Fighting to thrive via plant growth regulators: Green chemical strategies for drought stress tolerance
Abstract
As global climate change intensifies, the occurrence and severity of various abiotic stresses will significantly threaten plant health and productivity. Drought stress (DS) is a formidable obstacle, disrupting normal plant functions through specific morphological, physiological, biochemical, and molecular mechanisms. Understanding how plants navigate DS is paramount to mitigating its adverse effects. In response to DS, plants synthesize or accumulate various plant growth regulators (PGRs), including phytohormones, neurotransmitters, gasotransmitters, and polyamines, which present promising sustainable green chemical strategies to adapt or tolerate stress conditions. These PGRs orchestrate crucial plant structure and function adjustments, activating defense systems and modulating cellular-level responses, transcript levels, transcription factors, metabolic genes, and stress-responsive candidate proteins. However, the efficacy of these molecules in mitigating DS depends on the plant species, applied PGR dose, treatment type, duration of DS exposure, and growth stages. Thus, exploring the integrated impact of PGRs on enhancing plant fitness and DS tolerance is crucial for global food security and sustainable agriculture. This review investigates plant responses to DS, explains the potential of exogenously applied diverse PGRs, dissects the complex chemistry among PGRs, and sheds light on omics approaches for harnessing the molecular basis of DS tolerance. This updated review delivers comprehensive mechanistic insights for leveraging various PGRs to enhance overall plant fitness under DS conditions.
1 INTRODUCTION
The increasing global population demands a substantial rise in agricultural output. However, environmental factors induced by climate change, such as extreme temperatures, soil salinity, drought or water scarcity, nutrient imbalance, and metal toxicity, pose significant threats to global crop production (Zandalinas et al. 2021; Rivero et al. 2022; Terán et al. 2024). Among these challenges, drought stress (DS) stands out as a highly destructive abiotic stress worldwide, exerting a profound impact on crop yields (Cooper and Messina 2023; Raza et al. 2023c; Yang and Qin 2023; Vadez et al. 2024). Figure S1 illustrates the current and projected risks of DS on a global scale. The unpredictability of global climate patterns, characterized by irregular rainfall, contributes significantly to recurrent DS events worldwide. These events have extensive impacts, influencing socio-economic conditions, the environment, agricultural yields, food security, and rural livelihoods (https://www.fao.org/land-water/water/drought/en/; https://www.un.org/en/climatechange/science/climate-issues/water). Drought stress manifests at all growth stages, with the initial and most significant effects during seed germination. Reduced water influx during germination disrupts physiological and metabolic processes, setting the stage for a cascade of adverse impacts (Daryanto et al. 2017; Wahab et al. 2022; Cooper and Messina 2023; Raza et al. 2023c; Yang and Qin 2023; Vadez et al. 2024).
The root is the primary sensing organ for DS perception, and initiating DS signals induces a series of physiological and molecular responses in plants (Rahman et al. 2016; Mukherjee and Corpas 2020; Mukherjee and Corpas 2023; Vadez et al. 2024). Prolonged DS periods significantly reduce crop yield and productivity by disrupting photosynthetic performance, energy metabolism, phenology, growth, and reproduction. Under severe DS conditions, processes such as cell division, elongation, and expansion are impeded, reducing plant height, leaf area, stem extension, and root proliferation (Daryanto et al. 2017; Wahab et al. 2022; Cooper and Messina 2023; Raza et al. 2023c; Yang and Qin 2023; Vadez et al. 2024). Moreover, DS triggers the production of reactive oxygen species (ROS), including superoxide radicals (O2•-), hydroxyl radicals (•OH), and hydrogen peroxide (H2O2), intensifying lipid peroxidation, membrane degradation, and compromising biomolecule functionality in plants (Considine and Foyer 2021; Mittler et al. 2022; Wahab et al. 2022; Cooper and Messina 2023; Raza et al. 2023c; Yang and Qin 2023).
The sessile nature of plants necessitates adaptations in morphology, metabolism, and stress responses to withstand DS. To acclimate and survive DS, plants deploy diverse strategies, including morphological adjustments (such as escape, avoidance, and phenotype plasticity), physiological adaptations (including osmotic and redox adjustment and cell membrane stability), biochemical responses (involving proline, auxins, and ethylene), and molecular mechanisms (influencing stress-responsive proteins, transcription factors, and secondary messengers) (Kapoor et al. 2020; Bhardwaj and Kapoor 2021a; Riyazuddin et al. 2022; Cooper and Messina 2023; Raza et al. 2023b; Raza et al. 2023c). However, the effectiveness of these responses varies across species, growth stages, and environmental conditions.
The application of diverse plant growth regulators (PGRs) has emerged as a promising ‘green chemical’ approach to enhance plant growth and productivity under DS conditions. These PGRs encompass various categories: (1) phytohormones, including auxins, gibberellins (GA), indole-3-acetic acid (IAA), cytokinins (CK), abscisic acid (ABA), salicylic acid (SA), jasmonic acid (JA), brassinosteroids (BRs), and strigolactones (SLs) (Mubarik et al. 2021; Sabagh et al. 2021; Iqbal et al. 2022; Raza et al. 2022b; Raza et al. 2023a; Raza et al. 2023c); (2) neurotransmitters (NTs) including serotonin (SRT), melatonin (MLT), dopamine (DOPA), acetylcholine (Ach), and γ-aminobutyric acid (GABA) (Akula and Mukherjee 2020; Siddiqui et al. 2020; Martínez-Lorente et al. 2022; Raza et al. 2022b); (3) gasotransmitters or gaseous molecules such as hydrogen sulfide (H2S) and nitric oxide (NO) (Asgher et al. 2017; Fancy et al. 2017; Corpas et al. 2019; Thakur and Anand 2021; Raza et al. 2022c; Martínez-Lorente et al. 2022); and (4) polyamines (PAs), including putrescine (Put), spermine (Spm), and spermidine (Spd) (Gerlin et al. 2021; Sabagh et al. 2021; Shao et al. 2022). These chemical molecules play pivotal roles in plant stress biology, acting as protectants, ROS scavengers, and photosynthesis improvers and regulators of stress protein accumulation and various metabolic processes (Asgher et al. 2017; Pardo-Hernández et al. 2020; Sabagh et al. 2021; Iqbal et al. 2022; Martínez-Lorente et al. 2022; Raza et al. 2022b; Raza et al. 2023a). While some of these molecules might not be traditionally categorized as PGRs, this review collectively terms them as PGRs due to their contributions to plant growth, development, and stress management. Figure 1 provides an overview of the pros and cons of these PGRs. Whether termed PGRs or not, these natural and synthetic molecules, biosynthesized by plants, modulate growth and significantly contribute to DS mitigation by interacting with complicated signaling systems, harmonizing responses, and fostering the development of environmentally friendly strains. Plants, equipped with sophisticated mechanisms to perceive external signals, provide optimal responses to stress conditions with the support of PGRs (Iqbal et al. 2022; Sabagh et al. 2021; Raza et al. 2022b; Raza et al. 2023a). These regulators predominantly govern the defensive responses of plants through a complex interplay of synergistic and antagonistic activities.
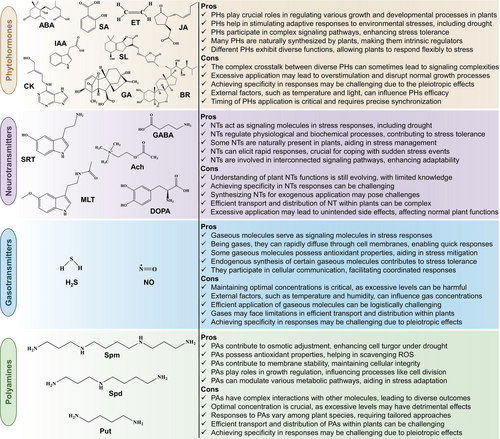
Abscisic acid (ABA); acetylcholine (Ach); auxins/indole-3-acetic acid (IAA); brassinosteroids (BRs); cytokinins (CKs); dopamine (DOPA); drought stress (DS); γ-aminobutyric acid (GABA); gibberellins (GAs); hydrogen sulfide (H2S); jasmonic acid/jasmonate (JA); melatonin (MLT); neurotransmitters (NTs); nitric oxide (NO); phytohormones (PHs); plant growth regulators (PGRs); polyamines (PAs); putrescine (Put); reactive oxygen species (ROS); salicylic acid (SA); serotonin (SRT); spermine (Spm); spermidine (Spd); strigolactones (SLs).
This review comprehensively examines DS responses and their impacts on plants, elucidating the beneficial effects of green chemical strategies, i.e., exogenously applied diverse PGRs. We examine how plants respond to DS, understanding their fitness, adaptation, and tolerance strategies. Moreover, we explore the complex interactions and crosstalk among various PGRs under DS conditions and highlight the utility of omics approaches in harnessing the molecular basis of PGR-mediated DS tolerance. By integrating these advanced techniques, researchers can gain deeper insights into the tricky mechanisms of enhancing plant DS tolerance. Overall, this review offers a comprehensive understanding of different PGRs for enhancing overall plant performance under DS conditions.
2 OVERVIEW OF PLANT RESPONSES AND IMPACTS OF DROUGHT STRESS
Drought stress poses a significant challenge to plant development, affecting various aspects from morphology to molecular processes. It also disrupts root morphology, photosynthetic activity, respiration, carbon assimilation rate, carbohydrate metabolism, and nutrient uptake (Kapoor et al. 2020; Bhardwaj and Kapoor 2021a; Riyazuddin et al. 2022; Cooper and Messina 2023; Raza et al. 2023b; Raza et al. 2023c; Vadez et al. 2024). Figure 2 provides an illustrative overview of plant responses and the effects of DS.
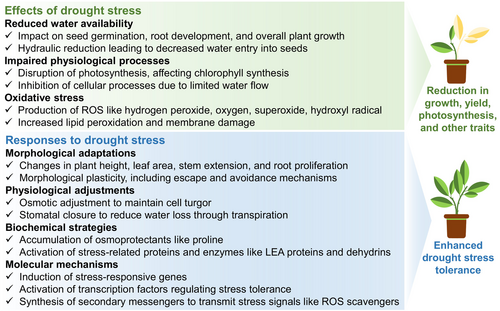
Under severe DS conditions, limited water availability impedes cell expansion, influencing plant water relations and factors like gaseous exchange attributes, leaf temperature, relative water content (RWC), leaf water potential, and canopy temperature. Reduced RWC is commonly observed in plants under DS, impacting processes like cell division and expansion, shoot development, leaf size, and water and nutrient uptake (Cooper and Messina 2023; Vadez et al. 2024). Aside from absorbing ions and water from the medium, roots play a crucial role in enhancing crop productivity and engaging in soil symbiotic interactions with microbes. Root length increased in saffron (Crocus sativus L.) under DS (Maleki et al. 2011), which is beneficial for improving soil water retention and nutrient uptake (Zulfiqar et al. 2020). In this sense, healthy roots are advantageous for sustaining accelerated plant growth, particularly during the primary plant growth phase (Smith and De Smet 2012). However, DS can also increase the root-to-shoot ratio, decreasing plant biomass (Akhtar and Nazir 2013). Drought stress adversely affects various plant growth attributes, photosynthetic pigments, gas exchange parameters, and antioxidant enzymes, including in maize (Zea mays L.) (Ahmad et al. 2022) and tomato (Solanum lycopersicum L.) seedlings (Altaf et al. 2022).
Photosynthesis is the most critical physiological aspect that determines crop growth and productivity. Alterations in photosynthetic rate are attributed to changes in photosynthetic pigments (Sallam et al. 2019), particularly chlorophyll (Chl) content, which significantly decreases under DS due to increased ROS levels (Allakhverdiev 2020). Plants respond to DS by regulating stomatal movement, adjusting osmotic balance, and activating antioxidant defense systems (Wahab et al. 2022). Stomatal closure is a primary indicator of plant response to DS, varying in extent among species based on their tolerance levels (Wahab et al. 2022). In sugar beet (Beta vulgaris L.), stomata closed more frequently as DS severity increased during the day (Islam et al. 2020). Severe DS conditions disrupt photosynthesis-related enzymes, such as Rubisco, further impacting photosynthetic efficiency (Brito et al. 2019) and reducing plant growth and productivity.
Drought stress induces oxidative stress by over-accumulating ROS, resulting in alterations in various biological and metabolic activities and disruption of the antioxidant defense system (Bhardwaj et al. 2021; Foyer and Hanke 2022; Mittler et al. 2022; Raza et al. 2023c). To counteract the adverse impacts of DS-induced oxidative stress, most plants have defensive, rapid, powerful, and effective antioxidant systems comprising enzymatic and non-enzymatic antioxidants (Mubarik et al. 2021; Sabagh et al. 2021; Iqbal et al. 2022; Raza et al. 2022b; Raza et al. 2023c) to scavenge ROS, maintaining the vitality and integrity of various organelles and cell membranes and ultimately reducing electrolyte leakage and lipid peroxidation (Considine and Foyer 2021; Foyer and Hanke 2022; Mittler et al. 2022). In tomato seedlings, the activities of antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX), along with non-enzymatic antioxidants like ascorbic acid (AsA) and glutathione (GSH), increase under DS (Rady et al. 2020). Similarly, in rice (Oryza sativa L.) cultivars, DS increased SOD, POD, CAT, and APX activities (Wang et al. 2019a; Wang et al. 2019b). Efficient osmolyte accumulation protects plants against water deficit-induced oxidative stress (Kabiri et al. 2018). The most prevalent osmolytes, including soluble sugars, proline, and proteins, work together at the cellular level to reduce membrane permeability during mild water shortages and preserve crop water balance during DS (Kabiri et al. 2018).
Plants sense and respond to external DS stimuli through plasmatic membrane sensors, triggering drought-responsive genes and adaptation mechanisms via multiple signal transduction pathways involving secondary messengers like transcriptional regulators, diacylglycerol, Ca2+, ROS, NO, H2S, and ABA (Riyazuddin et al. 2022; Raza et al. 2023c; Vadez et al. 2024). The transcriptional activation of drought-inducible genes is complex. Thus, transcription factors (TFs) like C-repeat/dehydration-responsive element-binding factors (CBF/DREB), mitogen-activated protein (MYB), cup-shaped cotyledon (CUC), no apical meristem (NAC) transcription factors, and zinc-finger proteins play critical roles in conferring DS tolerance (Cong et al. 2008; Kapoor et al. 2020). For instance, genetic engineering approaches targeting genes like GsZFP1 in alfalfa (Medicago sativa L.) showcase promising avenues for enhancing DS tolerance in plants (Tang et al. 2013). In tomatoes, DS downregulated the expression of DREB, LEA, HSP70, and HSP90 transcripts (Raja et al. 2020). In Achillea pachycephala, 7 days of DS upregulated the expression of CHS (chalcone isomerase), F3H (flavanone 3-hydroxylase), F3′H (flavonoid 3′-hydroxylase), and PAL2 (phenylalanine ammonia-lyase), suggesting their role in DS mitigation (Gharibi et al. 2019). Einkorn wheat (Triticum boeoticum) and Persian goatgrass (Aegilops crassa) exhibited higher expression of Wdhn13 (wheat dehydrin gene) and antioxidant genes, such as SOD, APX, and GPX, under DS than reference DS-tolerant cultivar (Mehrabad Pour-Benab et al. 2019).
3 APPLICATION OF DIVERSE PGRs HELPS TO IMPROVE PLANT PERFORMANCE UNDER DROUGHT STRESS
The application of diverse PGRs acts as ‘green chemical strategies’ and has been demonstrated to enhance plant performance under DS, as extensively explored in the subsequent sections covering different categories of PGRs. Each PGR, individually or in combined coordination, elicits a range of attributes associated with DS responses in plants (Figures 2 and 3; Table 1). In the ensuing sections, this review explores the coordinated effects of various PGRs involving DS tolerance in various plant species.
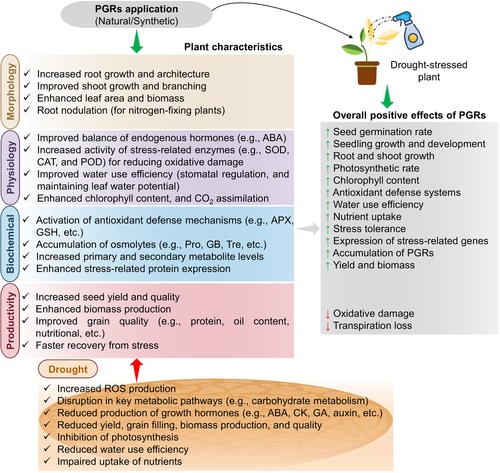
Abscisic acid (ABA); ascorbate peroxidase (APX); catalase (CAT); carbon dioxide (CO2); cytokinin (CK); drought stress (DS); glycine betaine (GB); glutathione (GSH); gibberellic acid (GA); plant growth regulators (PGRs); peroxidase (POD); plant growth regulators (PGRs); proline (Pro); reactive oxygen species (ROS); superoxide dismutase (SOD); trehalose (Tre).
| PGR name | Antioxidant properties | ROS scavenging | Photosynthesis | Photosynthetic pigments | Stress genes | WUE | Metabolic regulation | Endogenous synthesis | Hormonal balance | Cellular homeostasis | Signal transduction | Growth promotion | Yield | Biomass accumulation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IAA | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| GA | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| CK | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| ABA | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| SA | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| JA | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| BRs | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✘ | ✔ | ✘ | ✔ | ✔ | ✔ | ✔ |
| SLs | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✘ | ✘ | ✘ | ✔ | ✔ | ✔ | ✔ |
| SRT | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| MLT | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| DOPA | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Ach | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| GABA | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Put | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Spm | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| Spd | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| H2S | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| NO | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
- Note: ✔ and ✘ signify the presence and absence, respectively, of specific PGR-mediated outcomes on several mechanisms under stress conditions. ✔ suggests that the PGR is described or identified to have a positive impact on the corresponding mechanism. ✘ suggests that the PGR is described or identified to have no meaningful impact or a negative impact on the corresponding mechanism. Symbol allocation is based on existing data and scientific knowledge. It is noteworthy to highlight that the lack of a ✔ does not indicate the absence of impact; rather, it implies that the existing data is inadequate or limited. Furthermore, the specific notes of PGR-mediated influences may be modified according to plant species, experimental settings, and doses used. Therefore, we encourage readers to examine the related literature for specific insights into the positive or negative impact of each PGR on plant responses to stress.
- Abbreviations: Abscisic acid (ABA); acetylcholine (Ach); auxins/indole-3-acetic acid (IAA); brassinosteroids (BRs); cytokinins (CKs); dopamine (DOPA); γ-aminobutyric acid (GABA); gibberellins (GAs); hydrogen sulfide (H2S); jasmonic acid/jasmonate (JA); melatonin (MLT); neurotransmitters (NTs); nitric oxide (NO); phytohormones (PHs); plant growth regulators (PGRs); polyamines (PAs); putrescine (Put); reactive oxygen species (ROS); salicylic acid (SA); serotonin (SRT); spermine (Spm); spermidine (Spd); strigolactones (SLs); water-use efficiency (WUE).
3.1 Phytohormone-mediated drought stress tolerance
Phytohormone application effectively improves plant growth, development, and productivity under DS conditions (Mubarik et al. 2021; Iqbal et al. 2022; Raza et al. 2023c). Phytohormones are chemical messengers, synthesized at a specific site and transported to other plant tissues, regulating plant responses to stress conditions at minute concentrations (Mubarik et al. 2021; Iqbal et al. 2022; Raza et al. 2022b). Recent studies highlight the positive protective role of phytohormones in managing DS in various plant species. For instance, applying 5, 10, and 20 μM IAA to forage soybean plants significantly alleviated DS by improving leaf length and width and leaf proportional water content (Aksu 2022). Exogenous application of IAA (1 μM) improved white clover (Trifolium repens) tolerance to DS by increasing RWC, Chl content, and endogenous ABA and JA levels (Zhang et al. 2020). In maize plants, 20 μM IAA regulated relative apoplastic water flow under DS conditions (Quiroga et al. 2020).
Rapeseed (Brassica napus L.) cultivars treated with gibberellic acid (GA3, 500 mg L−1) and MLT (500 μM) exhibited positive effects on morphological attributes, proline accumulation, yield components, and enzymatic (SOD, POD, and CAT), and non-enzymatic (AsA and GSH) antioxidant activities under DS (Khan et al. 2020). Faba bean (Vicia faba L.) plants treated with 20 mg L−1 GA3 reversed DS-induced adverse effects by regulating RWC, photosynthetic pigments, photosystem II (PSII) efficiency, nutrient acquisition, osmolyte accumulation, endogenous IAA, GA3, and cytokinin contents, and antioxidant enzyme activities (Rady et al. 2021). Seed priming with 500 mg L−1 GA3 increased rapeseed germination rate, growth traits, and ROS scavenging by stimulating enzymatic (SOD, POD, and CAT) and non-enzymatic (AsA and GSH) antioxidant activities and increasing proline, soluble sugar, and soluble protein contents under limited water availability conditions (Zhang et al. (2023). In cowpea (Vigna unguiculata L.), GA3 application (60 and 120 ppm) alleviated the adverse effects of DS by regulating cell permeability, which increased photosynthetic pigment contents (Chl and carotenoids), RWC, and yield while reducing ion leakage (Miri et al. 2021).
Exogenous application of synthetic cytokinin [5 mg L−1 CPPU (N-2-(chloro-4-pyridyl)-N-phenyl urea)] increased the photosynthetic activity of rice under DS by enhancing stomatal conductance, photosynthetic pigments, and Rubisco activase activity (Gujjar et al. 2020). Soybean (Glycine max L.) cultivars treated with benzyladenine (BA) increased growth, biomass, and yield under DS (Mangena 2020). Similarly, in wheat (Triticum aestivum L.) under DS, 25 mg L−1 zeatin (a cytokinin), alone or combined with rhizobacteria, improved growth parameters, protein content, nutrient levels, and gas exchange attributes (i.e., stomatal conductance, photosynthetic rate, and transpiration rate), increasing grain yield (Zaheer et al. 2019).
The contribution of ABA to different plant growth and stress-related responses aids plants in adapting to various DS circumstances (Muhammad Aslam et al. 2022). For instance, 50 mg L−1 ABA significantly alleviated drought-induced damage in tea (Camellia sinensis L.) genotypes by stimulating specific metabolic pathways, such as lipid metabolism, amino acid metabolism, energy metabolism, and flavonoid biosynthesis (Gai et al. 2020). In cotton (Gossypium hirsutum L.), 0.5 μmol L−1 ABA, alone or combined with 200 μmol L−1 melatonin, mitigated DS-induced toxic effects by increasing RWC and enzymatic (SOD, CAT, GR, and POD) and non-enzymatic (AsA and GSH) antioxidant activities (Hu et al. 2021). In tropical carpet grass (Axonopus compressus), 100 μmol ABA, alone or combined with glycine betaine, increased photosynthetic pigments, osmoprotectants (proline, soluble sugars, phenolics, and proteins), and antioxidant enzyme activities (SOD, CAT, POD, and APX) under water deficit conditions, which increased ROS scavenging by reducing malondialdehyde (MDA) and H2O2 levels (Nawaz and Wang 2020). In drought-stressed barley genotypes, 100 μM ABA increased water content, PSII efficiency, and CO2 assimilation rate and preserved antioxidant potential by decreasing lipid peroxidation (Skowron and Trojak 2021).
Wheat cultivars treated with 150 mg L−1 SA and potassium increased RWC, water-use efficiency (WUE), photosynthetic pigments, and grain yield (Munsif et al. 2022). In drought-exposed pumpkin (Cucurbita pepo), exogenous application of SA (0.5, 1.0, and 1.5 mg L−1) stimulated antioxidant enzyme activities, reducing ion leakage, MDA, and H2O2 levels (Biareh et al. 2022). Similarly, SA application improved DS tolerance in tomato (Aires et al. 2022), wheat (Sapakhova et al. 2022), rice (Hussain et al. 2023), and sunflower (Helianthus annuus L.) (El-Bially et al. 2022). Radish (Raphanus sativus L.) treated with 100 μM SA exhibited increased growth traits, photosynthetic pigments, and gas exchange attributes under DS (Henschel et al. 2022).
Mustard (Brassica rapa L.) plants treated with 10 μM JA alone or combined with 0.01 μM 24-epibrassinolide (24-EBL) exhibited enhanced gas exchange parameters, photosynthetic pigments, antioxidant defense, osmolyte levels, and reduced membrane damage under DS (Ahmad Lone et al. 2022). In phalsa/falsa (Grewia asiatica), 0.5 mM JA significantly improved plant growth, CO2 assimilation, stomatal conductance, phenolics content, and antioxidant enzyme activities (SOD, APX, and GPX) under DS (Waheed et al. 2022). Wheat cultivars treated with 0.1 mM JA and 0.5 mM kinetin exhibited increased growth traits, biomass, total water content, osmoprotectants, and antioxidant enzyme activities under limited water availability (Abeed et al. 2021). Similarly, JA application improved DS tolerance in cotton (Gossypium hirsutum L.) (Nazim et al. 2021), strawberry (Fragaria × ananassa) (Yosefi et al. 2020), and sugar beet (Beta vulgaris L.) (Ghaffari et al. 2019).
Wheat plants treated with BR (1 and 3 μM), alone and combined with biochar, showed alleviated drought-induced phytotoxicity by enhancing growth parameters, photosynthetic pigments, nutrient content, and enzymatic and non-enzymatic antioxidant levels (Lalarukh et al. 2022). Chili pepper (Capsicum annuum L.) plants supplemented with 1 μM 24-EBL improved growth parameters and reduced oxidative stress by activating the antioxidant defense system under DS (Kaya et al. 2019). Timor white gum (Eucalyptus urophylla) plants treated with 50 and 100 nM 24-EBL exhibited increased photosynthetic pigment contents, PSII efficiency, net photosynthetic rate, and antioxidant enzyme activities (Barros Junior et al. 2021).
Napier grass (Pennisetum purpureum) treated with strigolactones (SLs; 1, 3, 5, and 7 μM) showed increased root length, fresh and dry weights, and improved photosynthetic performance under DS by increasing net photosynthetic rate, stomatal conductance, transpiration rate, and WUE (Li et al. 2022). Grape (Vitis vinifera) seedlings treated with synthetic SLs, specifically 1, 3, and 5 μM rac-GR24, significantly ameliorated the adverse effects of DS by reducing electrolyte leakage and ROS levels, closing stomata, and increasing RWC, Chl content, photosynthetic rate, and antioxidant defense potential (Min et al. 2019). Similarly, wheat genotypes supplemented with GR24 application under DS increased leaf water potential, gas exchange parameters (i.e., photosynthetic rate, transpiration rate, and stomatal conductance), antioxidant enzyme activities, and yield (Sedaghat et al. 2021).
In summary, phytohormone application appears to be a favorable strategy for enhancing plant DS tolerance. These phytohormones operate through various mechanisms, including regulating morphological traits, osmolyte accumulation, antioxidant enzyme activities, and photosynthetic efficiency. The observed positive effects across diverse plant species underline the versatility of phytohormones in mitigating harmful DS impacts, indicating golden opportunities for sustainable crop production under DS conditions.
3.2 Neurotransmitter-mediated drought stress tolerance
Neurotransmitters (NTs) like serotonin (SRT), melatonin (MLT), dopamine (DOPA), acetylcholine (Ach), and γ-aminobutyric acid (GABA), are regulatory molecules found in both mammals and plants (Zhao et al. 2019; Akula and Mukherjee 2020; Raza et al. 2022b). These compounds are important in several biological processes and aid in plant adaptation to environmental stresses, including DS (Akula and Mukherjee 2020; Raza et al. 2022b). Recent studies have discovered the involvement of NTs in shaping plant development, facilitating stress adaptation, and enhancing DS tolerance, as discussed below.
3.2.1 Serotonin
Serotonin, also known as 5-hydroxytryptamine (5-HT), is an indoleamine molecule and an intermediary in the biosynthesis of MLT. It has notable antioxidant properties (Mukherjee 2018) and plays crucial roles throughout different stages of the plant life cycle, including germination, vegetative development, stress adaptation, reproduction, and senescence (Erland et al. 2019). Studies have shown that exogenous application of SRT can mitigate the adverse effects of DS in plants. For instance, tomato plants treated with 5 μM SRT showed enhanced DS tolerance due to decreased tissue MDA contents and ion leakage levels while increasing RWC and antioxidant defense system under DS (Akcay and Okudan 2023). Moreover, SRT enhanced the expression of genes encoding antioxidant- and ethylene-related biosynthetic enzymes in response to DS (Akcay and Okudan 2023). In saffron (Crocus sativus L.) plants, 100 μM SRT enhanced DS tolerance by improving growth parameters and photosynthetic attributes and stimulating the antioxidant defense system (Gavyar et al. 2023). In rapeseed, exogenous SRT increased seedling growth, solute accumulation (proline and soluble sugars), and antioxidant enzyme activities (CAT, SOD, and APX) under DS (Shan-shan et al. 2019).
3.2.2 Melatonin
Melatonin is a key regulator that modulates plant growth and development. Exogenous application of MLT can induce morphological changes in plants and enhance physiological, biochemical, and molecular processes that enable plants to withstand and tolerate stress conditions (Siddiqui et al. 2020; Tiwari et al. 2021; Martínez-Lorente et al. 2022; Raza et al. 2022a; Raza et al. 2022b). Its application can also relieve DS symptoms through seed priming, seed coating, soil drenching, foliar application, mixing with nutrient solutions, hydroponic systems, irrigation supplementation, and root pretreatment. Melatonin doses vary significantly, from 50 nM to 1 mM, depending on the species involved (Moustafa-Farag et al. 2020; Raza et al. 2022a).
Melatonin application (0, 10, and 50 μM) alleviated drought-induced damage in tomato due to its protective impact on PSII through enhanced PSII photochemical features. Moreover, transcriptome analysis discovered that MLT modulated the expression profile of many genes linked to hormone signal transduction (Yang et al. 2022). Rice seedlings foliar-sprayed with 200 μM MLT decreased lipoxygenase (LOX) activity and MDA and ROS levels (measured as the generation rate of O2•- and H2O2). Moreover, MLT application improves DS tolerance by regulating osmoregulation (i.e., increased proline, fructose, and sucrose contents) and antioxidant enzyme activities (SOD, POD, CAT, and APX) (Luo et al. 2022).
Sugar beet seedlings foliar-treated with different doses of MLT (10, 50, 100, and 500 μM) improved their survival capacity under DS by increasing leaf RWC, water potential, and antioxidant enzymatic activities while decreasing electrolyte leakage and lipid peroxidation (He et al. 2023). Persian buttercup (Ranunculus asiaticus) plants treated with MLT (0, 50, 100, and 200 μM) at four specific time points (45, 60, 75, and 90 days) significantly decreased shoot length and leaf number, area, and fresh and dry weights while increasing other growth indicators under water deficit conditions compared to adequately watered plants (Eisa et al. 2023).
In Chrysanthemum (Chrysanthemum morifolium), 100 μM MLT applied via root irrigation or foliar spray decreased Chl and RWC but enhanced photosynthetic ability (photosynthesis rate and stomatal conductance), hormone levels (GA, SA, and CK), and enzyme activities (SOD, POD, and CAT) (Luo et al. 2023). Moreover, RNA-seq analysis of MLT-treated plants discovered increased sulfur, nitrogen, aspartate, glutamate, and alanine metabolism, offering insights for DS adaptation (Luo et al. 2023). A controlled greenhouse pot experiment explored the implications of foliar spraying of 100 μM MLT on DS tolerance of maize roots (Wang et al. 2023). The authors used a combination of transcriptome and metabolomic studies to identify genes and metabolites connected with DS resilience. Melatonin-treated plants exhibited decreased oxidative damage (reduced MDA levels) and enhanced root development. Furthermore, MLT increased the expression of genes involved in flavonoid biosynthesis, TFs, and ethylene response factors (Wang et al. 2023).
3.2.3 Dopamine
Dopamine is a widely distributed catecholamine chemical in several organisms, including mammals and plants (Ahammed and Li 2023). The effectiveness of this compound as a PGR is contingent upon its significant antioxidative capacity. Furthermore, it interacts with plant hormones to influence crop growth and development (Akula and Mukherjee 2020; Mohammadi Azni et al. 2021; Raza et al. 2022b). Plants benefit positively from applying exogenous DOPA, enhancing CO2 assimilation rate and maximal photochemical efficiency, especially under challenging environmental circumstances (Akula and Mukherjee 2020; Gao et al. 2021). Gao et al. (2020) reported that pretreating apple (Malus domestica Borkh.) seedlings with 100 μM DOPA in the nutrient solution mitigated DS by decreasing the degradation of photosynthetic pigments and enhancing the net photosynthetic rate. Similarly, 100 μM DOPA significantly improved DS tolerance in apple (M. hupehensis) by increasing free proline content, soluble sugar levels, and antioxidant enzyme activities (SOD, CAT, POD, and APX) while decreasing MDA content and superoxide anion production (Du et al. 2022). In another study, 100 μM DOPA reversed the adverse effects of DS on apple by improving seedling biomass, photosynthetic rates, Chl concentrations, stomatal functioning, and the concentrations, uptake, and transport of macro-, micro-, and trace elements (Liang et al. 2018).
3.2.4 Acetylcholine
Acetylcholine, another neurotransmitter found in various plant species (Roychoudhury 2020), has emerged as a potential promoter of plant growth and physiological processes, with the ability to mitigate the adverse effects of stress, including DS (Akula and Mukherjee 2020; Raza et al. 2022b). In soybean plants subjected to water constraints, exogenous supply of Ach (2.0 mM) through seed soaking, foliar spray, or both enhanced photosynthetic rate, biomass generation, and antioxidant activities (SOD, CAT, and APX) compared to non-treated plants (Braga-Reis et al. (2021). Similarly, tobacco (Nicotiana tabacum) seedlings treated with Ach (0.01 and 0.1 mM) under simulated DS conditions exhibited improved growth parameters and physiological performance of PSII and Chl fluorescence. Furthermore, exogenous administration of Ach mitigated oxidative stress induced by water scarcity by increasing antioxidant enzyme activities (SOD, POD, CAT, and APX) (Qi et al. 2023).
3.2.5 γ-Aminobutyric acid
γ-aminobutyric acid is a non-protein amino acid in plants involved in many physiological processes related to growth and development. Its role in mitigating the adverse effects of diverse abiotic stresses, including DS, is well recognized, primarily through efficient restriction of ROS generation in plant organs (Akula and Mukherjee 2020; Wang et al. 2021a; Raza et al. 2022b). Moreover, GABA can regulate stomatal aperture and closure, particularly in response to DS (Hasan et al. 2021a). For instance, in apple seedlings subjected to DS, exogenous GABA application increased leaf ABA synthesis, resulting in stomatal closure and subsequent decline in stomatal conductance (Liu et al. 2021a). This effect was accompanied by substantial changes in the expression levels of ABA-related genes, including PYL4, ABI1, ABI2, HAB1, ABF3, and OST1, indicating the involvement of GABA in ABA-mediated responses to DS (Liu et al. 2021a).
Furthermore, 40 mM foliar GABA spray to cucumber (Cucumis sativus L.) plants enhanced growth parameters and leaf proline content and facilitated ROS scavenging through antioxidant enzyme activation (Zahra et al. 2021). In another study, snap bean (Phaseolus vulgaris L.) seedlings treated with exogenous foliar GABA application at various concentrations (0.5, 1, and 2 mM) mitigated DS through GABA-induced pathways by enhancing various physiological factors, including growth stimulation, water status stabilization, membrane integrity preservation, osmotic equilibrium adjustment, antioxidant defense reinforcement, and nutrient acquisition optimization (Abd El-Gawad et al. 2021). Similarly, two chickpea (Cicer arietinum L.) cultivars exposed to varying water deficit levels (irrigated at 100, 60, 40, and 20% of field capacity) and GABA concentrations (0, 25, and 50 μM) exhibited decreased ROS generation and increased proline and endogenous GABA levels (Seifikalhor et al. 2022). Transcriptome alterations in cassava (Manihot esculenta) seedlings treated with 100 μM GABA under water deficit showed significant upregulation of genes associated with DS response pathways (DREB2A, NCED3, and CBF3) (Ma et al. 2022).
In summary, neuroregulatory molecules are essential in DS adaptation and tolerance in plants. These molecules exhibit diverse defensive effects, influencing physiological, biochemical, and molecular processes, and present new prospects for enhancing DS tolerance in plants, thus contributing to sustainable agricultural systems. However, the literature on Ach and DOPA remains limited, highlighting the need for more investigations to fully discover their dynamic roles in DS tolerance.
3.3 Gasotransmitters-mediated drought stress tolerance
Gasotransmitters, such as H2S, NO, and CO, are not only small gas molecules but also show features of PGRs as they adjust growth, development, and stress responses. These molecules are released by various organisms to transmit biological signals that regulate specific biological functions (Corpas et al. 2019; Yao et al. 2019; Mukherjee and Corpas 2020; Mukherjee and Corpas 2023). These dynamic and organic molecules can cross cell membranes and function independently of receptor systems. They are produced through regulated enzymatic processes and can be simulated through exogenous application. Gasotransmitters target specific cellular components, irrespective of whether they have downstream secondary signaling molecules (Kolupaev et al. 2019b). Accumulating evidence suggests that gasotransmitters, particularly H2S and NO, are crucial for enhancing plant tolerance to various stresses, including DS (Fancy et al. 2017; Corpas et al. 2019; Raza et al. 2022c; Corpas and Palma 2023).
3.3.1 Hydrogen sulfide
Hydrogen sulfide functions as a signaling moiety in plants, regulating various physiological processes such as seed germination, root architecture, photosynthetic activity, and flower senescence to alleviate the adverse impacts of environmental stresses (Corpas 2019; Bhardwaj and Kapoor 2021b; Thakur and Anand 2021; Raza et al. 2022c; Corpas and Palma 2023). Recent studies have demonstrated its role in improving DS tolerance through gene expression modulation and stomatal movement regulation, often interacting with ABA pathways (Chen et al. 2020; Yang et al. 2020; Mishra et al. 2021; Siodmak and Hirt 2021). Pretreating safflower (Carthamus tinctorius L.) plants with 0.5 mM sodium hydrosulfide (NaHS) increased plant growth parameters (root and shoot lengths, shoot fresh and dry weights), photosynthetic pigment contents (Chl and carotenoids), secondary metabolite levels (flavonoids and phenolics) and non-enzymatic antioxidant contents (anthocyanin and AsA) under DS (Amir et al. 2021). Similarly, rice seedlings treated with 100 μM NaHS exhibited enhanced DS tolerance by reducing lipid peroxidation and augmenting antioxidant defense systems and ABA accumulation (Zhou et al. 2020). Combined supplementation of NaHS (0.66 dose centipoise ha−1), zinc sulfate, and potassium phosphite significantly mitigated DS in soybean plants by activating antioxidant defense systems and osmotic adjustment mechanisms, which were associated with maintaining cell membrane integrity and photosynthetic performance (Batista et al. 2020).
Furthermore, 100 μM NOSH—a novel donor that simultaneously releases NO and H2S—improved physiological performance, proline accumulation, reactive oxygen/nitrogen species homeostasis, and transcriptional regulation of defense-related pathways in alfalfa plants under DS (Antoniou et al. 2020). Treatment with 0.3 mM NaHS enhanced growth attributes, photosynthetic pigments (Chl and carotenoids), metabolite contents (anthocyanin, flavonoids, and proline), and antioxidant enzyme activities (SOD, CAT, and GPO) while reducing MDA and H2O2 levels in wheat plants under water deficit (Kolupaev et al. 2019a). In another study, spinach (Spinacia oleracea) seedlings treated with 100 μM NaHS exhibited enhanced DS tolerance by increasing RWC, PSII efficiency, gas exchange parameters (net CO2 assimilation rate, stomatal conductance, transpiration rate), osmolytes (proline and glycine betaine (GB) and polyamine content (Chen et al. 2016). Moreover, the NaHS treatment upregulated the expression level of sugar biosynthesis-related genes, such as sucrose phosphate synthase (SoSPS1), fructose-1,6-bisphosphatase (SoFBPase), trehalose-6-phosphate synthase (SoT6PS), and PA-biosynthesis-related genes [arginine decarboxylase (SoADC), N-carbamoyl putrescine amidohydrolase (SoCPA), ornithine decarboxylase (SoODC), and spermidine synthase (SoSPDS)] (Chen et al. 2016). In wheat plants under DS conditions, exogenous application of 500 μM NaHS improved plant height, leaf RWC, antioxidant enzyme activities (SOD, CAT, and POD), and the expression of ABA metabolic pathway-associated genes (TaZEP, TaNCED, TaAAO, and TaSDR) while reducing MDA and H2O2 levels (Ma et al. 2016).
3.3.2 Nitric oxide
Nitric oxide is a free radical and redox signaling molecule considered crucial for regulating various physiological processes in plants (Nabi et al. 2019; Wei et al. 2020; Bhardwaj et al. 2021), including mitigating the adverse effects of environmental stresses like DS (Signorelli et al. 2013; Fancy et al. 2017; Pardo-Hernández et al. 2020; Corpas and Palma 2023). It exerts all these functions through a family of derived molecules called reactive nitrogen species (RNS), such as peroxynitrite (ONOO−), nitrogen dioxide (NO2) or S-nitrosoglutathione (GSNO) that exerts its regulatory functions through post-translational modifications such as S-nitrosation or tyrosine nitration. For example, in kiwifruit (Actinidia chinensis) under DS conditions, exogenous application of 100 μM sodium nitroprusside (SNP), an NO donor, increased RWC, photosynthetic pigment contents, leaf photosynthetic capacity, and glutamine synthetase functioning while reducing MDA and H2O2 levels and electrolyte leakage (Xia et al. 2022). Moreover, the SNP treatment regulated the expression of aquaporin genes (PIP1;1 and PIP2;2–1) and proline synthesis gene (P5CR1) (Xia et al. 2022). Similarly, a 200 μM SNP treatment increased plant biomass, leaf area, RWC, Chl content, stomatal conductivity, and L-proline content in apricot (Prunus armeniaca) under DS (Bakır et al. 2022). Nitric oxide-pretreated Myrobolan 29C rootstock (Prunus cerasifera Ehrh.) under DS increased root fresh and dry weights, relative shoot length, relative shoot diameter, stomatal conductance, leaf Chl content, and mineral nutrient contents (N, K, Ca, Mg, Fe, and Zn) (Bolat et al. 2022). In white clover (Trifolium repens), NO supplementation using 50 μM SNP as the NO donor enhanced nitrate reductase and L-arginine-dependent NO synthase-like activity, improving DS tolerance for 16 days (Peng et al. 2016).
Likewise, in Mexican lime [Citrus aurantifolia (Christ.) Swingle], SNP application at various concentrations (25, 50, and 100 μM) enhanced growth parameters (shoot number, shoot length, leaf number, and shoot fresh and dry weights), photosynthetic pigments (Chl and carotenoids), proline content, total soluble protein content, and antioxidant enzyme activities (SOD, CAT, and POD) and decreased electrolyte leakage and MDA content under in vitro DS conditions (Jafari and Shahsavar 2022). In another study, 100 μM SNP significantly mitigated the adverse effects of DS in tomato by enhancing plant growth attributes, Chl, RWC, osmolyte accumulation, and antioxidant enzyme activities (Elkelish et al. 2021). Zangani et al. (2021) reported that applying 100 μM SNP to milk thistle (Silybum marianum L.) decreased MDA and H2O2 levels and secondary metabolite contents (taxifolin, silychristin, silybin A and B, and isosilybin B) under DS (Zangani et al. 2021). A low SNP application (5 μM) improved banana (Musa paradisiaca Linn) root growth under water deficit conditions and increased the level of proteins involved in stress responses and carbohydrate and energy metabolism (Lau and Tan 2021). Soybean plants pretreated with 100 μM NO exhibited improved plant growth, antioxidant defense mechanisms, and osmotic adjustment by accumulating compatible solutes under DS conditions (Rezayian et al. 2020).
In gasotransmitters-mediated DS tolerance, H2S and NO are crucial regulators of plant physiological and molecular responses to water deficit. Their versatile roles include gene expression modulation, antioxidant defense system activation, and osmotic adjustment, collectively enhancing plant DS tolerance. Although substantial advancement has been made in recognizing the roles of H2S and NO in DS tolerance, the role of CO remains mainly unknown. Nearly no study has been performed on how CO influences plant DS tolerance. Harnessing the signaling properties of H2S, NO, and CO presents exciting prospects for advancing sustainable agriculture under DS conditions, emphasizing the need for further research to explore their new mechanisms and potential applications.
3.4 Polyamines-mediated drought stress tolerance
Polyamines, including Put, Spm, and Spd, are important compounds in all living organisms (Hasanuzzaman et al. 2019; Gerlin et al. 2021). These compounds participate in various physiological processes, including cell division, plant growth, floral development, secondary metabolism, leaf senescence, DNA synthesis, gene transcription, and protein translation (Shao et al. 2022; Kebert et al. 2023). Polyamines, a class of PGR, are considered crucial signaling molecules due to their antioxidant and osmoprotective properties with high ROS-scavenging capacity (Kebert et al. 2023). Consequently, PAs play a key role in improving biotic and abiotic stress responses in plants (Shao et al. 2022; Kebert et al. 2023). In the following sections, we focus on PAs-mediating DS tolerance in plants.
3.4.1 Putrescine
Putrescine, present in mammalian and plant cells (Handa et al. 2018), is synthesized from L-arginine through the consecutive action of three enzymes: arginine decarboxylase, agmatine amidohydrolase, and N-carbamoyl putrescine amidohydrolase (Hasanuzzaman et al. 2019). Its accumulation in response to DS helps mitigate oxidative injury by regulating cellular pH, ion homeostasis, and antioxidant defense mechanisms (Ahangir et al. 2020). Exogenous application of Put enhances DS tolerance by modulating physiological and molecular mechanisms. For example, low-level Put supplementation (1.0 ppm) alleviated DS in wheat by improving RWC and enhancing membrane stability index, leaf area index, and leaf area ratio (Wasaya et al. 2023). In another wheat study, 1 mM Put increased root length, plant height, leaf number, tiller number, flag leaf area, shoot and root dry weights, spikelet number, grain number, and spike weight and enhanced several physiological and biochemical parameters, including Chl a, Chl b, carotenoids, amino acids, soluble sugars, and phenols, which protected wheat plants from DS (Hussein et al. 2023).
Hassan et al. (2020) reported that 100 μM exogenous Put to wheat plants under DS improved growth, biomass yield, CAT activity, Rubisco level, and photosynthetic pigment content and decreased EL%, MDA content, and ROS markers, contributing to the mitigation of DS effects (Hassan et al. 2020). In another study, exogenous Put application to rapeseed under DS alleviated oxidative stress by improving POD, CAT, APX, SOD activities, RWC%, membrane stability, and photosynthetic pigment content, thus enhancing grain and oil yields (Ghassemi-Golezani et al. 2019). For two contrasting wheat cultivars (Katya and Zora), exogenous Put application enhanced photosynthetic performance, proline accumulation, and antioxidant activity in DS-tolerant Katya but decreased these parameters in DS-sensitive Zora (Doneva et al. 2021). In the same study, exogenous Put was potentially linked to reduced Put catabolism, suggesting a high Put level is required under DS (Doneva et al. 2021).
Putrescine also acts as an elicitor, inducing various physiological and metabolic alterations in plants. For instance, exogenous Put application (20 and 40 mg L−1) to common thyme (Thymus vulgaris) mitigated the adverse effects of DS by decreasing cellular injury and enhancing antioxidant enzyme activities (SOD, POD, APX, and CAT) and volatile components, including thymol, carvacrol, γ-terpinene and p-cymen (Mohammadi et al. 2018). Moreover, Put extends to altering plant anatomy (root, stem, and leaf) and improving WUE, as confirmed in T. vulgaris (Abd Elbar et al. 2019). Moreover, Put significantly increased total soluble phenolic compounds, PAL and polyphenol oxidase (PPO) activities, Chl (a, b, and a/b) concentrations, plant growth, and yield in T. vulgaris (Abd Elbar et al. 2019). In drought-stressed T. daenensis, 0.2 mM Put decreased drought-induced oxidative stress indicators (MDA and H2O2) and increased key enzymatic and non-enzymatic antioxidants (SOD, CAT, POD, PPO, total phenol, and flavonoid), accompanied by the accumulation of proline, PAL, tyrosine ammonia-lyase, IAA and MeJA content, improving plant fitness and DS tolerance (Shahroudi et al. 2023).
The effective dose of Put varies across different plant species. For instance, low doses of Put (0.01, 0.1, and 1.0 mM) to grapes increased SOD, POD, and CAT activities and AsA and GSH levels, alleviating DS (Zhao et al. 2021b). In sugar beet, low doses of Put (0.3, 0.6, and 0.9 mM) enhanced the expression trends of antioxidant candidate genes (Cu/ZnSOD, FeSOD, MnSOD, CAT, and APX) involved in antioxidant defenses against DS (Islam et al. 2022). Interestingly, 0.1 mM Put significantly changed the flow rate and direction of Ca2+, enhancing DS tolerance in lettuce (Lactuca sativa L.) plants (Zhu et al. 2019). In summary, low doses of Put can help stimulate plant growth, agronomic traits, plant fitness, and DS tolerance across diverse plant species.
3.4.2 Spermine
Spermine is the final product of PA biosynthesis in plants (Hasanuzzaman et al. 2019), playing a crucial role in counteracting oxidative stress-induced cellular damage by enhancing antioxidant enzymes and detoxifying methylglyoxal through glyoxalase I and II in plants (Hasanuzzaman et al. 2019). Spermine molecule has been explored as a key compound of DS tolerance in plants, showing promising effects across various species. For example, a drought-tolerant tomato genotype exhibited elevated Spm levels and antioxidant enzyme activities (SOD and CAT), providing better tolerance against DS-induced oxidative stress (Sánchez-Rodríguez et al. 2016). A low dose of Spm (1 mM) sprayed on garden nasturtium (Tropaeolum majus) seedlings enhanced plant fitness and tolerance against prolonged DS (60 days) by improving growth and yield traits, antioxidant enzyme activities (CAT, POD, and APX), and metabolite accumulation (free amino acids, phenolic compounds, reducing and non-reducing sugars) (da Silva et al. 2022). In wheat, foliar application of Spm (100 μM) improved the function and structure of mesophyll cells and chloroplast ultrastructure and reduced ROS-induced cellular damage and EL, mitigating the adverse effect of DS on leaf physiology (Hassan et al. 2020). Maintaining photosynthetic pigments (Chl a, b, and total), photosynthesis performance, plant growth, osmoregulation, and antioxidant defense are crucial for protecting DS in plants. Nahar et al. (2017) reported that 0.2 mM Spm maintained enzymatic and non-enzymatic antioxidants (COD, CAT, GPX, DHAR, GR, AsA, and GSH), reduced cytotoxic compound production (methylglyoxal) and enhanced DS tolerance in mung bean (Vigna radiata L.).
Drought induces significant changes in gene transcription levels in plants. For example, Heydari et al. (2023) reported that foliar application of Spm (1, 2, and 3 mM) alleviated DS in zinnia (Zinnia elegans L.) plants by improving various physiological traits (growth, photosynthetic performance, WUE, biomass yield, flower diameter, and vase life), accompanied by enhanced antioxidant enzyme activities (POD and APX), regulation of genes related to anthocyanin biosynthesis (ZeCHS, ZeCHI, ZeF3H, ZeF3′H, ZeDFR, ZeANS, Ze3GT, and ZeAT2) and reduced cellular lipid peroxidation (MDA) levels. Interestingly, the higher Spm concentration (3 mM) exhibited better DS tolerance than lower concentrations (1 and 2 mM) after 40 days at the 8-leaf stage, with 4–6 mM exhibiting adverse effects on zinnia vase life (Heydari et al. 2023). In orange (Poncirus trifoliata L.) seedlings, 1 mM Spm protected plants from combined heat and DS by upregulating the expression of Hsp70, Hsp90, Hsp100, ABF, and NCED3 genes and enhancing antioxidative performance (Fu et al. 2014). Recent research demonstrated that foliar application of Spm (1 mM) to nasturtium (Tropaeolum majus) triggered the metabolism of sugar and phenolic compounds, reducing ethylene production and protecting against the harmful effect of DS (Silva et al. 2023). In white clover seedings, 0.5 mM Spm regulated carbohydrate metabolism (sucrose, fructose, and glucose), the expression of key genes involved in dehydrins synthesis (Y2SK, Y2K, and SK2), and antioxidant enzyme activities and reduced EL and lipid peroxidation (Li et al. 2015a). In another white clover study, 0.5 mM Spm enhanced DS tolerance by altering endogenous PGRs (CKs, GA, and IAA), regulating stress-responsive gene expression (FeSOD, Cu/ZnSOD, MnSOD, CAT, GPOX, MDHAR, DHAR, GR, GPX, GST, ADC, ODC, SAMDC, PAO, and CuAO) and accumulating PAs by influencing various enzymes involved in their metabolism, such as arginine decarboxylase, S-adenosyl methionine decarboxylase, copper-containing amine oxidase and polyamine oxidase activity (Zhang et al. 2018). In barley, the efficacy of Spm application in alleviating DS depended on dose and time, influencing endogenous PA levels, protein content, and antioxidant activities (SOD, CAT, and APX), ultimately leading to better plant growth and fitness (Özmen et al. 2022).
These studies highlight the potential of Spm in enhancing plant tolerance to DS. However, the efficiency of Spm-mediated improvement may vary depending on the applied dose, plant species, cultivar or genotypic differences, and duration of DS exposure.
3.4.3 Spermidine
Spermidine is a small, ubiquitous, colorless, and nitrogenous compound that, like other PAs, plays a crucial role in plant growth regulation due to its involvement in promoting plant growth and developmental processes (Hasan et al. 2021b). Like Put and Spm, Spd's involvement in plant DS tolerance has been documented in recent studies (Tian et al. 2022; Gholizadeh et al. 2022), illuminating promising effects on physiological, biochemical, and molecular levels. For example, low levels of Spd (0.5–1.0 mM) significantly improved SOD and CAT activities and soluble protein, proline, and soluble sugar contents in the shoots of foxtail barley under prolonged DS (Tian et al. 2022). Similarly, in wheat, 10 mg L−1 Spd induced the genes encoding enzymes involved in PA biosynthesis [ornithine decarboxylase (ODC), arginine decarboxylase (ADC), S-adenosylmethionine decarboxylase (SAMDC), and spermidine synthase (SPDS)] and PA catabolism [polyamine oxidase (PAO)] (Gholizadeh et al. 2022). Recently, exogenous supplementation of Spd significantly improved DS tolerance in winterberry (Ilex verticillata) by enhancing SOD, POD, and CAT activities and soluble sugar and ABA contents, alleviating leaf chlorosis and dryness (Xie et al. 2023).
Exogenous application of Spd (20 μM) enhanced DS tolerance in white clover by improving morphological traits and RWC content and enhancing antioxidant enzyme activities (SOD, CAT, POD, and APX) and the expression of their corresponding genes (TrAPX, TrCAT, TrPOD, and TrAPX), effectively reducing lipid peroxidation (MDA content) and EL content (Peng et al. 2016). Liu et al. (2017) reported that Spd enhanced DS tolerance in centipede grass (Eremochloa ophiuroides [Munro] Hack.) by boosting enzymatic and non-enzymatic antioxidant activities (SOD, CAT, APX, GR, and AsA), with significant positive correlations between endogenous Spd accumulation and these enzyme activities. The coordination of endogenous Spd and the antioxidant defense system increased plant fitness in centipede grass exposed to 7 days of DS (Liu et al. 2017). In maize, Spd-mediated induction of endogenous PAs, increased IAA, GA3, SA, JA, and zeatin riboside (ZR) concentrations, and restored photosynthetic pigment contents (Chl a, b and total), photosynthetic rate (Pn), PSII efficiency (Fv/Fm), PSII operating efficiency (ФPSII), and non-photochemical quenching alleviated DS-induced impairments (Li et al. 2018c). Moreover, in creeping bentgrass (Agrostis stolonifera), Spd application (0.2 mM spray) significantly improved DS tolerance by enhancing antioxidant enzyme activities (SOD, APX, CAT, and POD) and endogenous polyamine accumulation (Put, Spm, and Spd) (Li et al. 2015b).
Polyamine metabolism in plants significantly regulates metabolic pathways associated with plant growth, survival, and stress tolerance. For example, Spd metabolism benefits plant tolerance by enhancing antioxidant compounds and raising the redox state of AsA, GSH, and endogenous PAs (Li et al. 2018b). Dong et al. (2022) found that exogenous Spd supplementation optimized N2 metabolism and improved yield by regulating plant growth and water and nutrient use efficiencies in maize. In wheat, Spd application (–50 to –60 kPa) promoted grain filling by modulating starch synthesis-related genes (TaIPT2 and TaIPT8), accumulating CK (zeatin and ZR), and enhancing antioxidant enzyme activities (SOD, POD, and CAT) (Li et al. 2020).
Numerous studies demonstrate that PAs, including Put, Spm, and Spd, are ubiquitous compounds complicatedly linked to plant growth, fitness, and stress tolerance. The mechanistic understanding of how Put, Spm, and Spd contribute to DS tolerance across various plant species is apparent. However, the efficacy of these molecules in mitigating DS depends on the plant species, applied PA dose, treatment type, duration of DS exposure, and growth stage.
4 INTERACTION AND CROSSTALK AMONG DIVERSE PGRs UNDER DROUGHT STRESS
Phytohormones are central PGRs governing various aspects of a plant's life cycle (Mubarik et al. 2021; Sabagh et al. 2021; Raza et al. 2022b; Raza et al. 2023a). While plants produce hormones to support their growth, they must also navigate environmental stressors. In response to these stress stimuli, plants evoke adaptive mechanisms regulated by a multitude of phytohormones, including auxins, ABA, CKs, JAs, CAs, BRs, and SLs (Mubarik et al. 2021; Sabagh et al. 2021; Raza et al. 2022b; Raza et al. 2023a). These phytohormones orchestrate plant adaptation to environmental stress by controlling the production of osmoprotectants (e.g., proline, and GB), sugar molecules, antioxidants (e.g., SOD, CAT, APX, and GR), and ion transport mechanisms (Sabagh et al. 2021; Rahman et al. 2022). Furthermore, phytohormones interact with signaling molecules (e.g., H2O2, NO, and H2S) (Asgher et al. 2017; Mukherjee and Corpas 2020), PAs (e.g., Put, Spm, and Spd) (Asgher et al. 2017; Napieraj et al. 2023), and NTs (e.g., SRT, MLT, DOPA, Ach, and GABA) to shape plant responses to environmental stressors, including DS (Pardo-Hernández et al. 2020; Raza et al. 2022a; Raza et al. 2022b; Martínez-Lorente et al. 2022). The following sections highlight the interactions and crosstalk among diverse PGRs that underpin plant responses to DS and the mechanisms underlying tolerance.
4.1 Phytohormones and other PGRs
The interplay among various phytohormones is crucial for coordinating plant growth responses under environmental stress. For instance, SA biosynthesis decreases in response to DS signals, inducing ABA production (Park et al. 2021), a key hormone involved in DS tolerance [see review by Muhammad Aslam et al. (2022)]. Abscisic acid induction stimulates ROS (H2O2), NO, and Ca2+ production, which initiates a cascade of signaling events culminating in stomatal closure, thereby regulating excess transpiration and enhancing DS tolerance (Bharath et al. 2021). In addition to ABA, DS triggers complex hormone regulations involving auxins, ET, JAs, BRs, and GAs while inhibiting CK accumulation (Figure 4A). Ethylene, for example, exhibits antagonistic behavior with ABA during root and shoot growth under DS (Kim et al. 2022). However, overexpression of ETHYLENE RESPONSE FACTOR1 (ERF1) induced by JA or ET synergistically or individually enhances DS tolerance (Li et al. 2018a) (Figure 4A). Abscisic acid also interacts with ABA-responsive TFs, including DEHYDRATION-RESPONSIVE ELEMENT-BINDING (DREB), 9-CIS-EPOXYCAROTENOID DIOXYGENASES1 (NCED), and myeloblastosis (MYB) to regulate DS-responsive genes and enhance DS adaptability (Muhammad Aslam et al. 2022) (Figure 4A). Furthermore, DS signals activate several TFs directly, such as AFs, ABA-responsive element-binding (AREB), ABRE, and ZIP, which play crucial roles in regulating DS-responsive genes. These complex hormonal interactions, coupled with coordinated physiological and molecular adjustments, synergistically promote enhanced DS adaptation in plants (Figure 4B).
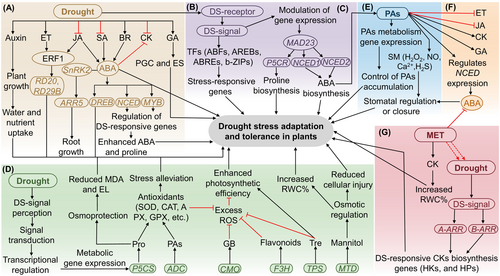
9-cis-epoxycarotenoid dioxygenases (NCED); ABA-responsive element-binding factors (ABFs/AREBs); ABA-responsive element-binding protein (AREB); abscisic acid (ABA); arginine decarboxylase gene (ADE); ascorbate peroxidase (APX); brassinosteroids (BRs); catalase (CAT); choline monooxygenase (CMO); cytokinins (CKs); dehydration-responsive element-binding (DREB); Δ1-pyrroline-5-carboxylate synthetase (P5CS); Drought stress (DS); energy saving (ES); ethylene (ET); ethylene response factor1 (ERF1); flavanone 3-hydroxylase (F3H); gibberellic acid (GA); glycine betaine (GB); guaiacol peroxidase (GPX); histidine kinases (HKs); histidine phosphotransferases (HPs); jasmonic acid (JA); mannitol dehydrogenase (MDT); melatonin (MET); myeloblastosis (MYB); phosphorylates type-A RR5 (ARR5); plant growth control (PGC); polyamines (PAs); proline (Pro); reactive oxygen species (ROS); salicylic acid (SA); signaling molecule (SM); superoxide dismutase (SOD); transcription factors (TFs); trehalose (Tre); trehalose-6-phosphate synthase (TPS).
Plants use various strategies to combat DS, including metabolite synthesis and osmotic adjustment (Rahman et al. 2016; Mubarik et al. 2021). DS triggers the accumulation of compatible solutes like proline, GB, and soluble sugars (Muhammad Aslam et al. 2022), which help to maintain water potential and osmotic adjustment and stabilize proteins and membrane lipid bilayer structures to sustain normal physiological processes. Molecular crosstalk exists among ABA, proline, and their regulatory genes. For example, the rice MAD23 gene acts as a positive modulator of ABA biosynthesis, increasing ABA acquisition by triggering ABA and proline biosynthesis genes (NCED1, NCED2, NCED3, NCED4, and P5CR), ultimately enhancing DS and salt stress tolerance (Li et al. 2021) (Figure 4C). Additionally, DS induces ABA that binds to PYR/PYL/RCAR receptors during the initial phase of ABA signaling, activating the SNF1-related protein kinase subfamily 2 (SnRK2s, SnRK2.2, SnRK2.3, and SnRK2.6), which phosphorylate ABA-responsive b-ZIPs and other TFs such as ABFs, ABRE, AREBs, and DRE (Muhammad Aslam et al. 2022). A transactivation study showed that TF b-ZIP72 activates the expression of genes involved in sucrose transportation (SWEET13 and SWEET15), helping rice plants to adapt to DS (Mathan et al. 2021).
4.2 Polyamines and other PGRs
The crosstalk between PAs and other PGRs under DS has been documented in various plants (Napieraj et al. 2023). For example, DS-responsive ABA modulated the expression of PA metabolism genes and subsequently controlled PA accumulation. Drought-responsive ABA and/or PAs regulate the production of several signaling molecules, including H2O2, NO, Ca2+, H2S, SA, JA, and MLT (Roychoudhury and Af 2021) (Figure 4E). These signaling molecules act synergistically or antagonistically to elicit specific responses. The interdependence of PAs, such as Put, Spd, and Spm, in plants is fascinating. For instance, in Arabidopsis, the acl5/Spms mutant, which lacks Spm production and is hypersensitive to DS, exhibits restored fitness upon pretreatment with Put and Spd, indicating the role of Spm deficiency in drought sensitivity (Yamaguchi et al. 2007). Moreover, a high ratio of endogenous (Spd+Spm)/Put has been associated with DS tolerance in mycorrhizal Pinus massoniana, where ectomycorrhiza adjust their Put biosynthesis and conversion, enhancing DS tolerance (Sánchez-Rodríguez et al. 2016). Furthermore, PAs coordinate with other PGRs in plants. Foliar supplementation of PAs can modulate various physiological and metabolic processes, including the accumulation of AAs, Pro, and sugars, thereby enhancing the efficiency of bioactive substances and antioxidants, ultimately protecting against DS-induced impairment of yield-related traits (Sánchez-Rodríguez et al. 2016). Additionally, DS-induced ABA promotes H2O2 production, which aids in stomatal closure and helps maintain water balance during DS adaptation (Napieraj et al. 2023) (Figure 4E). However, excessive H2O2 production can lead to oxidative stress, cellular injury, or even plant death. PAs also interact with other PGRs involved in DS adaptation. They regulate the accumulation of CKs and GAs but inhibit ET and JA. Moreover, PAs activate 9-CIS-EPOXYCAROTENOID DIOXYGENASE (NCED) gene expression for ABA biosynthesis, contributing to DS tolerance in plants (He et al. 2018) (Figure 4F).
4.3 Neurotransmitters and other PGRs
Researchers have identified interactions and crosstalk between several NTs (e.g., SRT, MLT, Ach, and DOPA) and other PGRs in enhancing biotic and abiotic stress tolerance in plants (Khan et al. 2022; Martínez-Lorente et al. 2022; Raza et al. 2022a; Raza et al. 2022b). While limited literature specifically addresses the multiple crosstalk of DS, NTs, and phytohormones, MLTs and other phytohormones have been extensively studied in the context of drought and other abiotic stress stimuli. In this context, DS induces endogenous MLT production, activating CK-responsive signaling components and several TFs (e.g., type-A ARR, type-B ARR), thus modulating drought-responsive CK biosynthesis genes, including HKs and HPs, ultimately facilitating DS adaptation in plants (Figure 4G). Melatonin has been shown to increase CK levels, enhancing RWC%, Chl level, photochemical performance, and DS tolerance (Ma et al. 2018). Conversely, MLT exhibited an antagonistic response to ABA accumulation, resulting in the downregulation of the ABA biosynthesis gene (NCED) and reduced ABA levels (Khan et al. 2022). Exogenous MLT application (100 and 150 mM) increased endogenous IAA levels and improved growth and yield potential in moringa (Moringa oleifera) plants under DS (Sadak et al. 2020). However, high doses of MLT decreased IAA levels. Melatonin application also increased GA accumulation under DS. Moreover, MLT regulates the expression of genes associated with PGRs, including hormone signaling pathway receptors (Khan et al. 2020). Catecholamine, a dihydroxyphenylalanine (DOPA), positively correlates with ABA in plants. For instance, exogenous ABA supplementation significantly elevated DOPA levels in potato plants (Świędrych et al. 2004). Gibberellins (GA3) induces hypocotyl growth in lettuce (Kamisaka 1973), and DOPA stimulates the GA3 response, as confirmed in hypocotyl root cells (Kamisaka 1979). γ-aminobutyric acid induces IAA, JA, L-arginine, and Spm production and enhances antioxidant activity, improving plant growth and DS tolerance in radiata pine (Pinus radiata) plants (De Diego et al. 2015). Moreover, functional coordination between GABA and CKs in transgenic barley plants has been observed, with GABA response genes (GAD and ALMT) influencing CK levels, leading to changes in root architecture and DS avoidance (Pospíšilová et al. 2016). While substantial research has been conducted on the coordination and crosstalk between NTs and plant regulators, specific interactions involving SRT and Ach with other PGRs in plants under DS remain less explored, presenting significant opportunities for further enrichment and understanding. In summary, the coordination between NTs and PGRs can exhibit synergistic or antagonistic effects on plant fitness under DS, highlighting the unique regulatory network governing plant responses to environmental stress.
4.4 Gasotransmitters and other PGRs
Gaseous molecules, mainly H2S and NO, are crucial plant signaling molecules and play significant roles in various developmental processes. Studies have documented the coordination between H2S and NO during DS and other stress conditions (Asgher et al. 2017; Mukherjee and Corpas 2020). For example, pretreatment of alfalfa with NO and an H2S donor (NOSH) enhanced survival rates under DS by inducing cellular accumulation of NO and H2S (Antoniou et al. 2020). Inhibition of NO and H2S accumulation by their respective inhibitors (cPTIO for NO, HA for H2S) reduced the effectiveness of the synthetic priming agent against DS, highlighting the potential of NO and H2S in mitigating DS in alfalfa (Antoniou et al. 2020). Drought induces PA accumulation, stimulating DS-responsive signaling molecules, such as H2O2, NO, Ca2+, and H2S (Figure 4E). This interplay among signaling molecules, including gasotransmitters and other PGRs, contributes to the plant's ability to adapt and tolerate various stresses during its development.
Polyamines play a multifaceted role in plants under DS, including controlling hormone homeostasis by enhancing CK and GA production while inhibiting ET and JA production under stress conditions. Signaling molecules such as H2O2 and NO actively participate in stomatal regulation, a crucial strategy for DS adaptation in plants. This regulation is tightly linked to ABA biosynthesis. For instance, exogenous NO supplementation induces stomatal closure in faba bean exposed to light but inhibits stomatal closure in the dark, apparently due to the natural absence of endogenous NO in dark conditions. This spectacle is further assisted by applying the NO scavenger cPTIO, which confirms the role of endogenous NO in modulating stomatal behavior. In the absence of exogenous NO or endogenous NO synthesis, stomatal closure persists, highlighting the key role of NO signaling in light-mediated stomatal regulation (Xiao-Ping and Xi-Gui 2006). Moreover, water deficit induced endogenous NO production in alfalfa, mediated by ABA-dependent pathways, suggesting a positive relationship between NO and ABA (Planchet et al. 2014). In contrast, NO negatively regulates ABA signaling in guard cells through S-nitroslation of Cys137 of the OPEN STOMATA 1 (OST1)/SUCROSE NONFERMENTING 1 (SNF1)-RELATED PROTEIN KINASE 2.6 (SnRK2.6) (Wang et al. 2015). Consequently, NO exhibits positive and antagonistic relationships with ABA during stomatal regulation under DS. Conversely, H2S functions as a downstream element of NO in ET-induced stomatal regulation in bean (Jing et al. 2012). Furthermore, NO acts downstream of H2S in ABA-mediated stomatal closure in plants (but not in all and is still under investigation), suggesting paradoxical coordination of NO, H2O2 and H2S in response to DS (Shen et al. 2020; Mishra et al. 2021). The complex crosstalk among gaseous molecules and other PGRs highlights their potential roles in plant development and DS adaptation. These studies suggest synergistic or antagonistic interactions among these or other molecules during plant DS adaptation. Further research is needed to elucidate the specific mechanisms underlying these interactions and their implications for crop improvement under DS conditions.
5 OMICS-DRIVEN MOLECULAR BASIS UNDERLYING PGRs-MEDIATED DROUGHT STRESS TOLERANCE IN PLANTS
Harnessing the molecular basis of PGRs-mediated DS tolerance is a dynamically evolving field, and omics approaches play a fundamental role in elucidating the complex mechanisms at the genetic, proteomic, metabolomic, ionomic, microbiomics, and phenomic levels (Raza et al. 2023c; Raza et al. 2024). Leveraging these advanced techniques allows us to identify promising key players coordinating plant responses and DS adaptation (Figure 5) (Raza et al. 2023c). Omics approaches deliver insights into the pathways and regulatory networks that are involved in PGR-mediated DS tolerance in plants. This integrative approach holds the key to gaining deeper insights into the molecular underpinnings of plant DS tolerance (Figure 5).
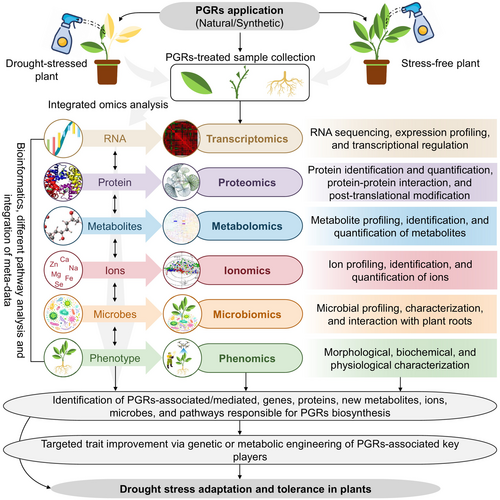
Plant growth regulators in plant cells control the expression of stress-responsive genes and their signaling mechanisms. For instance, IAA application to white clover cultivars improved the expression of DS-responsive genes (bZIP11, DREB2, MYB14, MYB48, WRKY2, WRKY56, WRKY108715, and RD22) and auxin-responsive genes (GH3.1, GH3.9, and IAA8), enhancing DS tolerance (Zhang et al. 2020). Moreover, ARF genes play an essential role in different cellular processes and metabolic pathways in rice, with the transcript abundance of several OsARF genes influenced by auxin treatment (Saibo et al. 2003; Zhang et al. 2020). In wheat, the expression of CALMODULIN, RPM1, CAT, SOD, and HSP70 genes was induced downstream by ABA and JA signaling pathways associated factors, i.e., SnRKs, JAZs, and MYC2, which subsequently improved DS tolerance (Wang et al. 2021b). Transcriptomic analysis suggested that the expression of various DS-related genes, such as CYP706A6, ACO4, ARK3, ABCG40, PDIL, NHL2, DDB2, and GPX8, was significantly regulated by BR signaling pathways, improving DS tolerance and promoting growth and development in WT and micro-tom (MT) tomato cultivars (Lee et al. 2018). Sahni et al. (2016) reported that DS tolerance improved in rapeseed due to the overexpression of AtDWF4, a BR-biosynthetic gene.
A comparative transcriptomic study discovered that MLT treatment positively regulated the expression of starch and sucrose metabolism-associated genes (SuS5 and SuS6), carbon fixation-related genes (TIM, RPI, and PCK) of photosynthetic organisms, and Ca2+ signaling pathway-associated genes (GAPA and CNGCs and CAM/CMLs), enhancing DS tolerance in loquat (Eriobotrya japonica) (Wang et al. 2021a). Another transcriptomic analysis identified 957 differentially expressed genes in MLT-treated maize cultivars, with major MLT-regulated genes associated with JA biosynthesis, glutathione metabolism, and Ca2+ signaling transduction to mitigate DS (Zhao et al. 2021a). Comparative RNA-seq profiling showed that MLT pretreatment stimulated 2,737 differentially expressed genes in dove-tree (Davidia involucrate) under DS, most of which were related to auxin signaling and BR biosynthesis (Liu et al. 2021b). In another transcriptome study, MLT improved DS tolerance in tomato genotypes by upregulating and downregulating the expression of linoleic acid synthesis (TGL4) and catabolism-related enzymes (CYP1A2 and LOX2S) genes, respectively (Yang et al. 2022). Transcriptome analysis of MLT-treated maize seedlings suggested the key role of genes associated with flavonoid biosynthesis (e.g., PAL, C4H, 4CL, HCT, CHS, CHI, F3′5′H, and DFR), DS-responsive TFs (ERFs, NACs, MYBs, and bHLHs), and plant hormone signaling (ERF4, ERF81, and ERF110) in DS tolerance (Wang et al. 2023).
The capacity of plants to maintain an optimum level of primary and secondary metabolites and defense responses often determines their DS tolerance. Metabolic analysis can play an important role in understanding and recognizing key mechanisms for DS tolerance in plants by analyzing phenotypic and genotypic changes (Kumar et al. 2021; Raza et al. 2023c). For example, a metabolomics study identified 53 differentially accumulated metabolites in tobacco seedlings treated with exogenous ABA, with the upregulated metabolites belonging to deoxyadenosine triphosphate, alkaloids (e.g., atropine, L-phenylalanine, cinnamic acid, inositol, keraxanthin, flaproone, hydroxy cinnamon acid, feruate), and some nucleotides (Deng et al. 2023). Improved DS tolerance in MLT-pretreated D. involucrate seedlings was achieved by regulating phenylpropanoid biosynthesis pathways, thereby increasing ROS-scavenging potential (Liu et al. 2021b). Another metabolome study identified 44 metabolites related to GABA shunt, tricarboxylic acid (TCA) cycle, and sugar and amino acid metabolic pathways in white clover under DS (Li et al. 2019). The authors found that β-sitosterol application promoted the accumulation of metabolites such as Glu, fructose, organic acids (e.g., glyceric acid, lactic acid, galacturonic acid, glycolic acid, shikimic acid, and quinic acid), and cysteine, with a lesser effect on the TCA cycle (Li et al. 2019). In maize, MLT treatment increased the DS tolerance by improving the higher accumulation of flavonoid metabolites, specifically apigenin, luteolin, and quercetin under DS (Wang et al. 2023).
A metabolome analysis in creeping bentgrass discovered that ABA promoted the accumulation of organic acids related to the TCA cycle, GABA enhanced amino acid and organic acid metabolism, and SA stimulated amino acid and carbohydrate biosynthesis, all contributing to enhanced DS tolerance (Li et al. 2017). Ramabulana et al. (2021) investigated metabolome changes in auxin- and CK-treated Bidens pilosa callus, identifying major metabolites such as mono- and di-acylated quinic acida (CGAs), primarily hydroxycinnamic acids (HCA) derivatives. Another non-targeted metabolomics analysis examined the effect of Spd on metabolite levels in wheat seedlings, identifying 43 metabolites belonging to five groups, including amino acids (18), organic acids (14), sugars (5), 3-3 polyols, and PAs (Put, Spd, and Spm), regulated by Spd to augment DS tolerance (Gholizadeh et al. 2022). Moreover, a metabolomics analysis conducted by Xu et al. (2022) showed that Spd improved carbohydrate and unsaturated fatty acid contents in tobacco, indicating its role in DS tolerance.
Interactions of proteins in different types of cells and complex structures are characterized and sorted using recent advanced proteomics approaches. These methods assess information associated with protein localizations related to stress signaling, stress tolerance, proteomes, and protein interaction maps (Alcázar et al. 2020; Hasan et al. 2021b). A study evaluating the proteomic responses in SA-applied wheat seedlings under DS found that SA increased proteins related to photosynthesis, energy production, carbohydrate, protein, and toxin metabolism, and stress defense systems, improving growth and DS tolerance (Kang et al. 2012). Another study in wheat identified 91 and 83 proteins in the Kundan variety and 90 and 46 proteins in the Lok1 variety at the vegetative and flowering stages, respectively, suggesting their role in SA-induced DS tolerance (Sharma et al. 2017). A proteomics study showed that SA maintained redox homeostasis and N remobilization in soybean due to the abundance of amino acid metabolism proteins, improving sink strength and DS tolerance (Sharma et al. 2018). In proteome analysis of maize leaves, MLT increased the abundance of key proteins involved in carbon fixation, photosynthesis, and biosynthesis of secondary metabolites and amino acids, suggesting their role in DS tolerance (Su et al. 2019).
In a recent integrated omics study (transcriptomic, metabolomic, and ionomic), the number of DEGs and DAMs increased with DS severity in rapeseed. Annotation analysis discovered that ion transport was notably squeezed, with early ionomic changes demonstrating decreased Mo, Fe, Zn, and Mn in rapeseed leaves before growth declined. Nutrient transport gene repression appeared before typical DS responses like ABA metabolism, proline accumulation, and oxidative stress defense (D'Oria et al. 2022). Exogenous proline application substantially modified the ionomic profile under DS in transgenic tobacco plants. Potassium was the most abundant nutrient, while micronutrients were largely higher in transgenic plants, except for Fe, probably because of competition with Cu. These findings advise that proline mitigates DS-induced nutrient defects by improving plant ion absorption and translocation (Cacefo et al. 2021).
Exogenous MLT application improved DS tolerance by enhancing non-structural carbohydrate metabolism enzymes and triggering antioxidative defense systems in the barley roots. Moreover, 16S/ITS rRNA gene-seq discovered that MLT significantly transformed the rhizosphere microbiome conformation, increasing bacterial abundance in carbohydrate and carboxylate degradation pathways, whereas decreasing fatty acid and inorganic nutrient metabolisms under DS, signifying the MLT power in DS tolerance via microbial interactions (Ye et al. 2022).
Harnessing the molecular basis of PGR-mediated DS tolerance is dynamically uncovered using diverse omics approaches. These tools have helped uncover key players in plant responses to DS (Figure 5). Diverse PGRs regulate stress-related genes and TFs, highlighting their role in improving DS tolerance. Metabolomics shows crucial phenotypic transformations, proteomics explains protein interactions in stress signaling, ionomics explains ion movement and homeostasis, and microbiomes show microbial interactions and compositions under DS. Future research should extend these studies to lesser-explored PGRs for comprehensive insights into DS tolerance in diverse plant species.
6 CONCLUDING REMARKS
The changing climate imposes diverse abiotic stresses, including DS, that significantly threaten global agriculture and food security. The new green chemical strategies, such as the supplementation of diverse PGRs, are a promising approach to address this challenge, enhancing plant fitness and DS tolerance and improving crop yields for sustainable food production. Our understanding of plant responses to DS has greatly advanced in recent years, emphasizing the unique network of multiple PGRs from various metabolic pathways involved in DS tolerance. This review focused on elucidating the responses and effects of DS on plants and the regulatory mechanisms mediated by key PGRs. We explored crosstalk and interactions among different PGRs, primarily regulated by stress-induced changes in plant defense systems. Moreover, omics analysis provided invaluable insights into the molecular basis of PGR-mediated DS tolerance, uncovering novel regulatory networks.
The judicious application of diverse PGRs suggests promising possibilities for modulating cellular homeostasis and enhancing plant performance. These regulators play crucial roles in activating antioxidant defense systems during drought-induced oxidative stress, while also acting as secondary messengers to initiate cascades of stress-related gene activation and inhibition. This complex interplay ultimately mitigates DS by boosting antioxidant activities and defensive compound biosynthesis. However, it is important to note that excessive or prolonged PGR application can disrupt plant metabolism, leading to adverse effects. Therefore, a balanced approach is necessary to harness the full potential of exogenous PGRs in enhancing DS tolerance without compromising plant health.
Overall, 'green chemical strategies' hold significant power for improving plant DS tolerance by regulating various morphological, physiological, biochemical, and molecular mechanisms. Further research is needed to fully elucidate the specific interactions and synergies among different PGRs, thereby maximizing their potential to enhance DS tolerance and ensure global food security.
7 WAY FORWARDS
Future research should focus on elucidating the dynamic roles of lesser-studied PGRs and their potential contributions to DS tolerance for a more comprehensive understanding of the regulatory mechanisms involved. Understanding the kinetics of PGR-induced changes is crucial, prompting their use in mitigating DS effects and necessitating investigations into the temporal dynamics of PGR responses. Moreover, bridging the gap between laboratory findings and field applications is essential: translating PGR-mediated DS tolerance strategies into practical and scalable agricultural practices for practical impact.
Exploring the crosstalk between DS and other stressors will be vital in developing robust stress management approaches, considering the interactive effects of PGRs with other abiotic and biotic stresses. Eco-physiological studies evaluating the long-term impacts of PGR application on plant ecosystems will contribute to ecological sustainability and address potential unintended outcomes. Furthermore, establishing standardized protocols for PGR application under DS conditions will facilitate comparative analysis across studies and aid in developing guidelines for optimal PGR application. In this regard, effective knowledge transfer strategies are crucial. Future research should also focus on closing the gap between scientific advancements and practical knowledge at the community level, ensuring that farmers and stakeholders benefit from 'green chemical strategies'-mediated DS tolerance insights.
Despite notable progress, challenges persist, necessitating a deeper exploration of plant developmental stages and their influence on metabolic regulation during DS. Moreover, elucidating precise genetic modifications to enhance DS tolerance, particularly by optimizing PGR biosynthesis, is crucial. Understanding stress response mechanisms, identifying key genes, and leveraging them to develop genetically engineered DS-smart plant production remain essential objectives. Accurate identification of multiple candidate genes is vital for improving the tolerance and yields of transgenic plants under DS conditions. Finally, genetic or microbial engineering of PGR-encoding genes/microbiomes emerges as a promising opportunity to enhance DS tolerance through targeted modifications in PGR biosynthesis and endogenous levels.
AUTHOR CONTRIBUTIONS
AR, KHMS, and ZH conceived the idea. AR, SB, MAR, PGC, RGRC, SC, RMR, and KHMS wrote the manuscript. AR and MAR designed the figures. AR, RGRC, RMR, FJC, SG, KHMS, and ZH critically reviewed and edited the manuscript. All authors have read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We thank all our colleagues whose contributions are cited in this article. We apologize to the authors of papers not mentioned in this manuscript due to space limitations.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
FUNDING
ZH received support from the Shenzhen Special Fund for Sustainable Development (KCXFZ20211020164013021), The grant from Development and Reform Commission of Shenzhen Municipality (XMHT20220104019), and Shenzhen University 2035 Program for Excellent Research (2022B010). RMR received support from the AGROALNEXT/MCIN with funding from the European Union Next Generation EU (PRTR-C17.I1). FJC research is supported by a European Regional Development Fund co-financed grants from the Ministry of Science and Innovation (PID2023-145153NB-C21), Spain.
DATA AVAILABILITY
No new datasets were generated or analyzed in this study.




