Fungal endophyte symbionts enhance plant adaptation in Antarctic habitats
Abstract
Despite their genetic adaptation to local conditions, plants often achieve ecological success through symbiotic associations with fungal endophytes. However, the habitat-specific functionality of these interactions and their potential to drive plant adaptation to new environments remain uncertain. In this study, we tested this using the vascular flora of the Antarctic tundra (Colobanthus quitensis and Deschampsia antarctica), an extreme environment where fungal endophytes are known for playing important ecological roles. After characterizing the root-associated fungal endophyte communities of both species in two distinct Antarctic terrestrial habitats—hill and coast—we experimentally assessed the contribution of fungal endophytes to plant adaptation in each habitat. The field reciprocal transplant experiment involved removing endophytes from a set of plants and crossing symbiotic status (with and without endophytes) with habitat for both species, aiming to assess plant performance and fitness. The diversity of root fungal endophytes was similar between habitats and mainly explained by plant species, although habitat-specific endophyte community structures were identified in D. antarctica. Endophytes significantly influenced C. quitensis homeostatic regulation, including oxidative stress and osmotic control, as well as plant fitness in both environments. By contrast, the effect of endophytes on D. antarctica was particularly evident in coastal sites, suggesting an endophyte-mediated improvement in local adaptation. Altogether, our results suggest that the two Antarctic vascular plant species follow different strategies in recruiting and developing functional symbiosis with root-associated fungal communities. While C. quitensis is more generalist, D. antarctica establishes specific interactions with habitat-specific microbial symbionts, predominantly in the most stressful environmental context.
1 INTRODUCTION
From seedling emergence to adult life, plants establish interactions with soil microorganisms that can alter their phenotypes to cope with a variety of changing environmental conditions (Jackson & Taylor, 1996; Hassani et al., 2018; O'Brien et al., 2021). The diversity of microorganisms and the spectrum of plant symbiotic interactions, whether mutualistic or pathogenic, can dynamically change over time, likely conditioned by the environment (Saikkonen et al., 1998; Feijen et al., 2018). Nevertheless, it is well known that plants can exert control over the associated community of soil microorganisms, eventually leading to mutual benefit (Delaux & Schornack, 2021; Mesny et al., 2023). Although modulated by environmental conditions (Rudgers et al., 2020; Angulo et al., 2022), root exudates play a fundamental role in shaping the structure and diversity of the soil-associated community of microorganisms, often resulting in highly species-specific interactions (Reinhardt et al., 2021; Kaur et al., 2022). Still, the challenge remains in comprehending the factors that determine the composition and function of natural root fungal communities, which are crucial for understanding their responses and interactions with plant hosts under changing environmental settings (Barnes & Tringe, 2022).
In a closely intertwined biotic interaction, such as that between a plant host and its endophytes, the identity of the plant—encompassing its species and genotype—is evidently pivotal in defining the structure and composition of its associated fungal community (Hughes et al., 2020; Whitaker et al., 2020; Trivedi et al., 2021). Indeed, as pointed out by Mesny, Hacquard and Thomma (2023), plant microbial symbionts that develop mutualist interactions with their host necessarily became adapted to the specific environments associated with their plant host. Accordingly, the environmental conditions of the local habitat, through their impact on plant physiology, also influence the composition of fungal endophytic communities (Latz et al., 2021; Kaur, Campbell and Suseela, 2022). This is exemplified by Higgins et al. (2007), who investigated the phylogenetic relationships and host affinities of fungal endophytes associated with three plant hosts from boreal and arctic ecosystems. Their study underscores that fungal endophytic communities associated with different boreal species exhibit greater similarity to each other than to those in arctic communities. Nonetheless, they also highlight the structuring influence of the plant host, as the majority of phylotypes from both boreal and arctic endophytes predominantly correspond to a single host (Higgins et al., 2007).
Complementarily, it has been proposed that relative to mesic habitats, plant-fungi interactions are more relevant for plant adaptation under stressful environments (Upson et al., 2009; Hill et al., 2019; Acuña-Rodríguez et al., 2020a). Thus, the specificity of plant-endophyte interactions could be expected to be high in response to a history of plant-fungal coexistence under such conditions (Delaux & Schornack, 2021). However, since harsh conditions may impose strong selection pressures on fungal endophyte communities, the potential for plant-endophyte feedback may be low, and there may be constraints on the plant's ability to maintain symbionts (Whitaker et al., 2020; Zhang et al., 2020; Casas et al., 2022). Hence, in highly stressful scenarios, fungal endophytes may become generalists regarding host specificity to cope with harsh local conditions. Consequently, in these environments, we might also expect a convergence toward common structural and functional communities of fungal endophytes across co-occurring plant species. Pointing in this direction, Ballesteros et al. (2024) showed that fungal endophytes can enhance the performance and fitness of new host plants in challenging conditions, irrespective of plant phylogenetic relationships. Indeed, regarding root-related endophytes, this phenomenon is expected to be particularly pronounced in low-productivity harsh environments, where the ability of endophytic fungi to acquire nutrients from organic sources may be highly advantageous (Ruotsalainen et al., 2022; Bruyant et al., 2024). For instance, it was elegantly demonstrated that dark septate root-endophytes (DSE) facilitate the acquisition of nitrogen from organic sources by Antarctic plants, an advantageous strategy for the Antarctic tundra, an ecosystem where decomposition and mineralization are very low (Hill et al., 2019).
This kind of environmentally driven convergence in the structure and function of plant-associated microorganisms underpins the concept of ‘habitat-adapted’ symbiosis (Rodriguez et al., 2008; Redman et al., 2011). Nevertheless, while we may expect maximal convergence in harsh environments, most studies comparing microbial communities associated with extreme habitat flora show strong spatial and taxonomic structuring of these symbiotic communities. For example, a study of the Antarctic fungal and bacterial communities in the rhizosphere of C. quitensis and D. antarctica on Byers Island (South Shetland Archipelago) showed that only 1% of the fungal types and 0.3% of the bacterial types were shared between all sampled sites for each plant (Guajardo-Leiva et al., 2022). Following this line, a comprehensive meta-analysis of plant-mycorrhizal interactions suggested that a shared history of co-occurrence among plants and mycorrhizae was the main factor predicting positive outcomes from specific plant-fungi interactions (Rúa et al., 2016). Therefore, identifying functional similarities among fungal communities of plant species from harsh environments across different habitats will help to unveil their contributions to plant performance and fitness.
With the preceding context in mind, here we investigated the structure of fungal endophytic endophyte communities associated with the two native Antarctic plant Angiosperm species, Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) and Deschampsia antarctica E. Desv. (Poaceae), in two contrasting Antarctic habitats and their role in plant adaptation. These species thrive across various islands in Maritime Antarctica and parts of the Antarctic Peninsula (Greene & Holtom, 1971; Parnikoza et al., 2007). Within this range, we identify two contrasting habitats: coastal areas heavily affected by sea activity and saline stress, and inland hills with limited nutrients and water, which are less influenced by the sea (Olech et al., 2002; Giełwanowska et al., 2005; Łachacz et al., 2018; Bokhorst et al., 2019). Coastal sites host dense but small patches of these species among large boulders, while inland hills feature extensive lawns with lichens and mosses, characteristic of the Antarctic tundra (Olech, 2002). However, due to the scarcity of water and nutrients in the inland areas, the individual plants observed at the hill sites are smaller (Giełwanowska et al., 2005; Androsiuk et al., 2015; Łachacz et al., 2018).
While it is known that these Antarctic plants exhibit morphological and physiological variations between local populations (Giełwanowska et al., 2005; Chwedorzewska et al., 2008; Androsiuk et al., 2021), it remains unclear whether these habitat-determined differences also influence the composition and structure of their associated fungal endophytic communities, and to what extent their effects on the plant hosts depend on their habitat of origin. To achieve this goal, we conducted a field experiment where we transplanted individuals of the two native plant species (C. quitensis and D. antarctica) with and without their fungal endophytes from one habitat (e.g., coast) to another (hill) and vice versa. This allowed us to control plant ecotypic identity and assess the role of endophytes in host performance in both original and new locations. We expected to unveil whether the structure of fungal endophytic communities reflects either the potential host plant specificity or the homogenizing pressure of the local environment to develop functional endophytic communities under specific abiotic conditions.
2 MATERIALS AND METHODS
2.1 Study site and local habitats
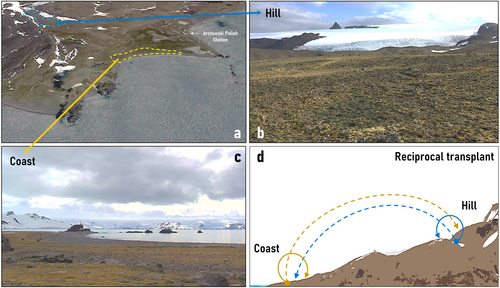
As part of the 54th Antarctic Scientific Expedition (ECA-54) from the Instituto Antártico Chileno (INACH), we visited the Antarctic Specially Protected Area N° 128 (ASPA 128) at King George Island (South Shetland Archipelago) during the austral summer season 2018–2019. Located at the western shore of Admiralty Bay-close to the Polish Antarctic Station “Henryk Arctowski” (62°09’ S; 58°28’ W), this area presents one of the largest plant communities of the Antarctic tundra with high abundance of the two native vascular plants, mosses, and lichens (Olech, 2002). This community is subjected to the typical harsh climate of Maritime Antarctica (Angiel et al., 2010), with a very short window for plant growth between late December and early March. Two habitats – hill and coast – were defined based on their positions in the landscape and previous ecological characterizations (Androsiuk et al., 2015; Łachacz et al., 2018). The “hill” habitat encompasses the first inland mounds ~500 m from the high-tide limit, while the “coast” habitat typically is encountered along the rocky shore, no more than 30 m away from the high-tide limit (Figure 1). A general soil chemical characterization (Carbon content, Nitrogen, Phosphorus, organic matter, water content, pH, and salinity) was performed on five soil cores of 250 g sampled from each habitat.
2.2 Plant sampling and symbiotic manipulation
On each habitat (hereafter: hill and coast), 30 healthy individual plants from each species, separated by at least 10 m, were collected along with their rhizospheric soils. They were placed in plastic boxes and maintained well-watered outdoors. At the “Arctowski” station laboratory, all plants were split into 10–12 clones, keeping their original genetic identity. This resulted in ~350 individual plants per species from each habitat. The plants were maintained outdoors with substrate from their specific sites in individual pots (50 cc). To generate plants free of fungal endophytes (E-), half of the clones from each originally collected plant were treated with the systemic fungicide Benlate© [Benomyl [methyl 1-[(1-butylamino) carbonyl]-1H-benzimidazol-2-yl] carbamate (DuPont). Every plant was treated with five 4.2 mL shots (dose: 2 g L−1 fungicide solution) every three days. All plant pots were maintained in plastic containers to prevent any accidental contamination of the local soil with fungicides. This protocol has been shown to be effective in removing endophytes from Antarctic plants with no apparent phytotoxic effects (Torres-Díaz et al., 2016; Barrera et al., 2020). Untreated plants (E+) were subjected to the same regime but sprayed with distilled water. For three weeks, the whole set of plants was allowed to grow and recover from the cloning. Before starting the experiment, 35 individual plants from each species (~10%) were randomly selected and evaluated for endophyte presence/absence. Following Vierheilig et al. (2005), newly grown root tissues were washed with distilled water, cut into pieces, and boiled in 10% KOH (w/v) for 5 min. Roots were then boiled in 5% blue ink (Pelikan) solution prepared in 5% acetic acid for 3 min, as recommended by Wu et al. (2012). Roots were finally rinsed several times with distilled water, and five prepared samples per plant were observed under an optical microscope. Since all the evaluated plants treated with fungicide (C. quitensis = 18; D. antarctica = 16) were free of fungal mycelia, we considered this group of plants as endophyte-free plants (E−).
2.3 DNA isolation and amplicon-based sequencing
To characterize the fungal endophytes from both plant species, root tissues were obtained from three hill and three coast individuals. Before DNA extraction, samples were pre-processed at the Arctowski station laboratory, accordingly, roots were gently rinsed with distilled water to remove the remaining soil particles and externally sterilized by submerging the samples for 2 min in alcohol (70%), one min in sodium hypochlorite (2%), and a final 2 min wash in distilled sterile water. The sample pre-processing ended by drying the root samples in an oven at 65°C for 24 h for safe transportation. Once at our laboratory at the University of Talca (Chile), total DNA from 0.25 g of dry roots was extracted from each sample using a PowerPlant® DNA extraction kit (Mo Bio Laboratories Inc.). DNA quality and concentration were assessed through a 1% agarose gel electrophoresis and spectrophotometry at 260/280 nm (Nanodrop Technologies). For each of the resulting 12 samples (3 individuals × 2 species × 2 habitats), an amplicon metagenomic library based on the internal transcribed spacer region 2 (ITS2) was prepared and sequenced using an Illumina MiSeq (Paired End, 2x300bp; Illumina). Briefly, amplicons were obtained by using single-indexed primers flanked by Illumina standard adapter sequences. Therefore, two PCR steps were carried out: first, the targeted gene region (ITS2) was amplified from DNA samples using Illumina overhang adapter sequences attached to locus-specific primers ITS3F (5’-GCATCGATGAAGAACGCAGC-3′) and ITS4R (5’-TCCTCCGCTTATTGATATGC-3′). Then, a second amplification step attached unique indexing primers (barcodes) to the initial PCR products, allowing the identification of multiplexed samples after sequencing (Kraler et al., 2016). Finally, all barcoded libraries were pooled in an equimolar way to be sequenced.
2.4 Field reciprocal transplant experiment
To evaluate the relevance of the local microbiome on the performance and fitness of each plant-host we carried out a field reciprocal transplant experiment in which endophytic (E+) and endophyte-free (E-) plants from each habitat (coast or hill) were “auto” transplanted in the same habitat of origin (i.e., from coast to coast, and from hill to hill) or “cross” transplanted in the other habitat (i.e., from coast to hill, and from hill to coast, Figure 1d). To account for local habitat variability, we implemented a design with three distinct blocks within each habitat (coast or hill), separated by a minimum of 5 meters. Within each block, we established 100 individuals per species (C. quitensis or D. antarctica), 50 auto- and 50 cross-transplanted. To counteract any microbiome loss due to the experimental manipulation, E+ plants were additionally reinoculated with their corresponding root endophytes (either from hill or coastal soil) by the addition of 5 mL of soil lixiviate solution from their respective origin.
To ensure randomness, these four groups of 25 plants per species were aleatorily arranged within their respective blocks in clusters of five plants each, in this way, every experimental group consisted of five sets of five individuals per block. This arrangement was considered to estimate the percentage of survival individuals within each five-plant cluster and average it per treatment per block (n = 5 clusters). All plants were transplanted in biodegradable pots (200 cc), which were carefully placed on the ground with the pot edge 1 cm above the soil surface. Although we cannot make sure other microorganisms enter into contact with the experimental plants, this latter procedure allowed us to delay, at least, the natural process of microbe colonization. The whole reciprocal transplant experiment used 600 plants per species (including the original genotypes and their clones), of which 300 were E+ and 300 E-. All plants were brought back to the Arctowski station laboratory after one month for processing and evaluation.
2.5 Physiological performance and fitness
To provide an overview of individual performance among the experimental plants of both species, we evaluated one indicator of physiological (oxidative) stress as lipid peroxidation, and two plant responses to osmotic stress: proline leaf content, and the relative expression of the NHX1 gene. The proline concentration in the leaves is associated with higher tolerances to osmotic stress, as the accumulation of organic osmolytes reduces the intracellular osmotic potential within plant tissues (Székely et al., 2008). In addition, since the protein derived from the NHX1 gene is a tonoplast-localized ion exchanger (Na+/H+), the expression levels of this gene have been related to the capacity of plants to tolerate saline stress (Sun et al., 2017). We focused on responses to osmotic stress conditions since it is a common environmental pressure for plants in Antarctic terrestrial ecosystems, either directly due to the unavailability of liquid water or because of their exposition to saline seawater. Complementarily, two fitness-related variables, namely plant survival and final biomass, were also considered in the field experiment. For the physiological and gene expression traits of both species, we selected the three healthiest individuals per experimental group on each block, avoiding taking two plants from a given cluster.
To quantify the impact of oxidative lipid degradation in plant cell membranes, we measured malondialdehyde (MDA) concentration in their leaves via the thiobarbituric acid (TBA) assay (Egert & Tevini, 2002). 0.5 g of foliar tissue from each plant was swiftly frozen and pulverized in liquid nitrogen. The resulting powder was mixed with 2 mL of trichloroacetic acid (TCA, 1%) and centrifuged at 10,000 g for 5 min. Subsequently, 250 μL of each sample's supernatant was combined with 1 mL of TBA (0.5%) in TCA (20%) and incubated at 100°C for 30 min using a dry bath (Thermolyne 16500; Marshall Scientific). After cooling to room temperature, thiobarbituric acid reactive substances (TBARS) content was determined by measuring absorbance at 532 nm and non-specific absorbance at 600 nm (Hodges et al., 1999). MDA content was calculated using its molar extinction coefficient of 155 mM−1 cm−1, and resulting lipid peroxidation values were expressed as mmol TBARS per gram of freshly weighted (FW) leaf tissues
In addition, to estimate the osmotic stress response of each plant, we determined their foliar proline concentration following Bates, Waldren and Teare (1973), with subtle modifications. Approximately 100 mg of frozen foliar tissue was ground in liquid nitrogen, homogenized with 2 mL of 3% sulphosalicylic acid, and centrifuged at 15,000 g for 20 min at 4°C. The supernatant (1 mL) was mixed with ninhydrin reagent (1 mL) and boiled at 100°C for 1 h. After cooling, toluene (2 mL) was added, and absorbance was measured at 525 nm using a spectrophotometer (Jenway 6300, Cole-Parmer). Proline concentration was determined on each sample by comparing absorbance with a standard proline curve, expressed as μmol g−1 tissue fresh weight
Complementarily, we also evaluated the NHX1 antiporter gene expression in experimental individuals. For this, total RNA was extracted from foliar samples following the protocol by Chang et al. (1993). DNA removal from RNA aliquots was conducted using TURBO DNA-free kits (Applied Biosystems). The cDNA strand was synthesized according to Ruíz-Carrasco et al. (2011). Quantitative PCR (qPCR) reactions included cDNA, 5 pmol of each primer, and 12.5 mL of Fast SYBR Green PCR master mix (Applied Biosystems, USA). For both species, the primer sequences for amplifying NHX1 amplicons (∼200 bp) were 5′-GCACTTCTGTTGCTGTGAGTTCCA-3′ (forward), and 5′-TGTGCCCTGACCTCGTAAACTGAT-3′ (reverse). PCRs were performed on a Step-One Plus 7500 thermocycler (Applied Biosystems) with an initial cycle of 30 min at 45°C and 2 min at 95°C, followed by 40 cycles at 95°C for 30 s, 60°C for 30 s, 72°C for 2 min, and a final cycle at 72°C for 10 min. Cycle threshold (Ct) values were obtained and analyzed using the 2−ΔΔCT method (Livak & Schmittgen, 2001). The Elongation Factor 1a (EF1a) housekeeping gene served as a reference gene for normalization. The relative expression ratio (log2) between the target gene and EF1a (fold-change) was calculated from qRT-PCR efficiencies and crossing point deviations using the mathematical model proposed by Pfaffl (2001).
As previously mentioned, plant survival and plant biomass were assessed at the end of the experiment as proxies of fitness. The former was assessed on every experimental group as the average percentage of live individuals among the respective five-plant clusters. For the latter, the dry weights of each alive individual (roots and shoots) were estimated at the end of the experiment. Accordingly, plants were dried in an oven (65°C) for about 24 h and weighed with a precision scale (±0.001 g) until constant weight.
2.6 Data analytics
2.6.1 Bioinformatic processing
Demultiplexed reads were imported into QIIME2 v.2019.7 (Bolyen et al., 2019) as “artifact objects”, from which primers were trimmed using the Cutadapt plugin (Martin, 2011). After trimming, reads were filtered, dereplicated and merged into amplicon sequence variants (ASVs) using DADA2-Qiime (Callahan et al., 2016). Taxonomic assignments were determined for all ASVs through the qiime2-feature-classifier with default parameters (Bokulich et al., 2018). For this, we used the UNITE ITS eukaryote database v.8.2 (Abarenkov et al., 2020), pre-trained and fitted with a Naïve Bayes classifier as the taxonomic reference (Rosa et al., 2020). After removing non-fungal ASVs from all libraries, sequences that were present in the UNITE database but without taxonomic information were classified as “fungi_unidentified”. To consolidate the communitarian structure in the observed samples, we conducted all downstream analyses using an agglomerated taxonomy dataset collapsed at the “genus” level. Multiple sequence alignment was performed with the AligSeq function from the “DECIPHER” R-package (Wrigth, 2016).
2.6.2 Metagenomic fungal community analysis
Following the “Rhea” processing pipeline for alpha diversity estimation (Lagkouvardos et al., 2017), the ASV read counts data, agglomerated by genus, were normalized by sample and converted to relative abundances. As suggested by Reitmeier et al. (2021), to avoid spurious taxa, we further calculated the effective number of species (effective-richness) and effective diversity values (effective Shannon counts) on each sample. For this, before calculating the referred effective-diversity measures, we removed low abundant ASVs, this is, those with counts <0.25% of the respective sample total reads. These represent the theoretical number of equally abundant species that would generate the same (observed) diversity value and have been recommended for metagenomic analyses to increase their robustness and replicability (Reitmeier et al., 2021).
For a general overview of the microbiome composition, a heat map of the root sample's ASVs abundances and a Venn diagram of shared ASVs was performed per species and habitat. Furthermore, to determine the influence of the local habitat on the structure and composition of these fungal communities, we executed in each species independent comparisons of the mean individual normalized richness and effective Shannon α-diversity using a non-parametric Mann–Whitney U test (coast vs. hill). Complementarily, the β-diversity distribution among species and habitats was explored through a principal coordinates analysis (PCoA) performed among all fungal communities (samples) using the ordinate function of the “phyloseq” R-package with a Bray–Curtis dissimilarity coefficient (McMurdie & Holmes, 2014). Finally, the grouping significance of the obtained ordination was tested permutationally (999 iterations) by means of a PERMANOVA analysis using the “microbiome” (Lahti et al., 2021) and “vegan” (Oksanen et al., 2020) R-packages. All the analyses were carried out in the R language and environment v.4.3.0 (R-CoreTeam, 2023).
2.6.3 Field statistical analysis
To compare the soil properties between sites, a t-test (hill vs. coast) was performed independently for each soil parameter, as described by field samples. Regarding the field experiment monitoring, all variables were analyzed independently using a Linear Mixed Model (LMM) framework, including the block as a random factor (Pinheiro & Bates, 2000). Accordingly, we performed for each variable an analysis of variance on the fitted model y ~ Endophyte x Habitat x Transplant type, using symbiotic status (E+, E-), habitat of destiny (hill, coast) and transplant type (auto-, cross-transplanted) as fixed factors. To explore the influence of the transplant direction (i.e., coast to hill, or hill to coast), a posteriori pair-wise comparisons between transplant groups (i.e., 1: coast-coast, 2: coast-hill, 3: hill-hill, and 4: hill-coast) were performed using a marginal means comparison analysis through the “emmeans” R- package (Lenth, 2021) as suggested for heteroskedastic data.
3 RESULTS
3.1 Sequencing report
Of the 12 planned samples, one belonging to the roots of a C. quitensis-hill individual was discarded due to the low quantity of extracted DNA for library construction. A total of 6′660,299 raw amplicon reads from the ITS region were obtained for the remaining 11 samples. After running DADA2, 5′277,861 amplicons were retained and subsequently clustered. From this, 265 Amplicon Sequence Variants (ASVs) were successfully assigned to a fungal Phyla, most of them to Ascomycota (79.6%), but also Basidiomycota (14.3%), Mortierellomycota (2.1%). Rozellomycota (2%) plus an undefined 2%. The final dataset, agglomerated by genus, was composed of 49 ASVs, from 4 phyla, 11 classes, 23 orders, 30 families and 33 genera, plus 16 ASVs with an unidentified genus (Table S1).
3.2 Fungal community analysis
Comparing plant hosts, the species richness of the root fungal endophytic community harboured by C. quitensis (38 ASVs) and D. antarctica (40 ASVs) is quite equivalent. Between them, 25 ASVs are shared, representing more than half (62–65%) of their fungal communities (Fig. 2ab). Interestingly, the influence of the local habitat (hill or coast) in structuring both plant species fungal endophytes could be observed on the site-specific ASVs, 12 for D. antarctica and 6 for C. quitensis, some of them exclusive of one plant species (Figure 2b). For D. antarctica only four genera might be denoted as “dominant”, this is, with abundances >2.5% relative to their local community (i.e., hill or coast). By contrast, for C. quitensis this group was more numerous in both habitats, but particularly among hill individuals (Table S2). Indeed, while for D. antarctica, the most dominant taxa in each local habitat represented half (or more) of their fungal endophytic community (hill: Plectosphaerella, 48.2%; coast: Acremonium, 68.1%), for C. quitensis they represented only one quarter of it (hill: undefined Heliotales, 25,7%; coast: Gibellulopsis, 27.6%). Notably, for both plant hosts, the dominant ASV of their fungal endophytic communities differed between local habitats, but for all of them, the genus Acremonium was present as an “abundant” ASV (>2.5% of relative abundance).
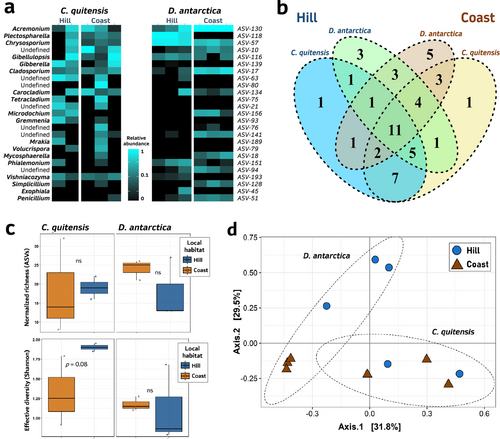
Within each plant species, neither the average richness nor the Shannon alpha-diversity denoted by their root fungal communities showed statistical differences between habitats, probably due to the number of replicates and the wide variability of some groups (Figure 2c). Nevertheless, the PCoA ordination, which describes the “shared” (i.e., beta) diversity among samples, and the posterior PERMANOVA validation, did find a significant role of the two analyzed factors (species × habitat: d.f. = 1, 7; F = 2.31; p = 0.039), suggesting that the clustering role of the habitat factor on the fungal root communities may vary depending on the plant species. This contrasting influence could be clearly inferred from the ordination plot, in which D. antarctica samples have a stronger segregation by habitat compared with those from C. quitensis (Figure 2d).
3.3 Reciprocal transplant experiment
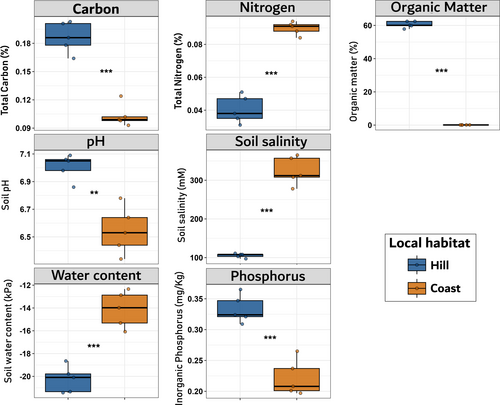
As a general baseline, the soil characterization of both habitats showed a strong divergence in their biochemical properties; in consequence, all the t-test comparisons turned out to be significant (Figure 3). As expected, soil water content, nitrogen concentration and soil salinity were higher on coastal soils, while carbon content, organic matter, phosphorus, and pH were on average higher among hill samples (Figure 3).
Our findings reveal that the main factor determining plant performance proved to be the presence of microbial endophytes, despite the fact that, in some cases, their relevance varies depending on both the transplant type and the habitat of destination. Hence, compared to sterilized (E-) individuals, experimental E+ plants were characterized by lower levels of lipid peroxidation (TBARS, Figure 3a), elevated proline concentrations in leaves (Figure 4b), and heightened levels of gene expression of the NHX1 gene (Figure 4c). Nevertheless, this positive influence on the plant physiology observed among plants growing with microbial symbionts, this is, less oxidative stress and better osmotic stress responses, was more evident in C. quitensis than in D. antarctica, for which the differential effect of the inoculation was only observed among cross-transplanted individuals (Figure 4).
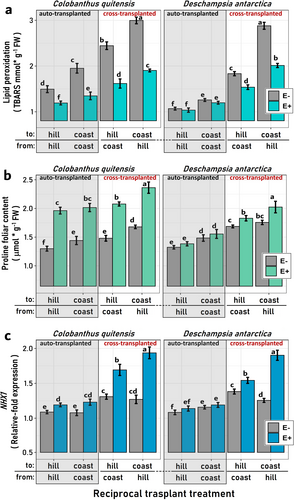
By contrast, significant distinctions between E- and E+ individuals for the three referred variables were evident among both auto- and cross-transplanted C. quitensis plants, albeit a tendency toward greater differences among cross-transplanted individuals was indeed observed (Figure 4). Accordingly, our results show that for some responses like TBARS and NHX1 gene expression, in the LMM models for D. antarctica the “Endophyte” factor is not significant by itself (i.e. alone in the model), but it is when interacting with the habitat and the transplant type (Table S3). Notably, among cross-transplanted individuals from both species, the destination habitat also appears to influence their average performance responses. This was evident among E+ plants cross-transplanted to either hill or coast habitats, where those transplanted to coastal locations typically exhibited physiological responses indicative of higher stress conditions relative to those assigned to the hills (Fig. 3abc).
The outcomes derived from the fitness-related variables (survival and final biomass) further underscore this differential impact of the experimental factors on each evaluated species. Specifically, the survival of C. quitensis plants was positively influenced solely by the presence of fungal endophytes. In contrast, for D. antarctica, the habitat and the type of transplant exhibited more relevance. Notably, cross-transplanted D. antarctica plants were particularly susceptible to mortality when destined for coastal locations (Figure 5a). Like which was observed with the physiological data, for D. antarctica, the “endophyte” factor alone was not a determinant of plant survival (Table S3). Nevertheless, as denoted by the significance of the interaction term “Endophyte × Transplant”, it was among cross-transplanted D. antarctica individuals where having fungal symbionts resulted in significant survival of this species (Table S3, Figure 5a). By contrast, for C. quitensis the “Endophyte” factor was the only significant term in the survival LMM model, for which E+ plants of this species almost triplicated E- average survival percentages across habitats and transplant types (Figure 5a).
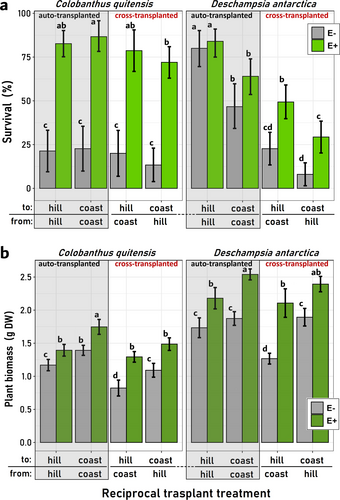
By the end of the growing season, the final biomass exhibited similarities to the patterns observed in the physiological data. Irrespective of the plant species, symbiotic plants (E+) consistently displayed higher average biomasses within each E+/E- transplant pair (Figure 5b). However, as indicated by the significance of the interaction terms “Endophyte x Habitat” and “Endophyte × Transplant” in the LMM models for both species (see Table S3), the net effect of fungal endophytes on the final biomasses of both plant hosts is contingent upon the contextual factors in which they operate. In this regard, it was frequent that hill plants of both species, either E+ or E-, denoted lower biomasses if compared with similar individuals at coastal locations. Similarly, auto-transplanted plants appeared, in general, with more biomass than cross-transplanted individuals (Figure 5b).
Interestingly, the mutual influence of the three experimental factors was also detected by the LMM models in the significance of the term “Endophyte × Habitat × Transplant type”, which suggests that the differences between E+ and E- plants, this is, the role of the fungal endophytes, depended not only on the local habitat conditions but also on the habitat correspondence of both plants and endophytes (Figures S1 & S2). Accordingly, the absence of fungal endophytes was, in general, more detrimental at coastal sites than at hill locations, and this was more evident among cross-transplanted individuals from both species (Figure 5b). However, contrary to C. quitensis, for D. antarctica the role of the fungal inoculum was rarely detected among auto-transplanted individuals, the significance of the triple interaction term in the LMM analyses was more frequent on D. antarctica models (TBARS, proline, NHX1 and Survival), than on those from C. quitensis (NHX1 and Biomass; Figures S1 & S2).
4 DISCUSSION
Using native Antarctic vascular plants as a study model, our results demonstrate the significant influence of local habitat on shaping the fungal endophytic community associated with these two plant species. Moreover, our study indicates that on the local scale, plant-microorganism interactions can be finely tuned to optimize host performance under specific habitat conditions. This conclusion is supported by the observation that when plants were translocated to a different habitat or their microorganisms were altered, the beneficial effects of symbiosis on plant host fitness diminished. As has been shown, local microorganisms endow hosts with novel and diverse biochemical mechanisms that become more relevant to plant fitness under abiotic stressful conditions (Upson, Read and Newsham, 2009; Petipas et al., 2020; Burg et al., 2024). Accordingly, plant-microorganism interactions play a crucial role in plant ecology in stressful environments through habitat-adapted functional symbiosis (Redman et al., 2011; Newsham, 2011; Revillini et al., 2016; Petipas et al., 2021). However, since these habitat-specific plant-microorganism interactions depend on a shared history of co-occurrence between the plant host and its associated symbionts under specific environmental conditions (Revillini et al., 2016; Rúa et al., 2016; Vahter et al., 2020), changes in the environmental context, for example when a plant grow in a new habitat, are likely to alter their outcomes (Acuña-Rodríguez et al., 2020a; Burg et al., 2024).
In regard to the Antarctic tundra, a recent work explored the factors behind the structure of the rhizospheric microbiome (fungi and bacteria) associated with C. quitensis and D. antarctica (Guajardo-Leiva et al., 2022). They found that, beyond the influence of distance and plant species, local environmental conditions significantly impacted their rhizospheric fungal communities more than those from bacteria. To explain this, it was proposed that factors such as moisture and exposure to sea spray likely drive greater fungal species turnover in the rhizosphere of these plants compared to the bacterial community (Guajardo-Leiva et al., 2022). Our findings on endophytic fungi align with those from this previous study, identifying habitat and host species as the main drivers of the root-associated fungal endophyte community structure. Additionally, these communities responded to the environmental conditions of the habitat in at least one of the plant hosts. Specifically, D. antarctica exhibited a strong dominance of a few taxa in both habitats, along with a high proportion of exclusive species. In contrast, C. quitensis had less structured fungal communities with a greater number of co-dominant taxa in both hill and coastal locations.
Analyzing the sensitivity of plants to translocation can shed light on the importance of root-associated fungal endophytes for plant performance and fitness, particularly in determining whether the benefits obtained from these symbionts are general or specific. In this regard, D. antarctica not only showed significant structural differentiation in the associated fungal communities between hill and coastal habitats but also exhibited high sensitivity to translocation compared to C. quitensis. Specifically, while C. quitensis exhibited reduced survival regardless of transplant destination (auto- or cross-transplanted) due to the elimination of fungal endophytes, D. antarctica individuals with endophytes experienced a decline in physiological performance, biomass production, and survival only when cross-transplanted. In contrast, the absence of fungal symbionts appeared to have only a marginal relevance among auto-transplanted D. antarctica plants.
This suggests that D. antarctica may possess specific stress response mechanisms to cope with the environmental conditions of the Antarctica continent and, consequently, be less reliant on symbiotic endophyte fungi. In support of this, several studies have shown that various molecular, biochemical, and physiological mechanisms of stress tolerance described in the Antarctic vascular flora are particularly efficient in D. antarctica (Alberdi et al., 2002; Bravo & Griffith, 2005; Clemente-Moreno et al., 2020). Indeed, these capacities have been proposed to be behind the broader distribution of D. antarctica in the Antarctic territory relative to C. quitensis (Komárková et al., 1985; Parnikoza et al., 2011). However, the significant sensitivity to translocation in this species, evident even among endophyte-symbiotic plants, suggests that the ecological functionality of plant-endophyte interactions may be linked to specific environmental contexts, likely through the existence of ecotypic differentiation.
Field manipulative experiments are crucial for revealing the real impact of symbiotic microorganisms on plant ecophysiology. Despite the significant experimental challenge of keeping plants free of endophytes, it is a worthwhile endeavour, even if the results must be interpreted with caution. Thus, the fungicide used to produce endophyte-free plants might have altered the homeostasis of the treated individuals. For example, besides killing pathogens and other fungal endophytes, a fungicide can induce plant defences through epigenetic regulation and alter primary metabolism and growth (Petit et al., 2012; Fang et al., 2024). Given the difficulty of maintaining axenic soils under field conditions, it is expected that a natural process of microbial colonization occurred in our experiment, likely at varying rates among endophyte-free and endophyte-symbiotic plants. Since we maintained all other experimental factors constant and observed consistent results across species, we can rely on the significant contribution of root-associated fungal endophytes to the plant adaptation to the environmental conditions of the two habitats. Even though all fungicide-treated plants had begun to be colonized by root endophytes in the field, this process would have differed among plant species and habitats. Certainly, the complex dynamics of root-associated fungal endophytes underscore the complexity of plant adaptation to diverse environmental conditions, warranting further investigation into their ecological implications.
Symbiont microorganisms provide their hosts with new and versatile biochemical mechanisms that enhance plant growth and tolerance to abiotic stress factors (Acuña-Rodriguez et al., 2020a; Burg et al., 2024). Some of the described benefits of fungal endophytes in the two Antarctic species include enhanced nitrogen acquisition from organic sources, which are highly available at coastal sites (Hill et al., 2019; Acuña-Rodriguez et al., 2020b), and improved control of oxidative stress (Barrera et al., 2020; Hereme et al., 2020) frequent at harsh environments. Given the greater local divergence in fungal endophytic communities between hill and coast for D. antarctica compared to C. quitensis, and the more detrimental fitness effect observed in D. antarctica during the reciprocal transplant, we hypothesize that the two native Antarctic angiosperms have different strategies regarding in their interactions with fungal symbionts. This might contribute new insights to resolving the “enigma” of the presence of these specific vascular plants in Antarctica, which remains under debate (Parnikoza et al., 2011; Biersma et al., 2020).
In this context, an apparent incongruence among Antarctic populations of C. quitensis and D. antarctica is their notable responsiveness to environmental changes despite their low genetic diversity (Chwedorzewska & Bednarek, 2011; Androsiuk et al., 2021). This is particularly intriguing for D. antarctica, which exhibits broad phenotypic variation across local habitats (Giełwanowska et al., 2005; Chwedorzewska et al., 2008). Despite its low overall diversity, the highly structured genetic variability observed in D. antarctica populations from King George Island in Maritime Antarctica has been proposed as evidence of the intrinsic genetic capacity of the species to cope with different environmental settings (Androsiuk et al., 2021). However, given the critical role of local fungal symbionts in the survival of this plant species in different habitats, our results demonstrate that the development of habitat-specific fungal endophyte communities is also part of its adaptive strategy. Therefore, the configuration of habitat-specific endophytic communities may be crucial for understanding the differences in local and regional distribution between the two plant species. Furthermore, this understanding could also be a key factor in predicting their future responses under climate change scenarios.
ACKNOWLEGEMENTS
We would like to thank Instituto Antártico Chileno (INACH), the Chilean Navy and the amazing crew of the Polish Antarctic Station “Henryk Arctowski” for their logistics and field support. We also extended our appreciation to Juan Carlos Soto from the Biology Laboratory at Universidad of Magallanes and Ernesto Teneb from the “Club de Montaña D'Agostini” for their valuable support in Punta Arenas. All samplings were conducted in accordance with international permits and authorizations provided by INACH.
FUNDING INFORMATION
ISAR was financed by the FONDECYT-ANID postdoctoral project 3180441, while ECN and MAMM were supported by INACH RG_21_18 and ANID-PIA-Anillo INACH ACT192057.
CONFLICT OF INTEREST STATEMENT
Authors declare that this work was carried out in the absence of conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All ASV sequences were assigned to samples using sample-specific barcodes and deposited in the NCBI Bioproject as FASTQ files under the code PRJNA830922. The data that support the findings of this study are available from the corresponding author upon reasonable request.




