred, and freezing tolerance was increased by 5°C. We characterized the transcriptomic response of endodormant buds to high and low temperatures and temperature cycling and identified new potential roles for the ethylene pathway, starch and sugar metabolism, phenylpropanoid regulation, and protein metabolism in the genetic control of endodormancy maintenance. Despite clear evidence of temperature-responsive transcription in endodormant buds, our current understanding of the genetic control of cold acclimation remains a challenge when generalizing across grapevine tissues and phenological stages.
1 INTRODUCTION
Climate change, a phenomenon attributed to direct or indirect human activities, is characterized by long-term changes in climate status and unseasonable weather events (Boadu 2016; Raza et al. 2019; Salama et al. 2021). As a consequence, the steady movement of optimum planting zones towards polar regions and more frequent weather extremes poses significant challenges to food production, particularly to the production of perennial crops such as grapevine, due to high reestablishment costs and slow breeding processes (Hong et al. 2020; Leisner 2020). Other than the expected migration of vineyards towards cooler regions and a shift to more adaptive cultivars in the future, the grape industry currently experiences major crop losses due to climate change-associated weather extremes such as extreme drought, precipitation, heat and cold (Schnabel & Wample 1987; White et al. 2006; Molitor et al. 2014; Mozell & Thach 2014; Mosedale et al. 2016; Droulia & Charalampopoulos 2021). Among abiotic stresses, cold-related damage in winter and spring is one of the leading constraining factors for the expansion of viticulture into cool and cold climates. When low temperatures surpass the freezing tolerance of grapevines at any time of the winter, damage can occur to specific organs or tissues such as the buds, phloem, cambium, and xylem, leading to reduced yields in the subsequent season and increased costs of replanting and additional management (Zabadal et al. 2007). The growing prevalence of late spring frosts, attributed to a phenomenon known as false spring (wherein warmer winters lead to early budbreak followed by a sudden frost), poses a major challenge to vineyards worldwide. These late frosts are particularly damaging to the delicate and highly productive primary buds of grapevines, often resulting in substantial losses in yield (Poling 2008; Wang & Dami 2020; Poni et al. 2022). Therefore, understanding adaptations to cold is key to the development of management methods for cold damage for the sustainability of grape and wine production.
To overcome low temperature stress in winter, perennials develop freezing tolerance through cold acclimation (Zabadal et al. 2007). As a three-phase process, cold acclimation is triggered by the short-day photoperiod in late summer and step-wise enhanced by low above-freezing and sub-freezing temperatures (Wake & Fennell 2000; Gusta et al. 2005). In general, the enhancement of freezing tolerance during this process is a largely unresolved consequence of a sophisticated combination of genetic control, metabolism amelioration and physiological adaption (Thomashow 1999; Hincha & Zuther 2020). Genetically, key regulatory pathways or cascades, such as the inducers of CBF Expression – C-Repeat Binding Factor/DRE Binding Factor – Cold Regulated Genes (ICE-CBF/DREB1-COR) cascade (ICE-CBF-COR), the abscisic acid (ABA) signaling pathway, the ethylene signaling pathway, and the jasmonate signaling pathway are activated to initiate the expression of functional proteins and metabolites (Shi et al. 2012; Hu et al. 2013; Jiang et al. 2017; Ding et al. 2019; Rubio & Pérez 2019). Metabolically, functional metabolites and proteins such as soluble sugars, proline, reactive oxygen species-detoxification proteins, ice-binding proteins, flavanols and anthocyanins accumulate in cells of overwintering buds (Guy et al. 2008; Korn et al. 2008; Bredow & Walker 2017; Hincha & Zuther 2020). Physiologically, adaptive mechanisms such as cell desiccation, modification of cellular lipid composition, glass formation and supercooling are thought to develop to either avoid or tolerate intracellular ice formation, which in turn enhances freezing tolerance (Gusta & Wisniewski 2013; Arias et al. 2015; Ritonga & Chen 2020). Despite these underlying mechanisms, the development of freezing tolerance in grapevines during cold acclimation is a dynamic process highly dependent on temperature. After the initial onset of cold acclimation in response to short-day photoperiods, grapevines further enhance their freezing tolerance in response to low above-freezing temperatures in early fall, followed by sub-freezing temperatures in late fall (Zabadal et al. 2007; Ferguson et al. 2011; Londo & Kovaleski 2017). Previous observations indicate that the most intensive enhancement of freezing tolerance typically occurs after the first freezing event (Ferguson et al. 2011; Londo & Kovaleski 2017; North et al. 2021; Kovaleski et al. 2023; Wang et al. 2024). In the cold or cool climate viticultural regions in North America, such as the Finger Lakes region of New York State, wine grapes (Vitis vinifera) typically attain a freezing tolerance following harvest of approximately -10°C before mid-October. Freezing tolerance is then progressively enhanced, presumably through a step-wise response to low above-freezing and subsequent sub-freezing temperatures, eventually reaching -22 to -28°C by mid-January, depending on the specific year and cultivar (Kovaleski et al. 2023; Wang et al. 2024). The physiological and transcriptomic mechanisms responsible for acclimation and the change of freezing tolerance remain unknown.
In parallel to cold acclimation, dormancy status also shifts from paradormancy during the growing season (inhibition of growth caused by apical dominance) to endodormancy (inhibition of growth caused by internal molecular constraint) (Lang 1987). Endodormant buds in woody perennials remain unbroken even under growth-permissive conditions, until transitioning to an ecodormant state (inhibition of growth due to environmental conditions), which occurs after being exposed to sufficient chilling length (Campoy et al. 2011; Kovaleski 2022). The response to chilling accumulation varies among Vitis species (Londo & Johnson 2014). Although many studies have explored aspects of endodormancy, the mechanism of growth inhibition is not understood. In some fruit crops, DORMANCY-ASSOCIATED MADS-box genes (DAMs) have been noted for their potential functionality during endodormancy. For example, a locus related to the deletion of tandemly repeated DAMs resulted in the loss of dormancy in ever-growing peach (Bielenberg et al. 2008). In Prunus persica, the expression of DAM1-4 peaked at bud set and might function during the initiation of endodormancy, while the expression of DAM5 and DAM6 peaked at the start of endodormancy and steadily decreased when exposed to prolonged chilling, suggesting their potential function in maintaining endodormancy (Li et al. 2009; Yamane et al. 2011; Yu et al. 2020). Functional studies suggest that the conservation of the amphiphilic repression motif in tandemly arrayed DAMs might lead to direct transcription repression, and DAMs might also interact with other dormancy-promoting hormones or proteins (Sasaki et al. 2011; Tuan et al. 2017; Wang et al. 2020b). Recent studies reported that the regulation of DAM expression is achieved by chromatin covalent modifications, DNA methylation or miRNA, suggesting the involvement of epigenetic control in DAM-mediated dormancy (Niu et al. 2016; Quesada-Traver et al. 2020; Rothkegel et al. 2020). However, orthologs of the DAM genes noted above have not been well-defined or are absent from the current grapevine genome annotation. Homologs of flowering time control genes (FTC) in A. thaliana and other model species were identified in grapevine, and some of these genes, including some MADS-box genes, were reported to be highly expressed during dormancy and lowly expressed during budbreak and flowering (Kamal et al. 2019). As climate change is predicted to lead to, on average, warmer winters with more frequent extreme warming or freezing events, grapevine survival during the dormant season and proper growth after the dormant season will likely be more challenging regarding the synergy of dormancy maintenance, chilling requirement fulfillment and sufficient freezing tolerance (Luedeling et al. 2011; Luedeling 2012; Mosedale et al. 2016; Salama et al. 2021). A thorough understanding of grapevine physiology and genetic control during cold acclimation and endodormancy, along with the alteration of these mechanisms under different temperature regimes, is urgently needed.
In this study, we conducted two experiments to investigate grapevine cold acclimation in endodormant buds under different temperature regimes. Given the observed relationship between low temperatures and the acquisition of freezing tolerance, our experiment tested the hypothesis that treatment of endodormant buds at low temperatures induces acclimation and increases freezing tolerance. To test this hypothesis, we exposed dormant grapevine cuttings to five stable temperatures ranging from 2 to 22°C and assessed changes in freezing tolerance over 10 weeks. A second experiment further assessed the role of temperature on acclimation by testing the effect of diurnal temperature cycling. In both time-course experiments, bud freezing tolerance was measured using differential thermal analysis (DTA) in parallel with RNA-seq-based transcriptome characterization to connect phenotypic change (freezing tolerance) with potential underlying genetic control. This work targets the gap of knowledge regarding grapevine cold acclimation and endodormancy and offers insight into developing novel viticultural management methods to sustain grape production in adaptation to climate change.
2 MATERIALS AND METHODS
2.1 Plant material
Dormant cuttings collected from field-grown V. vinifera ‘Cabernet Sauvignon’ cultivated at ‘Ravine's Wine Cellars’ in Geneva, NY (42.845° N, 77.004° W) were used for experimentation. ‘Cabernet Sauvignon’ was grafted on 3309C rootstock and subjected to standard vineyard management. Canes were collected on 26 October 2016 when grapevines were endodormant (chilling accumulation = 398 h, Utah model (Linvill 1990) and at Eichhorn Lorenz Stage 1 (EL1 – winter bud) (Eichhorn & Lorenz 1977). The first freezing event of this particular season occurred on 27 October 2016, and the second freezing event occurred on 28 October 2016. Thus, the grapevine cuttings used in this experiment had not experienced any sub-freezing temperatures before sample collection (Figure 1). After sample collection, canes were chopped into single-node cuttings, randomized, and placed in cups with cut ends in water. The cuttings were stored in a walk-in cold room at 7°C for 1 d until being used for experimentation. During the experiments, water was added to the sample cups periodically to maintain humidity.
2.2 Freezing tolerance measurement
Differential thermal analysis (DTA) was used to determine grapevine bud freezing tolerance following standard protocols (Mills et al. 2006; Londo et al. 2023). To perform a DTA, buds were excised along with surrounding tissue from single bud cuttings, loaded into thermoelectric modules in a programmable freezer, and subjected to a freezing rate of 4°C/h from 0 to -50°C. The release of heat during tissue freezing, a phenomenon known as the low temperature exotherm (LTE) was recorded via a Keithley 2700 data logger (Tektronix, USA). The temperature at which LTE occurs corresponds to the freezing tolerance of the grapevine bud (Pierquet & Stushnoff 1980; Londo et al. 2023). Freezing tolerance was determined for each treatment and collection timepoint (as described below) by recording the LTE values for five biological replications.
2.3 Experiment 1: cold acclimation under different stable temperatures
Single-node cuttings were divided into five groups, and each group was subjected to a temperature treatment in growth chambers: 22, 11, 7, 4 and 2°C. As the impact of temperature on grapevine cold acclimation is the major focus of this experiment, these treatments were set at stable temperatures without any diurnal fluctuation or light due to variable light controls across the five chambers and to avoid any confounding effect of light exposure. Previous studies to model grapevine dormant season freezing tolerance determined that temperature could be used as the only input to generate accurate prediction (Ferguson et al. 2014; North et al. 2021; Kovaleski et al. 2023; Wang et al. 2024), suggesting that temperature is the key driver of the dynamics of grapevine dormant season freezing tolerance. Similarly, treatments were restricted to temperature exposure above freezing due to growth chamber temperature limits and humidity controls. Sample collection for freezing tolerance assessments and RNA-seq was conducted between 10:00 am and 12:00 pm from growth chambers at eight sample times: at 7 d, 13 d, 20 d, 26 d, 34 d, 41 d, 55 d and 69 d of growth chamber temperature exposure.
2.4 Experiment 2: cold acclimation under temperature cycling
Experiment 2 was conducted in parallel with Experiment 1 but was temporally separated into two phases: phase I and phase II. The schematic presentation of the experiment is shown in Figure S1. During phase I, temperature exposure of the cuttings was held stable at 11, 7, or 2°C in the same growth chambers used in Experiment 1, starting at 0 d after field collection and continuing to 26 d after field collection. Following the 26-d freezing tolerance and RNA collection timepoint, a subset of cuttings were moved from each of the 11, 7, and 2°C stable treatments and placed into a shared growth chamber where the mean temperature was maintained at 7°C with a diurnal cycling of ±5°C (max = 12°C, min = 2°C). The cuttings were held at each temperature for 6 h, creating a 10°C oscillation analogous to daily temperature fluctuations (6 h at 2°C, 6 h at 7°C, 6 h at 12°C, 6 h at 7°C, repeat). The treatment is abbreviated as a ‘7±5°C’ treatment. Cuttings were maintained in the cycling chamber until 69 d after field collection. Sample collection for freezing tolerance assessments and RNA-seq were conducted in parallel with Experiment 1. Sample collection of the 7±5°C treatment and stable temperature treatments was conducted simultaneously over the experiment, and during sample collection, the temperature of the 7±5°C treatment chamber was 7°C.
2.5 RNA-seq library preparation and data processing
At each collection time point, bud samples were excised from single-bud cuttings and flash-frozen in liquid nitrogen. Three biological replicates, each consisting of five pooled buds, were collected per treatment. Frozen bud samples were stored at -80°C until extraction. The SpectrumTM Plant Total RNA Kit (Sigma Aldrich, USA) was used to extract total RNA from ground bud tissues. Cornell University Institute of Genomic Diversity (Ithaca, USA) provided technical support for library construction using Lexogen QuantSeq 3'mRNA-Seq Prep Kit (Lexogen, USA) following the manufacturer protocol. Sequencing of libraries was conducted at Cornell University Institute of Biotechnology (USA) using NextSeq500 (Illumina, Inc., USA) with 95 samples per lane. The read length was 85 bp, and sequencing was replicated three times in each library for technical replication.
As the first step of RNA-seq data processing, FastQC was applied to each library for quality control. BBDuk (Nordberg et al. 2014) was used to remove poly-A and adaptors following the default standard pipeline. STAR (Dobin et al. 2013) was used for the alignment of trimmed sequences against the V. vinifera 12X.v2 genome and VCost.v3 annotation (Canaguier et al. 2017). Transcript quantification at the gene level was accomplished via ‘-quantMode GeneCounts’. In each experiment, all gene counts (as rows) in all samples (as columns) were merged as a gene count matrix. Low-count genes (total gene count < total sample count) were excluded, and the remaining matrix was analyzed and normalized in DESeq2 (Love et al. 2014). In Experiment 1, the full model of DESeq2 contained days in treatments and treatment temperatures as continuous variables. In Experiment 2, the full model of DESeq2 contained the interaction of phase I treatment and phase II treatment as a discrete variable. Variance stabilization transformation (VST) and gene expression normalization were also conducted in DESeq2 for downstream gene co-expression network analysis and gene expression visualization, respectively.
2.6 Outlier filtering with PCA and gene co-expression network analysis with WGCNA
In each experiment, VST counts of all genes were analyzed using principal component analysis (PCA) to detect outliers through ‘prcomp’ (scale = True, which performs z-score normalization) in R package ‘factoextra’ (Kassambara & Mundt 2020). Samples showing apparent deviation from the main sample cluster in the first three principal components (PCs) were identified as outliers and excluded from the gene count matrix for downstream analysis. DESeq2 analysis was conducted again with the filtered gene count matrix using the same models, and resulting VST gene counts were used as input for weighted gene co-expression network analysis (WGCNA) (Langfelder & Horvath 2008). The co-expression network was constructed using the remaining samples and genes with ‘blockwiseModules’ (power = 12, networkType = "signed", TOMType = "signed", minModuleSize = 50, reassignThreshold = 0, mergeCutHeight = 0.25).
2.7 Identification of target co-expression modules and target DEGs
After the identification of co-expression modules through WGCNA, gene members in module ‘grey’ represented “noise” and were excluded from any downstream analysis. In each remaining module, module eigengenes (MEs), were tested for their correlation to each variable in the experiment with the Pearson method (Langfelder & Horvath 2008). MEs of samples were also visualized across the experiment. Target co-expression modules were determined based on the p-values of MEs-variable correlation and the identification of reasonable patterns in ME visualization per interest of the experiment (e.g., temperature effect in Experiment 1 or 7±5°C effect in Experiment 2). The genes in target modules were further subjected to correlation analysis (e.g., correlation of gene expression with temperature in Experiment 1) or contrasting (stable vs. 7±5°C in Experiment 2) in DESeq2. The genes with FDR (False Discovery Rate) < 0.05 in correlation analysis or FDR < 0.05 and log2 fold change (LFC) > 2 in DESeq2 were identified as target differentially expressed genes (DEGs) and were subjected to downstream analysis.
2.8 Pathway enrichment analysis with GSEA
Pathway enrichment analysis of target DEGs was conducted using gene set enrichment analysis (GSEA) (Subramanian et al. 2005). To obtain better functional annotation, the identification of target genes was derived from their CRIBI V1 annotation (http://genomes.cribi.unipd.it/grape), and gene models without V1 annotation were excluded for GSEA. The remaining genes were pre-ranked using the FDR from correlation analysis or contrasting in a decreasing order. The pre-ranked gene list was transformed into a.rnk file as a part of the input for GSEA. Pathway information was obtained from the VitisNet database (Grimplet et al. 2009), and all pathways were combined into a gene set file (.gmt) as another part of the input for GSEA. GSEA was conducted with ‘Run GSEAPreranked’ in weighted mode with 1000 permutations. Normalization mode was set as ‘meandiv’, and no max size or min size was chosen for any gene set.
2.9 Manual curation of top DEGs and genes of interest
In addition to pathways enrichment analysis, we manually investigated the top 200 DEGs (ranked based on LFC) that showed significance in the specific statistical analysis in each experiment, depending on the result of differential expression analysis. In Experiment 1, the top 200 DEGs that showed significant positive or negative correlations with temperatures were examined. In Experiment 2, the top 200 DEGs that showed significance in the contrast of stable temperature vs. 7±5°C were examined. During these manual examinations, when groups of genes were observed in the top 200 DEG list and were also repeatedly associated with known pathways, the DEGs were manually analyzed and visualized for their response to the specific temperature treatments during the experiment. Some previously noted genes of interest (the genes encoding for ICE or CBF/DREB) and some pathways of interest (ABA signaling pathway and carbohydrate metabolism pathways) were also subjected to manual examination.
3 RESULTS
3.1 Grapevine freezing tolerance dynamics at the sample collection site
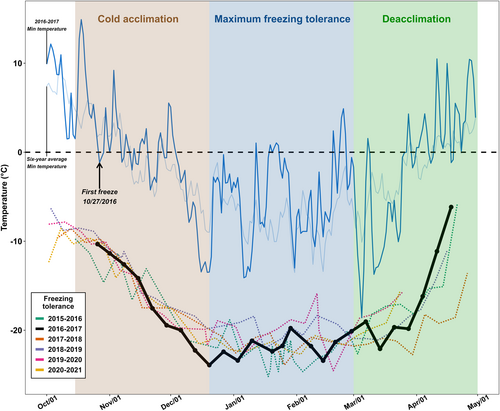
To contextualize the “normal” pattern of cold acclimation and freezing tolerance in ‘Cabernet Sauvignon’ under the climatic conditions in New York, the freezing tolerance of ‘Cabernet Sauvignon’ at the sample collection site was continuously measured over six dormant seasons from 2015-2016 to 2020-2021 (Figure 1). Typically, the minimum air temperature at the sample collection site follows a ‘U’-shaped pattern during the dormant season. This includes a decreasing phase from early October to late December, during which the first freezing event usually occurs between mid-October and early November; a plateau phase from late December to late February, where the minimum air temperature stabilizes around -5 to -10°C with occasional lower temperature events; an increasing phase from early March to the end of the dormant season, where the minimum temperature gradually rises (Figure 1). Correspondingly, the freezing tolerance of ‘Cabernet Sauvignon’ also exhibits a ‘U’-shaped pattern: a decreasing phase (indicating a gain of freezing tolerance) from approximately -10 to -23°C between mid-October and late December, representing cold acclimation; a stable phase between late December and March, representing the maintenance of maximum freezing tolerance at -23 to -25°C; and an increasing phase (indicating the loss of freezing tolerance) from March to the end of the dormant season, representing deacclimation (Figure 1). Based on the observations over these six dormant seasons, most of the freezing tolerance development in ‘Cabernet Sauvignon’ occurs between mid-October (or after the first freezing event) and late December (Figure 1).
During the 2016-2017 dormant season, the freezing tolerance of ‘Cabernet Sauvignon’ at the sample collection site on the date of sample collection (26 October 2016) was -10.3°C (Figure 1). The first freezing event occurred one day later (27 October 2016), and evidence of field cold acclimation is apparent in weekly assessments of freezing tolerance (Figure 1). As the winter season progressed, field freezing tolerance of ‘Cabernet Sauvignon’ continued to increase, reaching a maximum of -23.9°C by mid-December, and remained relatively stable during the mid-winter before deacclimating in mid-March (Figure 1).
3.2 Experiment 1: Cold acclimation in response to stable temperatures
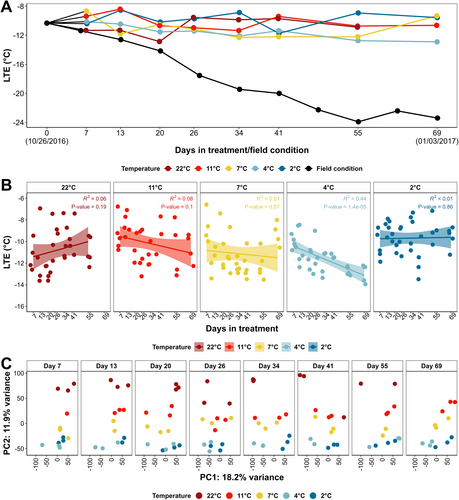
All buds remained dormant and unbroken during Experiment 1, except for the buds treated at 22°C at 69 d post-treatment, which were removed from the analysis. The freezing tolerance of buds from each collection is represented by the LTEs detected in DTA. The mean LTE of the buds of each treatment × timepoint during the experiment, along with the LTEs of the buds under field conditions, are shown in Figure 2A. The LTEs of each treatment were analyzed by the Pearson method for correlations with days in treatment (Figure 2B). The mean LTEs of all temperature treatments remained bounded within the range of -8.5 to -12.9°C during the experiment (Figure 2A and B). Tukey's HSD test of the temperature treatments at each sample collection timepoint indicates that no single temperature treatment induced consistently higher freezing tolerance throughout the experiment (Figure 2A and Table S1). In contrast, the mean LTE values recorded from field-collected material continuously decreased from -10.3 to -23.4°C between 26 October 2016 and 03 January 2017, corresponding to 0 d post-treatment and 69 d post-treatment in the growth chamber experiment (Figure 2A).
3.3 Experiment 2: Cold acclimation in response to diurnal temperature cycling
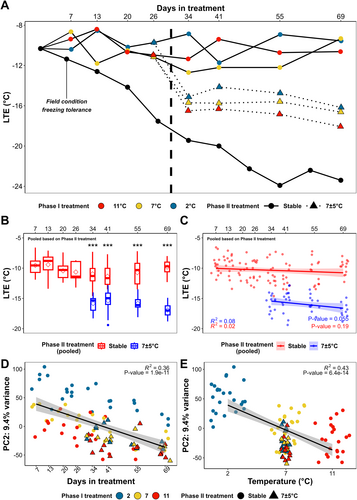
All buds remained dormant and unbroken during Experiment 2. The mean LTEs of the buds of each phase I treatment × phase II treatment × timepoint during the experiment, along with the LTEs of the buds under field conditions, are shown in Figure 3A. The mean LTEs of the buds treated with stable temperatures of 11, 7 and 2°C remained unchanged within the range of -8.7 to -12.7°C throughout the experiment (Figure 3A). In comparison, buds treated with 7±5°C in phase II acclimated to a lower range of mean LTE, -14.1 to -18.0°C, during phase II regardless of their previous phase I treatment (Figure 3A). There were no significant differences between LTE values for each collection in phase II, regardless of the original temperature treatments in phase I. Thus, LTEs were pooled based on phase II treatments (Figure 3B) and contrasted. For example, the samples in stable temperature treatments (11, 7 and 2°C) were pooled together, and the samples under 7±5°C treatment were pooled together regardless of phase I treatments. The LTEs of phase II stable treatment and phase II 7±5°C treatment were significantly different (p < 0.0001) at all the sample collections in phase II, indicating a differentiated phenotype of greater freezing tolerance during phase II. Pooled LTEs were also analyzed for their correlations with days in treatment in phase II (Figure 3C). The correlations are not significant, and the R2s are minimal (Figure 3C), indicating that the freezing tolerance of pooled samples did not change during phase II following the initial enhancement that occurred between 26 d and 34 d when samples were moved to the cycling temperature treatment.
3.4 RNA-seq data statistics
In total, 156 libraries were sequenced in the two experiments of this study. All libraries passed the FastQC per base quality test, indicating good quality of the raw reads. Mean read length per sequence of raw reads was 85 bp. After trimming to remove adaptor barcodes, the mean read length was 79 bp. The reads per library averaged 4.2 million and the uniquely mapped rate per library averaged 78.9%. One library, a replicate of phase I 2°C × phase II 7±5°C in Experiment 2, showed significantly lower uniquely mapped rate at 10.59% and was identified as an outlier in the sample PCA analysis and removed.
In Experiment 1, the gene count matrix was composed of 120 samples and after low-count filtering, and 16,346 of the total 42,413 genes (39%) in the VCost.v3 annotation were detected. These genes were analyzed by DESeq2 to generate VST counts. PCA of VST counts identified six outliers (Figure S2) in Experiment 1, and these outliers were excluded. The remaining genes and samples were subjected to downstream analysis. After the outlier filtering, only a single library from the buds treated under 11°C for 7 d was retained. However, since the systematic gene expression analyses in our study, such as PCA and WGCNA, required examining all libraries, and other specific analyses, such as correlation analysis with time or temperature in Experiment 1 and cycling vs. stable conditions in Experiment 2, required comparing specific groups of libraries, no direct comparisons were made between this specific treatment and timepoint combination and others. The statistical validity of the results should not be compromised by having only one library for this specific treatment and timepoint combination.
In Experiment 2, the gene count matrix comprised 111 samples and after low-count filtering, 16,028 genes (38%) were detected and analyzed by DESeq2 to generate VST counts. PCA of VST counts identified six outliers, including the library that showed significantly lower uniquely mapped rate (Figure S3) in Experiment 2, and these outliers were excluded. The remaining genes and samples were subjected to downstream analysis.
3.5 Transcriptomic analysis of temperature effects on cold acclimation
While no phenotypic change in freezing tolerance was observed during Experiment 1, clear differences in the transcriptome were observed that correlated with the different growth chamber temperatures. Initially, transcriptome characterization was conducted on VST counts of the detected genes through PCA. The top two PCs, PC1 and PC2, explain greater than 30% of the total variance, and the PC values of all the remaining samples are shown across the experiment in Figure 2C. Correlation analysis of PC value vs. temperature indicates that PC1 of the samples does not correlate with either treatment temperature or days in treatment (Figure S4A and B), whereas PC2 of the samples significantly correlate with treatment temperatures (Figure S4C).
After low count filtering, VST counts of detected genes (16,346 genes) in Experiment 1 were examined with WGCNA to construct a gene co-expression network and examine gene co-expression modules. In total, 24 gene co-expression modules (excluding module ‘grey’) with a number of genes ranging from 63 to 2,950 were identified. The MEs were tested for correlation with days in treatment and temperature and were visualized across the experiment (Figure S5). Among all, MEs ‘brown’, ‘cyan’ and ‘darkturquoise’ showed the most significant positive correlations with temperature (Figure 4A and B). MEs ‘red and ‘grey60’ showed the most significant negative correlations with temperature (Figure 4A and B). Thus, MEs ‘brown’, ‘cyan’, ‘darkturquoise’, ‘red’, ‘grey60’ were selected as target modules (temperature responsive modules) for further analysis. The 2,985 genes in these modules were analyzed for a correlation between expression and treatment temperature in DESeq2, and 2,856 genes with an FDR < 0.05. Among these 2,856 target DEGs, 1,798 were positively correlated with temperature, while 1,058 were negatively correlated with temperature. A full list of these temperature-responsive genes is available in Supporting Material 2.
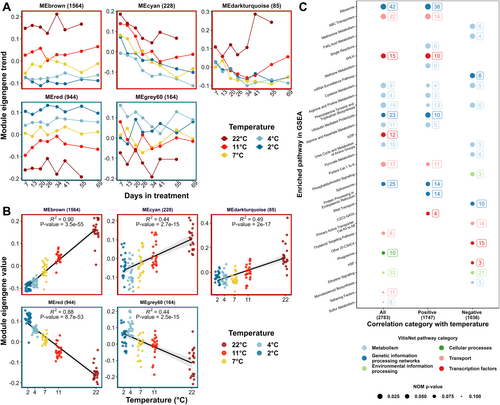
Target DEGs were cross-referenced with their corresponding CRIBI V1 annotation for pathway enrichment analysis. Three gene lists were generated based on their correlation categories with temperature (all, positive, or negative). Pre-ranked GSEA was separately conducted on three gene lists after ranking genes based on their correlation FDR. The results of the GSEA are shown in Figure 4C. The number of significantly enriched pathways (NOM p-value < 0.1) was 17, 14, and 14 in the gene lists for all genes, the genes positively and negatively correlated to temperature, respectively (Figure 4C). These pathways comprise all major pathway classifications in VitisNet: Metabolism (12), Genetic information processing networks (6), Environmental information processing (2), Cellular processes (1), Transport (5) and Transcription factors (5) (Figure 4C).
The top 200 DEGs that showed significant negative correlations with temperature were used to identify transcriptomic response to low temperature in endodormant grapevine buds. Among these 200 DEGs, 147 were functionally annotated. Two pathways, phenylpropanoid biosynthesis (vv10940, VitisNet) and galactose metabolism (vv10052, VitisNet) were repeatedly represented among the top DEGs. The expressions of these DEGs in Experiment 1 and Experiment 2 are shown in Figure S6. We also investigated the top 200 DEGs that showed significant positive correlations with temperature, but no pathway was repeatedly represented among these DEGs.
We next examined the structure of transcriptomic variation in response to diurnal temperature cycling (Experiment 2), and PCA was applied to VST counts of all genes after low count filtering (16,028 genes) in Experiment 2. PC1 and PC2 of all samples explain more than 27% of the total variance, and the PC values of these two PCs across the experiment are shown in Figure S7. No apparent treatment effect can be identified in PC1 (Figure S7). Correlation analysis of PC2 and mean treatment temperature (mean treatment temperature of temperature cycling treatment in phase II is 7°C) and days in treatment indicates that PC2 is not only negatively correlated with mean treatment temperature but also negatively correlated with days in treatment (Figure 3D and E). Among the top 100 genes with the greatest positive factor loading for PC2, 56 were categorized as temperature-responsive genes in Experiment 1. Among the top 100 genes with the most negative factor loadings for PC2, 38 were categorized as temperature-responsive genes in Experiment 1. Therefore, the variance in PC2 is likely affected by the genes showing a correlation with temperature.
The temperature-responsive genes (2,856 genes) identified in Experiment 1 were also examined for their behavior in Experiment 2 to detect if these genes were differently regulated in the cycling treatment. PC1 and PC2 of all samples explain more than 35% of the total variance (Figure S8A). Correlation analysis of these PC values vs. mean treatment temperature and days in treatment demonstrates that PC1 of the samples correlated with mean treatment temperature, whereas PC2 of the samples significantly correlated with days in treatment (Figure S8B and C).
VST counts of detected genes (16,028 genes) after low count filtering in the temperature cycling experiment were subjected to WGCNA to construct a gene co-expression network and detect gene co-expression modules. In total, 21 gene co-expression modules (excluding module ‘grey’) with the number of genes ranging from 74 to 3,628 were identified. The MEs were tested for correlations with phase II treatment, days in treatment, and mean treatment temperature and were visualized across the experiment (Figure S9). Phase II treatment was transformed to a dummy variable (7±5°C as ‘1’, stable as ‘0’) to facilitate correlation analysis. Although multiple MEs show significant correlations with days in treatment and mean treatment temperature, very few MEs (e.g., ME ‘black’, ‘royalblue’ and ‘greenyellow’) reveal significant correlations with phase II treatment, and the p-values of the significant correlations are high (Figure S9A). Based on ME visualization, the significance identified in these MEs-phase II treatment correlation analysis appears to be due to the segregation of the MEs of phase I 2°C treatment samples from the other samples, or the time effect that differentiates phase I and phase II samples (Figure S9B). Thus, no target module specifically associated with the phenotypic shift in freezing tolerance was identified in the experiment. Instead, we conducted a contrast (stable vs 7±5°C) on all genes after low-count filtering using DESeq2. This contrast resolved 87 genes with FDR < 0.05 and LFC > 2. The expressions of the 65 functionally annotated genes among these genes are shown in Figure S10. Due in part to the limited number of DEGs in this contrast, pathway enrichment analysis did not identify any overrepresented pathways associated with the observed shift in freezing tolerance.
4 DISCUSSION
Although the leading threat posed to the survival of grapevine in winter is low temperatures that exceed the lethal threshold, an emerging issue is the prolonged period of warmth in fall because of climate change (Luedeling 2012; De Rosa et al. 2021). As the first potential consequence, cold acclimation, which is progressively induced by low above and below freezing temperatures, may be compromised. Reduced acclimation in grapevine thus increases the susceptibility of grapevines to sudden temperature decreases (Howell 2000; Hincha & Zuther 2020). Secondly, as the accumulation of chilling is reduced under higher temperatures in grapevine (Dokoozlian 1999; Londo & Johnson 2014), prolonged maintenance of endodormancy under such temperatures might lead to unwanted physiological changes in overwintering buds (Kovaleski 2024). However, a comprehensive examination of the physiology and transcriptome of endodormant grapevine under such temperature conditions has not been explored.
The pattern of cold acclimation of ‘Cabernet Sauvignon’ at our sample collection site typically starts in mid-October, and most of the freezing tolerance is gained after the first freezing event. Our experimental material was intentionally collected before the first freezing event of the 2016-2017 dormant season to ensure that field temperatures would not confound the potential to artificially induce acclimation with our growth chamber experiment. The field level of freezing tolerance of the buds collected for this study (mean LTE) was -10.3°C. Maximum field freezing tolerance for these same vines in midwinter was recorded at -23.9, 13.6°C lower than at the start of our experiment. This result clearly indicates that the freezing tolerance of our vines was in the phase of early acclimation and had the potential to acclimate to much lower temperatures if provided with the correct stimulation.
Experiment 1 examined the impact of different stable temperatures from 2 to 22°C on the process of cold acclimation in grapevine endodormant buds with the overall hypothesis that lower temperature exposure treatments would induce the most acclimation and thus increase the freezing tolerance of the dormant buds. As lower temperatures in the field typically result in enhanced freezing tolerance (Zabadal et al. 2007; Davenport et al. 2008; Londo & Kovaleski 2019; De Rosa et al. 2021), we thus hypothesized that the buds in the growth chambers with low, stable temperatures (2, 4, 7°C) would gain freezing tolerance, relative to warm chambers (11, 22°C). Temperature treatments lasted for 69 d, well exceeding the period needed in the field for acclimation to occur (Figure 1), and all buds remained unbroken except for the buds treated at 22°C, which broke at 69 d post-treatment. As it has been shown that grapevine buds with sufficient chilling exposure (complete transition from endodormancy to ecodormancy) break bud within four weeks under forcing conditions (Pérez et al. 2007; Mathiason et al. 2009; Londo & Johnson 2014; Camargo Alvarez et al. 2018), the budbreak of buds in the 22°C treatment in our experiment at 69 d post-collection is likely a consequence of prolonged forcing under high temperatures rather than true transition of dormancy status or dormancy release (Zabadal et al. 2007; Davenport et al. 2008; Londo & Kovaleski 2019; De Rosa et al. 2021). During the experiment, the buds at 22, 11, 7 and 2°C did not demonstrate any improvement in freezing tolerance from the original field collected levels of freezing tolerance, even after 10 weeks of exposure (Figure 2B). LTEs of the buds at 4°C appeared to decrease during the experiment (showed significant negative correlation with time) (Figure 2B). However, this gain of freezing tolerance at 4°C is more likely to be a spurious result given that 1) the gain was not consistently statistically significant across the experiment, 2) there was no gain of phenotype at 2°C, and 3) the gain is minimum compared to field conditions (Figure 2A). Researchers demonstrated a similar lack of cold acclimation in endodormant buds of the cultivar ‘Thompson Seedless’ treated with constant 4°C treatment (Rubio et al. 2019), though over a shorter treatment time of 16 days. Thus, we conclude that exposure to stable temperatures, both warm and cool, is not sufficient to trigger acclimation in endodormant buds. It is possible that the process of acclimation in our experiment was affected by our use of detached single node cuttings, the lack of a below-freezing treatment, or that the experiments were conducted without photoperiod effects. However, the results of Experiment 2 argue against these caveats. By exposing the cuttings to diurnal temperature treatments, we were successful in triggering acclimation with an increase of freezing tolerance for all samples by about 5°C (Figure 3A). This shift in freezing tolerance occurred quickly, within one week of the change in temperature exposure and was triggered by temperature cycling but not exposure to actual freezing temperatures. This result suggests two new pieces of information. First, diurnal temperature variation appears critical to the acclimation process, and second, the gaining of freezing tolerance, at least to the levels we detected, does not require a freezing event. It is clear from examining field freezing tolerance data (Figure 1) that freeze exposure further drives freezing tolerance to levels lower than those detected in our study, and freeze exposure is likely critical to achieving maximal freezing tolerance. Future studies using growth chambers that can achieve much lower temperatures (below freezing) would help determine how both the diurnal cycle and minimum temperature work together to achieve maximum freezing tolerance. It is also interesting that the previous stable temperature exposure (phase I treatment) did not seem to influence the magnitude of the impact of diurnal cycling (phase II treatment) on the artificial acclimation, resulting in increased freezing tolerance. Despite the temperature direction of the oscillation treatment, the change in freezing tolerance resulting from diurnal oscillations from each of our stable temperature treatments was about 5°C. For example, the 11°C chamber went from experiencing 11°C constant temperature exposure to a new minimum temperature of 2°C (a drop of 9°C) and a mean of 7°C. In contrast, the 2°C experienced the opposite change with temperature warming of 10°C. Despite these contrasting directions, they acclimated and converged on the same level of freezing tolerance. This result suggests that the critical aspect of the diurnal cycle is the shifting of temperature itself, not necessarily the direction of the change. The physiological driver of this response is unknown, but our results suggest a mechanism that may involve shifting patterns of gene expression or perhaps changes in membrane fluidity at different temperatures (Los & Murata 2004; Cano-Ramirez et al. 2021). In our study, we only tested a single diurnal temperature oscillation, and it remains unknown if the oscillations with greater or lesser changes in temperature or oscillations that reach minimum temperatures below freezing would be more effective at driving freezing tolerance. Taken together, these results enhance our understanding of the process of cold acclimation in grapevine and suggest that depending on future climate change impacts, acclimation remains a critical portion of winter physiology.
Despite a lack of changes in the acclimation phenotype in Experiment 1, clear differences in the transcriptome were observed in the buds under different treatment temperatures. A total of 2,856 temperature-responsive genes were identified (1,058 and 1,798 genes negatively correlated and positively correlated with temperature, respectively), comprising twenty-eight significantly enriched biological pathways (Figure 4C). Our analysis explored these genes in the context that they are likely a part of temperature sensing and cold response systems in dormant grapevine buds.
4.1 Low temperature-responsive genes
Among the enriched pathways of the genes negatively correlated with temperature (upregulated when temperatures are low), the ethylene signaling pathway contained the greatest number of DEGs (Figure 4C). Ethylene has been reported for its importance in the control of freezing tolerance, however, the role of ethylene is inconsistent among different plant species (Shi et al. 2012; Catalá & Salinas 2015; Sun et al. 2016; Londo et al. 2018; Wang et al. 2021; Hwarari et al. 2022). In our study, eight genes in the ethylene signaling pathway and 13 ERFs were upregulated under low temperatures (Figure 5 and Figure S11). The DEGs in ethylene signaling include three genes (Vitvi05g00684, Vitvi06g00518 and Vitvi04g00115) encoding ethylene receptors or co-receptors (two ETR2 and one RTE1), four genes (Vitvi05g00573, Vitvi18g00533, Vitvi18g00534 and Vitvi11g00475) encoding negative regulators (all three CTR1 and one EBF1) and one gene (Vitvi13g01126) encoding a transcription factor that stimulates the expression of ERF (EIN3) (Figure 5). Ethylene signaling has been linked to plant freezing tolerance through its regulation of cold-responsive gene expression in various plant species (Sun et al. 2016; Robison et al. 2019; Hwarari et al. 2022). In the absence of ethylene, ethylene receptors also inhibit ethylene signaling by promoting the activity of CTR1 (Binder 2020), thus, the upregulation of these genes most likely induces repression of ethylene signaling. The upregulation of these ethylene signaling components under low temperatures suggests that the repression of ethylene signaling might be a protective mechanism to enhance low temperature stress tolerance. Further manual curation of the top 200 DEGs that negatively correlated with temperature in Experiment 1 identified the enrichment of genes in two pathways that have been noted as important in previous cold-related studies, galactose metabolism and phenylpropanoid biosynthesis (Figure S6A). A detailed discussion of these pathways and their associated genes is included in Note S1.
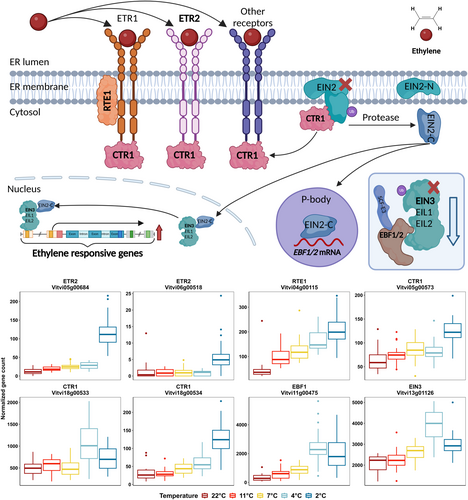
Besides these overrepresented pathways, we also specifically examined gene expression patterns of two key, previously identified cold response pathways; the ABA pathway and ICE-CBF-COR regulatory cascade. ABA signaling is known for its concomitant rhythm with the dynamics of grapevine freezing tolerance, with increased signaling during cold acclimation and reduced signaling during deacclimation (Kovaleski & Londo 2019; Rubio & Pérez 2019; Wang et al. 2020a). Low temperatures can induce ABA signaling, and treatment of grapevines with exogenous ABA stimulates freezing tolerance, which is a potential result of the onset of ABA signaling (Li & Dami 2016; Rubio et al. 2019; Wang et al. 2020a; Wang et al. 2022; Ren et al. 2022, 2023; Nai et al. 2022; Noriega et al. 2024). In this experiment, several ABA signaling genes were upregulated by low temperatures (e.g., Vitvi18g00784, encoding AREB2 and Vitvi13g01083, encoding AREB3), however, most of the pathway remained unresponsive (Supporting Material 2). None of the genes in the ABA signaling pathway showed differential expressions under temperature cycling treatment in Experiment 2, despite a 5°C enhancement of freezing tolerance (Figure 3A). This result contrasts with the observation that exogenous ABA, combined with low-temperature exposure, can increase freezing tolerance in grapevine (Rubio et al. 2019). These findings suggest that exogenous ABA may be able to trigger a parallel molecular response to that of the temperature oscillations used in this study. However, the gain in freezing tolerance was lost after 16 d of treatment exposure, suggesting that the promotive effect of exogenous ABA was not sufficient to produce sustained freezing tolerance (Rubio et al. 2019). Thus, while ABA signaling has a promotive role, it may not necessarily play this role in the development and/or persistence of freezing tolerance in grapevines under field conditions.
The ICE-CBF-COR cascade is known for its regulatory and functional role in enhancing freezing tolerance under low temperatures in plants, even though the involvement of ICE in the expression of CBF is still not clear (Tang et al. 2020; Thomashow & Torii 2020). The function of the ICE-CBF-COR cascade has been intensively studied over the last two decades, and the findings are mainly based on annual model plants. The results from most of these studies suggest that ICE-CBF-COR is an early participant in plants’ cold response, which is upregulated within hours under cold exposure to stimulate downstream functional metabolite biosynthesis, while the upregulation of the cascade gradually diminishes within days (Cook et al. 2004; Shi et al. 2017; Liu et al. 2019; Li et al. 2019; Hwarari et al. 2022). Some studies specifically examining CBFs in grapevine tissues indicate that CBFs could be upregulated for weeks under cold exposure but may not be associated with increased cold acclimation in bud tissues (Xiao et al. 2006, 2008; Karimi et al. 2015; Rubio et al. 2019; Noriega et al. 2024). Our study did not identify any statistically different changes in the expression of any ICE, DREB or CBF genes in either experiment presented here. Among the 14 ICE and CBF/DREB-related genes annotated in VitisNet, six genes passed the low-count filtering. None of these six genes are CBF genes, and their expression patterns did not correlate with any treatment effect in our experiments (Figure S12 and Figure S13). This finding does not agree with many previous studies of grapevine reporting the upregulation of CBF genes in response to low temperature treatment as a long-term consistent response that lasts for more than a week (Xiao et al. 2006; Karimi et al. 2015; Rubio et al. 2019; Wang et al. 2019; Noriega et al. 2024). It is possible that in the context of dormant bud physiology and our experiment, ICE-CBF-COR signaling occurred in the field material prior to our collection and subsequent temperature treatments. However, we believe this is unlikely as we collected dormant cuttings from the field prior to any freeze exposure, so this result would suggest that a role for ICE-CBF-COR signaling in dormant buds would be triggered by temperatures above freezing. Relatedly, we were successful at inducing acclimation and increasing freezing tolerance in the diurnal cycling experiment, yet we did not detect a role of ICE-CBF-COR signaling in this experiment either. Given our transcriptomic sampling occurred during the 7°C mean temperature, we cannot rule out the potential that the ICE-CBF-COR signaling pathway played a role during other portions of the temperature cycle. However, that potential signaling would have to have occurred and stabilized far more rapidly than has been observed in other low temperature stress studies (Liu et al. 2019). Similarly, our experiments were conducted in the dark and it is possible that light exposure may be critical for the induction of the ICE-CBF-COR cascade as it relates to cold response. The undetectable expression of CBF genes might also be an artifact of the sequencing method or low-temporal resolution of sample collection in our study. However, another interpretation of these results is that the experimental material used in previous studies where ICE-CBF-COR are shown to be induced by low temperature are either green tissues (leaves, shoot tips or paradormant buds) in actively growing grapevines or overexpressing lines of these genes in A. thaliana, whereas we used endodormant single-bud cuttings. The understanding of ICE-CBF-COR cascade regulation in the cold acclimation of dormant woody perennials is still limited (Wisniewski et al. 2018). Researchers have observed changes in gene expression for ICE and CBF genes in endodormant grapevine in response to stable low temperature, ABA, and synergistic expression enhancement in combined treatments, which resulted in a gain of freezing tolerance (Rubio et al. 2019; Noriega et al. 2024), although it is important to note that the phenotypic response was not persistent and was lost after 16 days. Thus, we conclude that it is possible that the signaling cascade needed to elicit cold acclimation in green tissues may differ from that of dormant tissues, and the importance of the ICE-CBF-COR cascade in cold acclimation requires deeper analysis between different tissues and phenological stages of grapevine.
4.2 High temperature-responsive genes
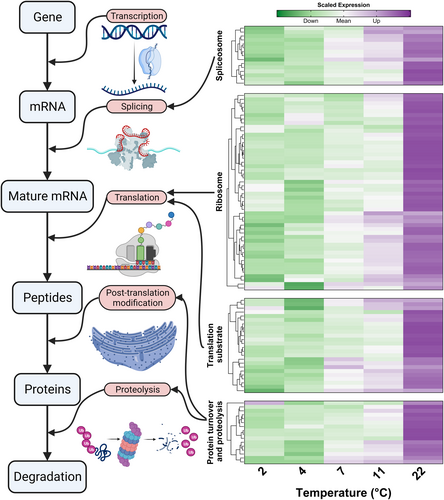
While the goals of this study were to induce cold acclimation in dormant buds and associate transcriptional change with this process, our experiment also gives us the opportunity to examine the potential mechanism of maintaining endodormancy under higher temperatures. Among the 2,856 temperature-responsive genes, 1,798 genes were positively correlated with temperature, indicating the increase of transcript abundance under higher temperatures. Pathway enrichment analysis of the genes upregulated in higher temperatures revealed 14 significantly enriched pathways, which are primarily associated with the categories of amino acid metabolism and genetic information processing (Figure 4C). Many of these pathways in the major steps connecting DNA to mature proteins, splicing, translation and post-translation peptide modification were upregulated, suggesting the activation of some growth-related pathways as a potential ubiquitous response under growth permissive conditions. The ubiquitin-mediated proteolysis pathway, a major protein degradation mechanism in plants, was also found to be enriched (Note S2). Taken together, these findings support a genetic control model for the maintenance of endodormancy at higher temperatures (Figure 6). This model posits that endodormant buds remain transcriptionally and potentially metabolically active under growth-permissive temperatures, but the metabolites and proteins produced may be degraded. Since all the steps from pre-mRNA splicing to ubiquitin-mediated proteolysis require energy in the form of ATP (Peth et al. 2013; Lynch & Marinov 2015; Tseng et al. 2017; Martinez-Seidel et al. 2020), maintaining endodormancy under high temperature could result in a waste of energy. The potential consequences of such energy wastage, particularly through unnecessary carbohydrate catabolism, are further discussed in Note S3 and Figure S14.
We successfully demonstrated increased acclimation and enhanced freezing tolerance in response to temperature cycling (Figure 3A). When examining the transcriptomic response in this experiment, our first approach focused on the temperature-responsive genes identified in Experiment 1 to determine if their expression is impacted by temperature cycling. Interestingly, when the treatment was shifted from stable temperatures in phase I to cycling temperature in phase II, the expression patterns of these genes in the 2°C and 11°C treatments converged on the expression profile observed in the stable 7°C treatment (Figure S8) while the buds that were in stable 7°C (phase I) and shifted to the cycling treatments (7±5°C, phase II) remained unchanged. Three co-expression modules were identified, and the genes in the two major modules exhibited similar converging gene expressions, as shown in PCA (Figure S15). Similar convergence in gene expression was also observed in most of the DEGs identified in Experiment 1 in the galactose metabolism pathway and phenylpropanoid biosynthesis pathway (Figure S6B). These results suggest that the temperature-responsive genes identified in this study are likely associated with the mean temperature (mean temperature of the cycling treatment is 7°C), but not diurnal temperature cycling, and the alternation of the expression of these temperature-responsive genes are likely not the mechanism contributing to the increase of freezing tolerance.
We applied multiple methods to analyze the transcriptome data to identify the transcriptomic mechanism driving the change in acclimation and increase in freeze tolerance, however no individual gene or gene cluster was found to be strongly linked with the shift in freezing tolerance. Instead, we contrasted stable versus cycling conditions (stable vs. 7±5°C) for all genes and all samples. Most of the resulting DEGs are likely influenced by factors that limit their potential as candidate genes for changes in freezing tolerance (Note S4 and Figure S10). However, four of these contrast DEGs (Vitvi07g01832, Vitvi01g00994, Vitvi00g02173 and Vitvi14g1819) showed patterns corresponding to temperature cycling treatment, suggesting that they may be reasonably worth considering as candidate genes for cold acclimation or at least for responsiveness to diurnal temperature cycling. Assuming the enhanced freezing tolerance is a result of transcriptomic change, it is possible that mechanistic changes in gene expression needed for a change in freezing tolerance occurred within the 8 d between the last timepoint in phase I (26 d) and the first timepoint in phase II (34 d). For these four genes, expression was significantly upregulated for all phase II time points with a greater magnitude change in regulation that correlated with the initial stable temperature: 11 > 7 > 2°C. Vitvi07g01832 is a gene homologous with CYP78A11, a cytochrome P450 type gene shown to have roles in developmental timing of leaf initiation in rice (Miyoshi et al. 2004) and fruit size differences in cherry (Qi et al. 2017) and eggplant (Zhou et al. 2023). Vitvi01g00994 is a gene homologous with phytochrome kinase substrate genes, which have been shown to be important in the development of plant organs in response to light as well as contributing to organ positioning (Fiorucci et al. 2022; Lopez Vazquez et al. 2023). Both of these genes function downstream of light response pathways, and it is unclear why temperature cycling would impact these genes. Perhaps diurnal temperature fluctuations in dormant bud tissues trigger similar pathways to those activated in green tissues exposed to fluctuating light conditions. Vitvi00g02173 is a gene encoding beta-expansin precursor. Beta-expansin genes are involved in modulating cell wall rigidity and have been linked to resistance against salt and low temperature stress in rice. When overexpressed in Arabidopsis, these genes contribute to enhanced low temperature resistance (Marowa et al. 2016; Feng et al. 2019; Jadamba et al. 2020). The final candidate gene is Vitvi14g1819, a gene encoding a GASA protein. GASA proteins are a class of proteins that are associated with the plant hormone gibberellin, and their function in plants is relatively unknown. However, they have been found to be associated with the interface between biotic and abiotic stress response (Bouteraa et al. 2023) and have been found to play a role in fruit and seed development in grapevine and in dormancy response in pear (Yang et al. 2019; Ahmad et al. 2020). Given the buds used in our study were endodormant, perhaps the GASA gene's function and the regulation of gibberellin play an important role in reinforcing dormancy during the acclimation process. Despite this short list of candidate genes, we are unable to definitively identify the putative mechanism or transcriptomic variation that drove the enhancement of freezing tolerance under the temperature cycling treatment. It is possible that the promotive effect of temperature cycling on the freezing tolerance of endodormant buds is transient and was not captured with the temporal resolution of sample collections used in this study. Since our temperature cycling treatment used 6-hour setpoints and our sample collection occurred at the midpoint of this setpoint, our results suggest any significant transcriptomic response that would occur in response to the temperature shift from 11 to 7°C, or from 2 to 7°C, might have been resolved and stabilized by the time of our sampling. An experiment designed with higher collection resolution, perhaps every 1-2 h is needed to uncover any potential transcriptomic component of the phenotypic change we observed in Experiment 2. Alternatively, the enhancement of freezing tolerance may result from translational, proteomic, lipidomic or fluidic response rather than an upstream transcriptomic response since the alternation of these mechanisms were also reported to affect plant freezing tolerance (Takahashi et al. 2013; Song et al. 2020; Cano-Ramirez et al. 2021).
In this study we examined freezing tolerance and transcriptomics of endodormant grapevine buds and the impact on cold acclimation under different temperature regimes. Our result shows that non-freezing stable temperatures are insufficient to induce cold acclimation of endodormant buds, even when temperatures are low (e.g., 2°C). However, we also determined that diurnal temperature cycling seems essential for the enhancement of freezing tolerance, and some cold acclimation is possible even without exposure to freezing temperatures. This result is perhaps not surprising given the natural diurnal fluctuation of temperature that grapevines are exposed to in field conditions. It is however surprising to note that the movement of all three stable temperature treatments into a shared cycling treatment resulted in comparably similar freezing tolerance. This suggests that the cycle we used induced a specific maximum depth of freezing tolerance, and this information could be valuable for the modeling of the freezing tolerance of grapevines. The effects of other temperature cycles on the cold acclimation process, both in amplitude and frequency, as well as the potential effects of freezing exposure and light, remain to be studied. Examination of the transcriptome validated the cold-responsive role of previously reported pathways such as ethylene, ABA, galactose and phenylpropanoid pathways but also failed to support a major role of the ICE-CBF-COR cascade in endodormant buds. Disappointingly, our analysis of the transcriptomic response of the samples where acclimation was promoted was inconclusive, presumably due to the resolution of sample collection. While the results of this study shed light on the critical role of temperature cycling on acclimation and the potential for temperature responsiveness of dormant buds during endodormancy, future high-resolution studies are needed to directly link transcriptomic and other physiological changes and are necessary for the development of mitigation methods to enhance cold acclimation as climate changes.
AUTHOR CONTRIBUTIONS
JPL and APK designed and conducted the experiment and collected LTE and RNA-seq data. HW conducted the analysis. HW and JPL wrote most of the manuscript, with contributions from all co-authors.
ACKNOWLEDGEMENT
This study was supported in part by the USDA-ARS appropriated project 1910–21220–006–00D and by the National Institute of Food and Agriculture, U.S. Department of Agriculture, through the Northeast Sustainable Agriculture Research and Education program under subaward number GNE16-130. We would like to acknowledge Hanna Martens, Amy Swezc-McFadden, and Kathleen Deys for assistance in collecting dormant cuttings and processing samples for LTE analysis.
Open Research
DATA AVAILABILITY STATEMENT
All RNA-seq raw data along with processed gene count matrix and sample metadata are available in NCBI-GEO (accession: GSE232062).




