The function of an apple ATP-dependent Phosphofructokinase gene MdPFK5 in regulating salt stress
Abstract
Salt stress severely affects the growth and yield of apples (Malus domestica Borkh). Although salt-tolerant genes have been extensively studied, documentation on the role of the ATP-dependent phosphofructokinase gene MdPFK5 in salt stress is limited. This study conducted an evolutionary tree and three-dimensional structure analysis of the PFK gene family in Arabidopsis thaliana and MdPFK (MD01G1037400), revealing a close phylogenetic relationship between MdPFK (MD01G1037400) and AtPFK5. Given the similarity in their protein tertiary structures, MdPFK was designated as MdPFK5, suggesting functional similarities with AtPFK5. Further investigation revealed elevated expression levels of MdPFK5 in apple leaves and flowers, particularly showing significant upregulation 120 days after blooming and differential expression beginning at 3 hours of salt stress. Overexpression of MdPFPK5 conferred salt tolerance in both apple calli and transgenic lines of Arabidopsis thaliana. Moreover, NaCl treatment promoted soluble sugar accumulation in apple calli and transgenic lines of Arabidopsis thaliana overexpressing MdPFK5. This study provides new insights into the salt tolerance function of MdPFK5.
1 INTRODUCTION
Salt stress poses a significant threat to global agricultural development (van Zelm et al., 2020). Soil salinization is a global challenge, with salt adversely affecting plant growth and crop yield (Hou et al., 2023). The accumulation of sodium ions in the body of plants under salt stress leads to growth and development obstruction. Cultivation of salt-tolerant crops is one of the key measures to alleviate soil salinization (Zhao et al., 2021). The salt overly sensitive (SOS) pathway has been widely studied in plants as an important regulatory mechanism after salt stress (Liu et al., 2020). Mutating SOS3, the first gene in the SOS family to be identified, makes Arabidopsis more sensitive to high salt concentrations (Liu & Zhu, 1998). SOS3 activates the serine/threonine Ca2+ binding protein of SOS2 in a Ca2+-dependent manner (Halfter et al., 2000). SOS1 is phosphorylated by the SOS2-SOS3 protein kinase complex, which reduces the toxicity of salt ions (Mahajan & Tuteja, 2005). The close relationship between SOS1, SOS2, and SOS3 plays an important role in plant salt tolerance. Apples, as a globally important fruit, are highly sensitive to salt stress, significantly constraining their yield and quality (Chen et al., 2019). High salt levels notably inhibit apple plant growth, affecting root development, plant height, stem thickness, and root-to-shoot ratio, while also disrupting photosynthetic pigment content and chloroplast ultrastructure, thus impacting photosynthesis (Jia et al., 2019; Xian et al., 2023). The soluble sugar content is closely related to apple salt tolerance (Jiang et al., 2021). Yet the influence of sugar metabolism on regulating plant salt resistance remains to be explored, particularly in terms of identifying key genes involved.
The glycolytic pathway (glycolysis) serves as a crucial metabolic pathway within organisms, holding significant importance in cellular energy provision, carbon source supply, metabolic balance, and environmental adaptation, thus constituting one of the key metabolic pathways that are essential for sustaining life and functionality (Fernie et al., 2004). Research indicates a close correlation between glycolysis and abiotic stress. In plants, an important drought regulatory network has been identified, capable of initiating dynamic metabolic flux conversion from glycolysis to acetate synthesis, ultimately activating the jasmonic acid signaling pathway to regulate plant drought resistance (Kim et al., 2017). Under phosphorus deficiency, plants activate the cytoplasmic glycolytic pathway to alleviate phosphorus stress, thereby controlling plant growth (Bechtaoui et al., 2021). Phosphofructokinase (PFK) catalyzes the second step of the glycolytic pathway and is one of its key rate-limiting enzymes. In plants, PFK exists in two types: ATP-dependent PFK (ATP-PFK) and pyrophosphate-dependent PFK (PPi-PFK). ATP-PFK and PPi-PFK coexist and function in the cytoplasm, with ATP-PFK catalyzing the irreversible conversion from fructose-6-phosphate to fructose-1,6-bisphosphate (Kuil et al., 2023).
In Arabidopsis thaliana, the PFK family comprises seven distinct isozymes. Among these, two isozymes localize in chloroplasts, while the remaining five localize in the cytoplasm (Winkler et al., 2007). This gene family is divided into three subgroups, with subgroup PFK_A primarily containing cytoplasmic isozymes and subgroup PFK_C primarily containing chloroplastic isozymes (Mustroph et al., 2013). Variations in subcellular localization result in different functionalities among PFK family isozymes. PFK1, PFK3, PFK6, and PFK7 in Arabidopsis thaliana localize in the cytoplasm. Studies on these four isozymes show reduced PFK activity in the pfk1/7 double mutant compared with that in the pfk3/6 single mutant, exhibiting distinct growth and metabolic phenotypes, including slowed leaf growth, early senescence, and elevated hexose levels. In addition, alterations in short−/long-chain fatty sulfur metabolite ratios and root-to-shoot distribution were observed. However, no significant changes were observed in short-term carbon flux and hexose phosphate levels in this mutant. Thus, PFK1 and PFK7 were suggested to play crucial roles in leaf sugar metabolism and source–sink relationships, while PFK3 and PFK6 have minor effects under normal growth conditions (Perby et al., 2022). AtPFK5 localizes in chloroplasts and is regulated by redox (Mustroph et al., 2013). AtPFK5 activity decreases under thioredoxin-f reduction but can also be oxidatively activated through the Thioredoxin-like2/2-Cys peroxiredoxin pathway. Cys152 and Cys157 are critical sites for intramolecular disulfide bond formation in AtPFK5, regulating its function. AtPFK5 modulates light/dark metabolic transitions and, together with fructose-1,6-bisphosphatase, constitutes a crucial regulatory node (Sugiura et al., 2019). Research on the PFK gene family in Arabidopsis thaliana is extensive, whereas studies in other species are relatively limited. Studies on cotton indicate the critical role of PFK in drought stress. Through RNA-seq, drought-tolerant central genes GhPFK11 and GhPFK17 were identified. KEGG enrichment analysis revealed that under drought stress, most amino acids were upregulated, whereas some sugars were significantly downregulated, indicating the potential role of PFK genes in cotton drought tolerance and sugar metabolism, yet specific mechanisms have yet to be verified (Mehari et al., 2022). In apples, high nitrogen levels cause changes in PFK-related genes, resulting in decreased soluble sugar content in apple fruit (Wang et al., 2021). Despite existing research on PFK genes in plants, further exploration is needed to understand how PFK genes interconnect sugar metabolism and salt stress and function within them.
Limited research on PFK5 in apples is available, and its related functions require further elucidation. In this study, using the Gala apple cDNA as a template, one ATP-dependent PFK gene, MdPFK5, was obtained via PCR amplification. The study indicates that this gene responds to salt stress, promoting soluble sugar accumulation. The salt-resistant function of MdPFK5 was confirmed in transgenic apple calli and transgenic Arabidopsis thaliana. This research improves our understanding of the salt-tolerance mechanisms of apples and provides a theoretical basis for the creation of new salt-tolerant apple varieties.
2 MATERIALS AND METHODS
2.1 Experimental materials
We sampled five-year-old Gala apple trees planted in an experimental farm. These trees were covered with 10 μm nylon fabric to prevent pollen contamination from other trees. Samples of roots (growing roots), branches (one-year-old non-woody shoots), leaves (functional leaves), and flowers (bellflower stage) of the Gala apple trees were collected. Fruits were collected 10, 20, 30, 60, 90, and 120 days after flowering for follow-up studies. All samples were rapidly frozen in liquid nitrogen and stored at −80°C.
Gala apple tissue-cultured seedlings were planted in Murashige and Skoog (MS) solid culture medium containing 0.2 mg/L IAA (indole-3-acetic acid) and 0.8 mg/L 6-BA (6-benzylaminopurine) agar and placed in a growth chamber at 25°C under a 16-hour light/8-hour dark cycle. Tissue-cultured seedlings after rooting were treated with 100 mmol·L−1 NaCl. Samples were collected from tissue-cultured seedlings treated with NaCl for 0, 3, 6, 12, 24, and 48 hours, followed by rapid freezing in liquid nitrogen and low-temperature storage.
‘Orin’ apple calli was induced from young embryos. They were cultured in MS solid culture medium containing 0.5 mg·L−1 6-BA +0.2 mg·L−1 2,4-D, subcultured three times at three-week intervals and placed in the dark at 25°C for cultivation.
2.2 Cloning and promoter analysis of MdPFK5
The conserved protein domains of MdPFK5 were analyzed using the SMART online software. An evolutionary tree of the protein sequences of the AtPFK family and MdPFK (MD01G1037400) was constructed using MEGA7.0 for phylogenetic analysis. The tertiary structures of Arabidopsis thaliana AtPFK56 and apple MdPFK5 proteins were analyzed using the PHYRE 2 online software.
2.3 RNA extraction and qRT-PCR analysis
Total RNA was extracted from the stored samples by using the RNA Plant Plus reagent kit (Tiangen), and cDNA was synthesized using the PrimeScript® RT reagent kit (Perfect Real Time, TaKaRa). Apple 18S was used as the reference gene, and the relative expression level of MdPFK5 was analyzed using real-time fluorescence quantitative reverse-transcription polymerase chain reaction (qRT-PCR).
qRT-PCR experiments were conducted using the UltraSYBR Mixture (with ROX) kit (Kangweishiji) on a BIO-RAD IQ5 instrument. The reaction system (20 μL) included 10.0 μL of 2× UltraSYBR Mixture, 1.0 μL of upstream primer (MdPFK5-F: ACGAAGAGTGGCACGGCTAC), 1.0 μL of downstream primer (MdPFK5-R: GCATTCAGCACCAGCAGACG), 1.0 μL of cDNA, and 7.0 μL of ddH2O. Each sample was tested in triplicate. The qRT-PCR reaction conditions were as follows: pre-denaturation at 95°C for 10 min, denaturation at 95°C for 15 s, annealing at 56°C for 15 s, and extension at 65°C for 10 s, for a total of 40 cycles. Quantitative data analysis was performed using the 2-ΔΔCT method (Livak & Schmittgen, 2001).
2.4 Construction of MdPFK5 expression vector and genetic transformation of apple calli tissue and Arabidopsis
The cloned MdPFK5 was sequenced after being inserted into the pMD19-T vector (TaKaRa), which allows efficient cloning of PCR products (based on pUC19 vector; TA Cloning), and a plasmid containing the correct sequence was selected as templates for new PCR amplification with upstream (ATGGCCACTCTCTCCCACG) and downstream primers (CTAGATAAAGTCCGGCTGACCTG) (see Table S1 for the list of all primers used in this study). The PCR products were then ligated into the expression vector pCXSN-Myc to form the construct 35S:: MdPFK5-Myc. After confirmation of correct sequencing, plasmids were extracted and transformed into Agrobacterium tumefaciens strain GV3101 (empty vector used as control) for infiltration in ‘Orin’ apple calli following a previously published protocol (Hu et al. 2016; Zhao et al. 2019). To be specific, two-week-old ‘Orin’ apple calli were cultivated in liquid medium (MS + 1 mg/L 2,4-dichlorophenoxyacetic acid [2,4-D] +1 mg/L 6-BA+ 30 g/L sucrose, pH 5.8–6) containing A. tumefaciens by agitation at 120 rpm at 25°C for 10–20 min. The infected transgenic calli were spread on solid medium (MS + 1 mg/L 2,4-D + 1 mg/L 6-BA+ 30 g/L sucrose+8 g/L agarose, pH 5.8–6) for dark culture. After 2–3 days of dark culture, the transgenic calli were washed 2–3 times with sterile water and then transferred to the selection medium (MS + 1 mg/L 2,4-D + 1 mg/L 6-BA+ 30 g/L sucrose+8 g/L agarose+30 mg/L kanamycin) for further culture. The transgenic calli were cultured on the selection medium for 20–30 days and identified by PCR. Transgenic callus was identified for follow-up experiments. Agrobacterium suspension was used to infiltrate Arabidopsis flower buds, and harvested seeds were screened for transgenic Arabidopsis using MS medium supplemented with 60 mg/L kanamycin. Pure transgenic Arabidopsis lines were obtained after three generations of consecutive selection for use in subsequent experiments.
2.5 Physiological parameter determination of apple calli tissue and Arabidopsis
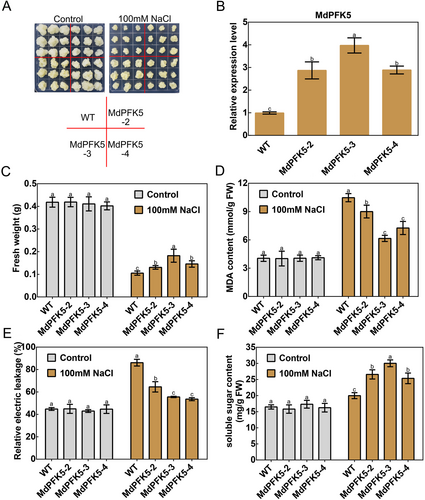
Three MdPFK5-overexpressing lines (MdPFK5-2, MdPFK5-3, and MdPFK5-4) and wild-type (WT) controls were treated and replicated three times in apple calli tissue MS solid culture medium supplemented with 100 mmol·L−1 NaCl. After 15 days, a portion of the calli was weighed, and another portion was used for physiological parameter measurement, including malondialdehyde (MDA) content and relative electrolyte leakage (REL). MDA content is an important parameter reflecting the potential antioxidant capacity of the body, which can reflect the rate and intensity of lipid peroxidation in the body, and also indirectly reflect the degree of tissue peroxidation damage (Kumar et al., 2023). REL is also an important physiological index to evaluate the integrity of plant cell membranes and the response to stress (Li et al., 2022b). So, we used MDA and REL to study the degree of tolerance to salt stress of apple calli overexpressing MdPFK5 compared with the control. MDA was quantified as described by Ma et al. (2017), and REL was determined following the method of Hu et al. (2013) with slight modifications.
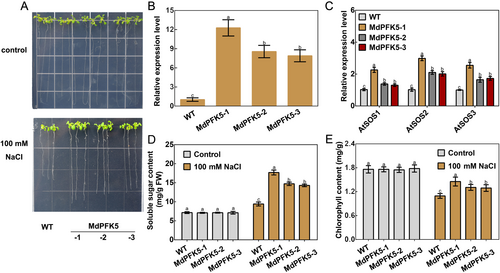
In Arabidopsis MS solid culture medium supplemented with 100 mmol·L−1 NaCl, three MdPFK5-overexpressing lines (MdPFK5-1, MdPFK5-2, and MdPFK5-3) and WT controls were treated and replicated three times. A portion of the transgenic seedlings was weighed, and root length was measured using a ruler. Another portion was used for physiological parameter determination, including chlorophyll content and soluble sugar content, following the methods described by (An et al. 2017) and the instructions provided by Beijing Solarbio Science & Technology Co., Ltd. for soluble sugar content measurement.
2.6 Data statistical analysis
Data were processed using SPASS 19.0 statistical software. Results were expressed as mean ± standard deviation of three independent experiments. Differences among treatments were analyzed using Tukey's test (p < 0.05). Different letters indicate significant differences (p < 0.05).
3 RESULTS
3.1 Evolutionary tree and subcellular localization prediction of MdPFK5
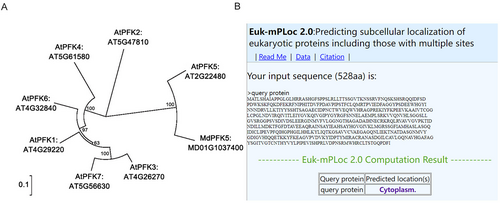
The protein sequences of the seven AtPFK family members in Arabidopsis and the MdPFK (MD01G1037400) protein sequence in apple were downloaded from the TAIR website. With the use of MEGA 7.0, an evolutionary tree analysis was conducted on MdPFK and the seven PFK sequences in Arabidopsis, revealing a close phylogenetic relationship between MdPFK protein and AtPFK5 in Arabidopsis. The similarity percentage between AtPFK5 and MdPFK5 is 100%. Consequently, the gene was named MdPFK5 (Figure 1A). Subsequently, the protein sequence of MdPFK5 was inputted into a website for subcellular localization prediction, indicating that MdPFK5 is localized in the cytoplasm (Figure 1B).
3.2 Conservation domain and three-dimensional structure analysis of MdPFK5
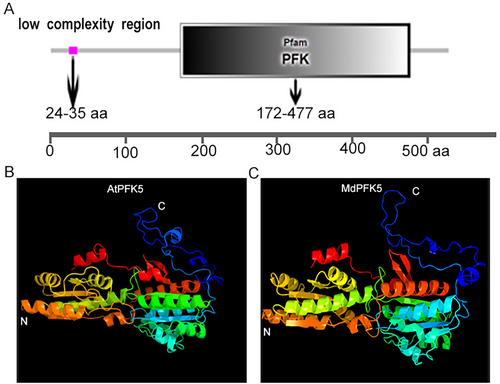
The protein sequence of MdPFK5 was subjected to conservation domain analysis using the SMART online tool. MdPFK contains a conserved PFK domain near the C-terminus, spanning amino acids 172 to 477, with a low-complexity region located at amino acids 24 to 35 toward the N-terminus (Figure 2A). A low-complexity region is a protein fragment consisting of one or a few types of amino acids and is correlated with the rate of evolution. Studies have shown that low-complexity regions behave differently in different protein families, with low conservation and structural flexibility (Kastano et al., 2021; Li & Kahveci, 2006). The three-dimensional structures of Arabidopsis AtPFK5 and apple MdPFK5 were analyzed using Phyre2 online software (Figures 2B,C). Comparative analysis of their 3D structures suggests potential functional similarities between AtPFK5 and MdPFK5. The physicochemical properties of the amino acids encoded by this gene were retrieved using the website (http://web.expasy.org/protparam/), revealing that MdPFK5 encodes 528 amino acids with a molecular weight of 57688.73 Da and an isoelectric point of 6.90.
3.3 Expression of MdPFK5 in different tissues and fruit maturation
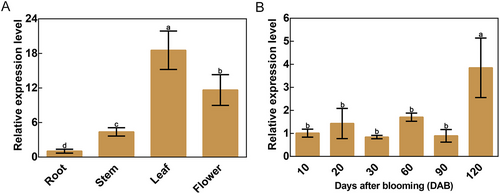
The relative expression of MdPFK5 in various apple tissues was analyzed using qRT-PCR (Figure 3A). The results indicate that MdPFK5 is expressed in the roots, stems, leaves, and flowers of apple, with relatively higher expression levels observed in the leaves and flowers. Subsequently, the expression of MdPFK5 was examined at different time stages during apple fruit development (10, 20, 30, 60, 90, and 120 days after blooming [DAB]) (Figure 3B), revealing differential expression starting from 120 DAB. These findings suggest that the functional domain of MdPFK5 is primarily concentrated in the leaves and flowers.
3.4 Response of MdPFK5 to abiotic stress
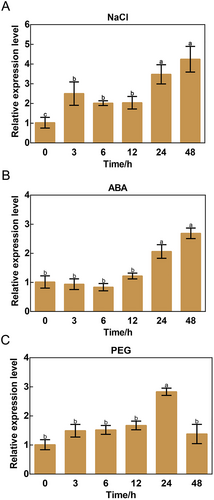
In a tissue culture system, apple does not have environmental influences and experimental errors are small; it is hence often used to study stress response (Sun et al., 2018; Ma et al., 2020; An et al., 2023). In this study, apple tissue cultures were treated with 100 mmol·L−1 NaCl, and samples were collected 0, 3, 6, 12, 24, and 48 hours after treatment. The response of MdPFK5 to NaCl was analyzed via qRT-PCR. The results indicate that MdPFK5 expression levels increased with the duration of NaCl treatment. Compared with the 0-hour treatment, MdPFK5 expression significantly increased at 3, 6, and 12 hours, with no significant differences between 3-, 6-, and 12-hour treatments. However, at 24 and 48 hours, MdPFK5 expression significantly increased compared with 3-, 6-, and 12-hour treatments, with no significant differences between 24- and 48-hour treatments (Figure 4A). This result suggested that MdPFK5 rapidly responds to NaCl induction. In addition to NaCl treatment, we subjected apple seedlings to ABA and PEG (simulating drought) treatments to investigate the response of MdPFK5 to hormones and drought stress. Seedlings were treated with 100 μmol·L−1 ABA, and samples were collected at 0, 3, 6, 12, 24, and 48 hours post-treatment (Figure 4B). The results showed that compared with the 0-hour treatment, MdPFK5 exhibited differential expression after 24 hours of ABA treatment. Similarly, seedlings were treated with 4% PEG4000, and samples were collected at 0, 3, 6, 12, 24, and 48 hours post-treatment (Figure 4C). The results indicated that compared with the 0-hour treatment, MdPFK5 exhibited differential expression after 24 hours of PEG treatment, with expression levels returning to baseline by 48 hours. Overall, these results demonstrate that MdPFK5 responds to salt, drought, and ABA treatments, with the fastest response observed for NaCl.
3.5 Phenotypic characterization of MdPFK5 transgenic apple calli for salt tolerance
Transgenic apple calli lines overexpressing MdPFK5 (MdPFK5-2, MdPFK5-3, and MdPFK5-4) were obtained using Agrobacterium-mediated transformation (Figure 5A). Real-time fluorescence qRT-PCR analysis revealed a significant increase in MdPFK5 expression levels in the three transgenic apple calli compared with the WT (Figure 5B). The WT and the three transgenic apple calli lines were treated with 0 and 100 mmol·L−1 NaCl, respectively (Figure 5A). Results showed that compared with the control, the transgenic apple calli overexpressing MdPFK5 exhibited significantly reduced sensitivity to NaCl, increased fresh weight, decreased levels of MDA and REL, and accumulation of soluble sugars (Figures 5E–F). These findings suggest that overexpression of MdPFK5 enhances salt tolerance in apple calli under NaCl treatment.
3.6 MdPFK5 enhances salt tolerance in Arabidopsis
Leaves were collected from WT Arabidopsis and three MdPFK5 transgenic lines (MdPFK5-1, MdPFK5-2, and MdPFK5-3). qRT-PCR analysis revealed a significant increase in MdPFK5 expression levels in the transgenic Arabidopsis lines (Figure 6B). Both WT and the three MdPFK5 transgenic Arabidopsis lines were treated with 0 and 100 mmol·L−1 NaCl, respectively, and their phenotypes are shown in Figure 6A. Compared with the WT, the three MdPFK5-overexpressing Arabidopsis lines exhibited significantly reduced sensitivity to NaCl, increased whole-plant fresh weight, and enhanced root length (Figure S1). Expression levels of SOS pathway genes, including AtSOS1, AtSOS2, and AtSOS3, were significantly upregulated in the MdPFK5-overexpressing Arabidopsis (Figure 6C), indicating that MdFPK5 partly enhances salt tolerance through the SOS pathway. Further analysis of soluble sugar and chlorophyll content before and after NaCl treatment revealed a significant increase in both parameters in the transgenic Arabidopsis (Figure 6D, E) These results demonstrate a significant enhancement in salt tolerance in MdPFK5 transgenic Arabidopsis compared with the WT.
4 DISCUSSION
As one of the world's significant fruits, apples are subjected to various abiotic stresses, such as salt, drought, and heat stress, during their growth and development (Bu et al., 2022; Li et al., 2022a; Li et al., 2022c). Salt stress, in particular, is a crucial constraint to the development of the apple industry. Studies have indicated that MdMYB46 enhances the salt and osmotic tolerance of apples by promoting lignin synthesis and activating stress-related gene expression (Chen et al., 2019). Meanwhile, MdNAC047 promotes ethylene production to resist salt stress (An et al., 2018). In Arabidopsis, overexpression of the zinc finger protein gene MdZAT5 reduces the expression levels of salt stress-related genes, and MdZAT5-overexpressing apple calli exhibit increased sensitivity to salt stress, suggesting MdZAT5 negatively regulates the salt tolerance of apples (Wang et al., 2022). Proteomic studies on salt-treated sugar beets have identified PFK5 as a novel salt-responsive protein, indicating a connection between salt stress and PFK5 (Wu et al., 2018). Previous studies showed that the apple transcription factor MdbHLH3 binds to the MdPFP-β promoter and activates the expression of MDPFP-β, thereby promoting the accumulation of soluble sugars in apples (Yu et al., 2022). Overexpression of PbPFP1 in pears can significantly increase the fruit's sugar content (Lü et al., 2019). Although PFP and PFK belong to the same glycolysis pathway, their catalytic substrates are different, and they vary in their specific regulatory functions. However, reports on PFK's effect on the sugar content are few. Therefore, the question of whether MdPFK5 overexpression in apples can increase the sugar content of fruits needs further investigation. However, research on the PFK family's role in abiotic stress in apples is scarce.
This study demonstrates that MdPFK5 responds not only to salt stress but also to drought and ABA (Figures 4 and 5). It also reveals the role of PFK5 in abiotic stress in apples. Under salt treatment, transgenic Arabidopsis overexpressing MdPFK5 showed reduced sensitivity to salt stress compared with the WT, as evidenced by measurements of fresh weight, root length, and chlorophyll content. In addition, PEG was used in this study to simulate drought stress. In the experimental environment, PEG molecules are large and cannot enter cells, so they will not cause toxicity to cells. PEG has good hydrophilicity, and the addition of water can reduce the solution water potential, and the water potential is inversely proportional to the amount of PEG added. The water absorption of plant roots is carried out by the principle that the cell water potential is lower than the soil water potential. Therefore, drought stress is caused, so PEG is generally used to simulate drought stress. However, PEG cannot fully simulate drought; the decrease of soil water content is a slow process, while PEG treatment quickly causes osmotic stress. We speculated that MdPFK5 gene may also promote resistance to osmotic stress. This finding indicates that the transgenic Arabidopsis experienced less stress than the WT did (Figures 6 and S1). This study also found that NaCl treatment promoted soluble sugar accumulation in MdPFK5 transgenic apple calli and transgenic Arabidopsis plants (Figures 5 and 6). MdPFK5 belongs to the PFK gene family, playing a crucial role in plant sugar metabolism (Zhu et al., 2013). Therefore, the increased salt tolerance in Arabidopsis with ectopic expression of MdPFK5 might be due to MdPFK5 being able to enhance soluble sugar content in plants to resist abiotic stress.
Since MdPFK5 belongs to ATP-dependent phosphofructokinase, which can catalyse glycolysis rather than gluconogenesis, this study showed that the soluble sugar content in Arabidopsis and apple calli overexpressing MdPFK5 increased under salt stress, which may be due to the increase of chlorophyll content in transgenic Arabidopsis leaves caused by salt stress. More soluble sugar was produced, but the growth of plants was inhibited under salt stress, the transport of soluble sugar slowed down, and the accumulation of soluble sugar in leaves increased. Another possibility is that the osmotic potential of plants increases under salt stress, and plants would have to adjust the osmotic potential by increasing soluble sugar to regulate the osmotic potential in the body. Accumulation of sugars has been shown to enhance plant resistance. Studies have demonstrated that under salt stress, sucrose accumulates in salt-sensitive rice varieties, whereas no difference in sugar content is observed compared with controls in salt-tolerant varieties, indicating that sucrose accumulation contributes to salt stress resistance (Pattanagul and Thitisaksakul, 2008). In Arabidopsis, the abscisic acid pathway, which is dependent on osmotic stress, can enhance sugar transport, promote SWEET11/12 phosphorylation, improve root growth, and increase the root-to-shoot ratio, providing a new strategy for breeding drought-resistant crops. This finding reveals the significant role of SWEET (Sugars Will Eventually be Exported Transporters) genes in plant adaptation to drought conditions (Chen et al., 2022). Sugars not only regulate drought stress but are also involved in the response to salt stress. It has been found that mutants of COP1 (CONSTITUTIVE PHOTOMORPHOGENIC 1) in Arabidopsis are more tolerant to salt stress, but their growth is affected in salt-containing media. Exogenous sucrose can restore them to WT levels, correlating with high sucrose content. The cop1 mutant roots are more sensitive to sucrose stimulation, but they do not exhibit a significant difference in root length. Thus, COP1 has an important influence on plant salt stress and is closely related to sucrose metabolism (Kim et al., 2022).
Plants have two types of PFK members, and PFK5 belongs to the ATP-dependent PFK (Yoshida and Hisabori, 2021). AtPFK5 is located in chloroplasts, but the predicted subcellular localization of MdPFK5 is in the cytoplasm. This difference in localization reflects species diversity, and genes with similar protein structures may have different functions due to their different localization. Although AtPFK5 is known to play a significant role in redox regulation in Arabidopsis (Hess et al., 2021), a similar role was not observed in apple. However, MdPFK5 plays a function in glycolysis.MdPFK5 primarily functions in glycolysis, but compared with the WT, the chlorophyll content significantly increased in MdPFK5 transgenic Arabidopsis under NaCl treatment, indicating that MdPFK5 may also regulate photosynthesis (Figure 6). Glycolysis and photosynthesis may jointly regulate the salt tolerance of MdPFK5. However, further exploration is needed to understand how these two processes are connected to exert salt tolerance mechanisms.
5 CONCLUSION
This research highlights the role of the fructokinase MdPFK5 in enhancing the salt tolerance of apples. NaCl treatment induces the expression of the MdPFK5 gene, leading to increased fresh weight and soluble sugar content in apple calli under NaCl stress. Measurement of MDA and REL before and after treatment indicates that overexpression of MdPFK5 enhances the salt tolerance of apple calli. Ectopic expression of MdPFK5 in Arabidopsis results in significantly increased expression of genes related to the SOS pathway under NaCl treatment compared with the WT. Physiological measurements reveal a significant increase in soluble sugar and chlorophyll content in MdPFK5 transgenic plants under NaCl treatment. This finding suggests that MdPFK5 may enhance the salt tolerance of plants by increasing the accumulation of soluble sugars and improving photosynthesis. However, further research is needed to elucidate the mechanisms underlying its salt tolerance enhancement. This study provides new insights into the function of PFK genes in apples, offering a basis for breeding new salt-tolerant apple varieties.
AUTHOR CONTRIBUTIONS
J.-Q. Y. and L.-L. Z. analyzed the data and wrote the manuscript. L.-L. Z., H. Z., C.-Y. C., N.-N. S. and L.-X. S. provided technical assistance to J.-Q.Y. and helped with field data collection. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
Authors gratefully acknowledge Da-Gang Hu and Kai-Di Gu for providing the logistic and technology support for conducting the experiments.
FUNDING INFORMATION
This research was supported by the Natural Science Foundation of Jiangsu Province (BK20230574) and the Basic science (Natural science) research project in universities of Jiangsu Province (23KJB210015).
Open Research
DATA AVAILABILITY STATEMENT
All other data supporting the findings of this study are available within the article and its supplementals.




