Regulatory effect of pipecolic acid (Pip) on the antioxidant system activity of Mesembryanthemum crystallinum plants exposed to bacterial treatment
Abstract
The presented study aims to elucidate the regulatory role of Pipecolic acid (Pip) in modulating the antioxidant system activity of Mesembryanthemum crystallinum plants exposed to Pseudomonas syringae infestation. M. crystallinum, known for its semi-halophytic nature, can transition its metabolism from C3 to CAM under salt stress conditions. The research encompasses the antioxidant system of the plants, covering both enzymatic and low molecular weight components. The findings indicate that Pip supplementation confers a beneficial effect on certain elements of the antioxidant system when the plants are subjected to stress induced by bacteria. Notably, during critical periods, particularly in the initial days post-bacterial treatment, M. crystallinum plants supplemented with Pip and exhibiting C3 metabolism display heightened total antioxidant capacity. This enhancement includes increased superoxide dismutase activity and elevated levels of glutathione and proline. However, in plants with salinity-induced CAM, where these parameters are naturally higher, the supplementation of Pip does not yield significant effects. These results validate the hypothesis that the regulatory influence of Pip on defence mechanisms against biotic stress is contingent upon the metabolic state of the plant. Furthermore, this regulatory effect is more pronounced in C3 plants of M. crystallinum than those undergoing CAM metabolism induced by salinity stress.
1 INTRODUCTION
In the complex interaction between plants and their environment, biotic stress poses a significant threat to survival and productivity. Plants have a complex, multilayered defence system to fend off pathogens and limit disease spread. However, keeping this system constantly activated is energetically costly, which can reduce both growth and reproductive potential. To balance defence with growth and development, plants have evolved mechanisms to induce defence responses only when a pathogen attack occurs, minimizing unnecessary energy expenditure (Dangl and Jones, 2001).
Induced defence response involves local reactions occurring at the site of infestation and reactions in distant organs, known as systemic reactions or systemic acquired resistance (SAR). The latter restricts the spread of pathogens within the plant and enables effective combat against subsequent infection. However, this requires long-distance communication between infected and uninfected, distant parts of the plant, involving significant transcriptional reprogramming in remote locations, priming them for a rapid and often stronger defence response in case of subsequent infection (Hartmann and Zeier, 2019; Návarová et al., 2012; Yildiz et al., 2021).
Among the array of signalling molecules involved in plant defence mechanisms, pipecolic acid (Pip) has emerged as a central player. Pip is a non-protein amino acid derived from the catabolism of lysine, naturally occurring in the world of plants, animals, and microorganisms (Koc and Dinler, 2022). In the biosynthesis of Pip, two enzymes are involved: AGD2-Like Defense Response Protein 1 (ALD1) and SAR-Deficient 4 (SARD4). ALD1 is responsible for transferring the amino group from L-lysine, resulting in the formation of 2,3-dihydro Pip (2,3-DP). SARD4 then reduces this product to Pip (Ding et al., 2016; Hartmann et al., 2017; Návarová et al., 2012). Recently, it has been shown that Pip serves as a crucial precursor for N-hydroxypipecolic acid (NHP), produced by Pip hydroxylation by flavin-containing monooxygenase 1 (FMO1) and directly involved in the induction of SAR (Chen et al., 2018; Hartmann and Zeier, 2018; Shan and He, 2018). Therefore, both Pip and NHP might be considered important mediators of defence mechanisms induction in plants. There is ongoing discussion about whether these compounds may serve as key systemic signals. This concept is supported by systematically published research results that currently demonstrate the presence of Pip and NHP in various groups of angiosperm plants, both dicotyledonous and monocotyledonous (Al-Rooqi et al., 2022; Brambilla et al., 2023; Schnake et al., 2020; Vogel-Adghough et al., 2013).
Pip and its derivative NHP interact with other signalling pathways to establish an efficient plant defence strategy against pathogens. Among the signals involved in defence via systemic acquired resistance (SAR) is salicylic acid (SA) and several components of the SA pathway, including methyl salicylate (MeSA), a methylated derivative of SA (Gondor et al., 2022). However, it is worth emphasizing that MeSA is a molecule whose role in SAR is still under discussion. In the case of the model species Arabidopsis thaliana, it was found that this molecule is dispensable for SAR, as plants defective in MeSA production can still accumulate SA in distant leaves and develop SAR upon local Pseudomonas syringae inoculation (Attaran et al., 2009). Pip prepares plants for optimal phenolic defensive signal SA production and orchestrates SAR and defence priming through both SA-dependent and SA-independent signalling mechanisms (Bernsdorff et al., 2016). Beyond the pathway induced by salicylic acid, reactive oxygen species (ROS) are also involved in the formation of defence mechanisms in response to pathogen attacks. Importantly, unlike salicylic acid, ROS function in a concentration-dependent manner because they can provide SAR only when present at an optimal concentration (Wang et al., 2014). Therefore, maintaining a subtle balance between ROS elimination and production is crucial for effective defence mechanisms. Plants are thus equipped with both enzymatic (e.g., superoxide dismutase (SOD), catalase (CAT), and peroxidase (POX)) and non-enzymatic antioxidant systems (ascorbic acid, tocopherols, proline, glutathione, and carotenoids), which allow for maintaining ROS homeostasis. These antioxidant systems act synergistically, and various molecules are abundantly produced during pathogen attacks to enhance plant defence responses. Recent research by Stahl et al. (2019) highlights this synergistic action. The tocopherol biosynthetic pathway in Arabidopsis enhances salicylic acid accumulation, crucial for resistance against P. syringae. ROS generated by pathogens can damage cellular components, but tocopherols act as lipid-soluble antioxidants, protecting membrane lipids from oxidation. This role supports cellular integrity, facilitates salicylic acid signalling, and activates defence mechanisms, ultimately bolstering plant resistance. In several previously published studies, it has been presented that Pip may also regulate the level of ROS and the activity of the antioxidant system under both biotic and abiotic stress conditions (Zhang et al., 2020; Wang et al., 2021), enabling effective plant defence against adverse factors.
Given this background, our research focuses on Mesembryanthemum crystallinum, a semi-halophytic species that can switch its photosynthetic metabolism from C3 to Crassulacean Acid Metabolism (CAM) during its growth and development. This switch is a defence strategy to adapt to harmful environmental conditions caused by high light, salinity, and drought stress. Due to this ability, M. crystallinum species is often used as a model plant to study plant defence response. Experimental data so far indicate that the CAM mode of photosynthesis in these plants promotes greater resistance to biotic stress compared to C3 plants. It is suggested that the microenvironmental conditions within the plant tissue contribute to the reduced effectiveness of pathogen infection in CAM plants compared to C3 plants. The differences in reactions between C3 and salinity-triggered CAM plants to the pathogen likely stem from photorespiratory activity, which is associated with changes in reactive oxygen species (ROS)-redox signalling and salicylic acid (SA)-mediated local and systemic responses (Libik-Konieczny et al., 2019).
Research conducted so far on halophytes has primarily focused on identifying genes related to salt and drought tolerance, as well as investigating the phytoremediation potential of these plants (Rahman et al., 2021). The research gap we aim to fill involves understanding the mechanisms that induce defence responses to biotic stress, which are not yet fully explained. This knowledge seems essential, especially in the context of a changing climate and the invasion of pathogens into new environments. The results obtained are crucial for predicting the effects of pathogen infections under environmental pressures caused by abiotic stress. One of the key strategies being studied is the production of Pip as a precursor of signalling molecules that induce defence responses. Our research explores the effectiveness and regulatory impact of this molecule through its supplementation during plant growth. We hypothesize that Pip supplementation can significantly influence the functioning of the antioxidant system, modifying its activity depending on the metabolic pathway exhibited by the studied plants to efficiently combat the effects of bacterial infection.
2 MATERIALS AND METHODS
2.1 Plant material and experimental design
The procedure for cultivating plants and preparing bacteria was based on previous research by Libik-Konieczny et al. (2011). The M. crystallinum seeds were sown directly into the universal soil with peat. After 2 weeks from sowing, the seedlings were each planted into a separate pot and placed in a growth chamber (Producer: THERMOCOLD, Święta Katarzyna, Poland) at 12 h light (24°C) /12 h dark (18°C) cycle, 60/80% RH (relative humidity), and irradiance of 200–250 μmol quanta m−2 s−1 (PAR; λ = 400–700 nm, LED Lamps by Plantalux).
Samples from four independent plant cultures were used in the described experiments (see Appendix S1 in Supporting Information). Each time, 100 2-week-old seedlings without damaged root systems were used for further development. After the next 2 weeks of normal growth, 50% of 4-week-old plants with fully expanded leaves were irrigated with 400 mM NaCl solution for seven days, which is needed for inducing metabolism change from C3 to CAM. The other 50% were still treated with water for 7 days. After the metabolic change, plants were divided into the next subgroups for each metabolism state and prepared for Pip supplementation. Half of the plants in each metabolism variant were supplemented with 10 mL of 1 mM Pip solution applied directly to the soil.
One day after Pip supplementation, the plants were treated with bacteria. For successful infestation of plants with P. syringae, the bacterial solution was introduced into the leaf using a syringe with a needle. This requirement stems from the specific anatomy of Mesembryanthemum crystallinum leaves, characterized among others by specialized epidermal cells, that have a water and NaCl storage function for osmotic adjustments and for some substances that might be involved in defence against pathogens (Loconsole et al., 2019). This feature renders other methods, such as syringe pressure infiltration or spray inoculation, ineffective (Adams et al., 1998). The procedure for bacteria treatment of M. crystallinum has been previously utilized in our studies, with a detailed description of the method published by Libik-Konieczny et al. (2011).
In the experimental design, samples were collected from wounded plants as well as from plants infested with bacteria using a needle to distinguish the defensive response to mechanical damage versus pathogen presence. Therefore, two treatment variants were prepared. To observe changes in defence mechanisms due solely to physical damage, a 2 mL solution of 10 mM MgCl2 was injected into the plants. This ensured that observed responses were from mechanical injury, not pathogen presence. To examine the response to bacterial presence, a 2 mL bacterial suspension in 10 mM MgCl2 was injected into the plants. This approach allowed us to differentiate between the responses to mechanical damage and bacterial treatment (Ślesak et al., 2008).
For both variants, the injection with MgCl2 or bacterial solution was performed on the underside of one leaf from the third pair, specifically between the veins. This standardized location ensured consistency across all samples. Samples were collected at three different time points: 1 day, 2 days, and 3 days post-treatment. The treated leaf was carefully cut from the plant at each designated time point, immediately placed in liquid nitrogen to halt any biological processes, and then stored at −70°C to ensure stability until analysis.
2.2 Bacteria culture
Solution of Pseudomonas syringae DC3000 strain was prepared by establishing a liquid culture from a 1-week-old bacteria culture in an NGYA medium (Bacto Proteose Peptone 0.5%, yeast extract 0.3%, glycerol 2% (v/v), agar 1.5%) with rifampicin (50 mg l−1). The liquid bacteria culture was grown in darkness at 28°C for 24 h with shaking (100 rpm). The suspension was then centrifuged at 5000 g for 5 min at room temperature (RT). The pellet cells were collected and resuspended in 10 mM MgCl2. The culture density was measured, and bacteria were diluted up to OD600 = 0.1 (106 colony-forming unit – CFU ml−1).
2.2.1 Titration of bacteria
To investigate the effect of Pip supplementation of plants on bacterial growth in infested leaves a titration of bacteria was performed. This experiment was designed to analyze the correlation of the intensity of bacteria growth with morphological changes observed in the bacteria-treated leaves. Bacteria were extracted in 10 mM sterile MgCl2 from three ground leaf discs collected from plants of each variant. Serial dilutions (starting with a 1:10 dilution) of the extracted solution were prepared in the same 10 mM MgCl2 solution. The bacteria were grown on NGYA medium agar plates (containing Bacto Proteose Peptone 0.5%, yeast extract 0.3%, glycerol 2% (v/v), and agar 1.5%) at 28°C. The colony counting units (CFU) per milligram of fresh weight (FW) were counted to quantify bacterial growth. Titrations of bacteria were conducted at three different time points: 1 day, 2 days, and 3 days post-inoculation.
2.3 Preparation of the samples
The procedure for preparing leaf extracts for specific experiments varied depending on the intended use/test. Therefore, a description of the preparation of individual extracts can be found in the descriptions of the methods.
2.4 Analysis of pipecolic acid and proline content
Proline (PRO) and Pipecolic acid (PIP) were estimated employing a stable isotope-labelled internal standard of [2H3]proline (PRO-D3, Sigma-Aldrich). Samples of about 10 mg were spiked with PRO-D3 and extracted, as reported by Wiszniewska et al. (2019). The cleaned-up procedure was simplified to exchange the final solvent of 1 M formic acid to mobile phase B after evaporation.
The separation of analytes was achieved utilizing hydrophilic interaction liquid chromatography (HILIC) on Poroshel HILIC-Z (2.1 × 100 mm, 2.7 μm, Agilent) at 50°C. The mobile phase was (A) 20 mM ammonium formate in H2O and (B) ACN with 5% 20 mM ammonium formate. A gradient program at a flow rate of 0.5 mL min−1 was used. Initial conditions were 90% B, then in 3 min 50% B, then to 4.5 min 25% B (flushing column), and 5 min return to 90% B and 4 min column re-equilibration. The injection volume was 1 μL.
An Agilent 1260 coupled with a 6410 triple quadrupole mass spectrometer with electrospray ionization in the positive ionization mode (+ESI) was used. Mass Hunter software was used to control the LC–MS/MS system and for data analysis. Monitored MRM transitions: PRO 116.1\70.1 and 116.1\43.1; PRO-D3 119.1\73.1 and 119.1\46.1; PIP 130.1\84.1 and 130\56.1.
2.5 Trolox Equivalent Antioxidant Capacity (TEAC) and lipid peroxidation (MDA-malondialdehyde) measurement
The procedure for TEAC was based on a method described by Molyneux (2004) and Eriotou et al. (2014) but optimized for 96-well plates to simplify the measurement. Therefore, concentrations and proportions of reagents were maintained, adjusting them to the capacity of a single well. The procedure began with the preparation of the 100 μM DPPH reagent and TROLOX stock (1:1 with methanol), which were used to prepare the standard curve (a series of dilutions 0–0.6 mg ml−1), samples consisted of 195 μL of DPPH solution and 5 μL of each concentration of TROLOX.
The plant extracts were prepared by homogenizing 100 mg of tissue on ice in 300 μL of 100% methanol and centrifuging at 4500 g for 10 min, 4°C. First, different amounts of extracts were tested to fit the curve, and the most optimal was selected for the reaction. The reaction was started using 15 μL of plant extracts mixed with 185 μL of DPPH solution. All the samples and standards were incubated in the dark at RT for 20 min and then measured spectrophotometrically at 515 nm.
A modified method described in the Lipid Peroxidation (MDA) Assay Kit by Sigma-Aldrich manual was used for MDA measurement. The standards were prepared according to the product manual. Preparation of plant samples was carried out by homogenization of 100 mg of tissue on ice with 300 μL MDA Lysis Buffer containing 3 μL BHT (butylated hydroxytoluene) (100x concentrated), sample centrifugation at 13 000 g for 10 min, 4°C, and collecting 200 μL of supernatant. The standards were prepared according to the product manual.
The reaction was performed by adding 600 μL of thiobarbituric acid (TBA) solution to each sample and standard vial, incubating at 95°C for 120 min, and cooling for 10 min on ice. Then samples were centrifuged at 13 000 g for 3 min, 4°C, pipetted into a 96-well plate, and measured at 532 and 553 nm.
2.6 Visualization and measurement of antioxidant enzyme activities
2.6.1 Preparation of protein extracts
To isolate fractions of soluble proteins, 1 g of fresh plant tissue was homogenized at 4°C, with a mortar in a 2.5 mL buffer (17.9 g l−1 Tricine, 0.74 g l−1 MgSO4, 0.155 g l−1 DTT, 1.14 g l−1 EDTA (pH 8.0)). Non-soluble material was removed by centrifugation for 3 min at 3000 g.
2.6.2 Determination of protein content in plant extracts
The protein concentration was then determined according to Bradford (1976) using the BioRad protein assay with Bovine Serum Albumin (BSA) as a standard (Libik-Konieczny et al., 2011).
2.6.3 Visualization of antioxidant enzyme activities on polyacrylamide gels
Activity staining of polyacrylamide gels after native electrophoretic separation of isolated proteins was performed. The percentage of the gel depended on the currently measured enzyme (12% for SOD and 10% for CAT, POX) as well as the time of electrophoresis and staining procedure of the gel (Lee and Lee, 2000). An equal amount of protein extract was loaded on the gels (12 μg for SOD and POX, 7 μg for CAT).
For SOD, the gels were placed in a staining solution, i.e. 50 mmol l−1 potassium phosphate buffer (pH 7.8) in which 0.2 g l−1 NBT, 7.5 mg l−1 riboflavin, 0.372 g l−1 EDTA and 31% (w/v) TEMED were added. The gels were incubated in the dyeing solution in the dark at RT for half an hour and then exposed to white light to visualize the bands (Beauchamp and Fridovich, 1971).
For CAT, the gels were incubated for 15 min in 0.03% (v/v) H2O2 and then stained in a solution containing 20 g l−1 FeCl3 and 20 g l−1 K3Fe(CN)6 for less than 1 min and finally washed out of the solution in water (Woodbury et al., 1971).
For POX, the gels were incubated in a staining solution, i.e. potassium phosphate buffer (pH 5), containing 0.0068 g l−1 KH2PO4, 0.0175 g l−1 Na2HPO4, 0.4285 g l−1 3,3’-Diaminobenzidine (DAB) in DMSO for 20 min. Then, 25 mL of an aqueous 30% hydrogen peroxide solution was added. After the appearance of the bands, the gels were transferred to water (Christensen et al., 1998).
2.6.4 Measurement of antioxidant enzyme activities visualized on polyacrylamide gels
After electrophoretic separation, the ImageJ program was used to measure the differences in enzyme activity between the variants based on gel images. Gels were scanned with the Epson Perfection V700 Photo scanner (Epson America). The intensity of the bands corresponding to SOD, CAT, or POX activity was determined through densitometric analyses using ImageJ software (National Institutes of Health).
The values are presented as arbitrary units (A.U.), which correspond to the area under the densitometric curves. Three biological replicates were used for analyses, and three measurements were performed on each of the replicates.
2.6.5 Spectrophotometric analyses of antioxidant enzyme activities
Total SOD activity was analyzed spectrophotometrically by measuring the inhibition of the superoxide radical anion-dependent reduction of Cytochrome c at 550 nm, according to the method described by Vives-Bauza et al. (2007). One unit of SOD activity was defined as the amount causing 50% inhibition of Cytochrome c reduction at 25°C. The results of the enzymatic assay were given in units of SOD activity per milligram of total proteins (U mg−1 protein).
CAT activity was measured according to the method described by Aebi (1984). The disappearance of H2O2 [initial concentration: 0.04% (v/v) H2O2] in a phosphate buffer (50 mM NaH2PO4, 50 mM Na2HPO4 pH 7.0) was monitored at 240 nm. Enzyme activity was defined as 1 μmol of H2O2 decomposed per minute. For calculation, the absorbance coefficient 43 L M−1 cm−1 was used.
Total POX activity was determined spectrophotometrically according to Bergmeyer (1974). The conversion of 3,3′-Diaminobenzidine (DAB) to its oxidized form was monitored at 470 nm, in 300 mM potassium phosphate buffer, pH 6.1, in the presence of 2.10 mM H2O2. POX activity was calculated using the absorbance coefficient 26.6 mM−1 cm−1.
2.7 Determination of glutathione and glutathione disulfide levels
The enzymatic recycling method for measuring the reduced form of glutathione (GSH) and its oxidized form - glutathione disulfide (GSSG) described by Rahman et al. (2006) which was based on (Luwe et al., 1993) was used (96-well microtiter plate assay version). The experiment began with the preparation of the GSH stock (1 mg ml−1) in the KPE (potassium phosphate) buffer and a series of standards for the standard curve (concentrations between 0.103 nM ml−1–26.4 nM ml−1), which was made through a series of dilutions.
The plant tissue was homogenized in 5% metaphosphoric acid with the addition of triethanolamine (TEA) (1:6 in KPE buffer). The homogenized material was centrifuged at 10 000 g, 4°C, for 20 min, and collected supernatant was used for both assays.
To determine the total content of glutathione, 20 μL of standard or prepared samples were applied to the plate, and then 120 μL of GR and DTNB solution (1:1) were added to the wells. After 30 s (conversion of GSSG to GSH), 60 μL of β-NADPH was added to each well. Absorbance measurement at 412 nm was taken every 20 seconds for 3 minutes.
To determine the GSSG form, 2 μL of vinyl pyridine (covalently reacts with GSH) was added to the 100 μL of previously prepared supernatant, and then samples were incubated for an hour at RT with occasional stirring. The oxidized form remains free in the sample and can be reduced by the GR enzyme. The rest of the procedure was performed as described above. Total glutathione and GSSG contents were estimated from the standard curves. GSH was calculated as the difference between total glutathione and GSSG concentrations.
2.8 Determination of ascorbate and dehydroascorbate levels
The ascorbate contents were determined in leaf extracts according to the method described by Foyer et al. (1983) and Harrach et al. (2008). The plant tissue was homogenized in 5% metaphosphoric acid. The homogenized material was centrifuged at 10,000 g, 4°C, for 10 min, and the collected supernatant was used for both assays.
To determine the reduced form of ascorbate, 125 μL of supernatant and 25 μL of 1.5 M triethanolamine (TEA) were mixed on a vortex in the Eppendorf. Then 150 μL 150 mM phosphate buffer (pH 7.4) and 75 μL water were added to the same Eppendorf. 200 μL of the above mixture and 2 mL of 100 mM phosphate buffer (pH 5.6) were added to the quartz cuvette and measured immediately at 265 nm. 1 unit of ascorbate oxidase (AO) was added to the cuvette, and the decrease in absorbance was measured for 2 min until the lowest value was achieved.
To determine the DHA form, the plant extract was stabilized as before by adding 1.5 M TEA. Then DHA was reduced with 75 μL of 10 mM dithiothreitol (DTT) (non-enzymatic reduction) and incubated for 15 min at room temperature. The rest of the procedure was performed as described above. The total ascorbate content was calculated as a difference between the beginning extinction and the minimal extinction.
2.9 Statistical analyses
All statistical analyses (ANOVA, post-hoc Duncan's tests, Pearson correlation) were conducted using Statistica 13.1 software (StatSoft). The Tukey test was also used to verify the significance of the differences between the values.
3 RESULTS
To compare the impact of bacterial infestation versus injection, measurements were made on both groups of plants. The injection control gave results similar to the primary control, therefore, these results are not shown in this article.
3.1 Differences in Pip concentration
The results regarding the level of Pip concentration showed a significant difference between plants with C3 and CAM metabolism. The concentration of this molecule was nearly ten times higher in CAM plants compared to C3 plants (Figure 1). Upon supplementation with a 1 mM Pip solution, the concentration of Pip increased significantly in both metabolic groups of plants. In the days following bacterial treatment, a similar pattern of Pip level fluctuations was observed in both supplemented and untreated C3 plants. Initially, the concentration of Pip in C3 plants decreased within the first 24 h post-bacterial treatment (pbt), followed by a significant increase 3 days pbt, particularly notable in plants supplemented with Pip. Interestingly, unlike C3 plants, the Pip concentration remained unchanged after bacterial treatment in CAM plants that were not supplemented. Remarkably, in supplemented CAM plants, the concentration continued to increase daily post-bacterial treatment, reaching its highest level 3 days pbt.
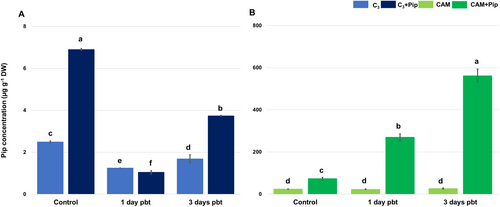
3.2 The effect of Pip supplementation on bacterial colony number and morphological symptoms of disease development on infested leaves
Within the first 24 h post-inoculation of M. crystallinum leaves a higher number of bacterial colonies was noted in C3 plants compared to CAM plants. However, differences in colony numbers formed by bacteria isolated from C3 and CAM leaves diminished over the next 24 h, (see Appendix S2 in Supporting Information). The number of bacterial colonies in C3-infested leaves was kept at a similar level for the whole experimental period, while in CAM plants, an increase in colony numbers was observed. The reduction in bacterial colony numbers was observed in both metabolic types after Pip supplementation compared to not-supplemented plants. This reduction was particularly notable 1 day pbt in CAM plants and 2 days pbt in C3 plants. Morphological symptoms of bacterial presence in leaf tissue differed between C3 and CAM plants, with more visible necrotized areas in CAM plants (see Appendix S3 in Supporting Information). Both metabolic groups showed smaller areas of lesions caused by infestation when pre-treated with Pip, indicating its positive effect in ameliorating symptoms of bacterial presence.
3.3 TEAC and MDA Levels
In the Trolox Equivalent Antioxidant Capacity (TEAC) assay, it was confirmed that CAM plants have a higher total antioxidant capacity compared to C3 plants, especially in the control groups (Figure 2A). In non-supplemented C3 and CAM plants, the bacterial treatment led to a slight increase in antioxidant potential after 1-day pbt (Figure 2B). Still, this potential decreased in C3 plants during the following days. Pip supplementation did not influence the antioxidant potential of C3 plants, even after bacterial infestation. However, in CAM plants, Pip supplementation increased the antioxidant potential in non-bacterial-treated plants compared to those without supplementation, but it decreased significantly after 3 days of bacterial treatment (Figure 2C).
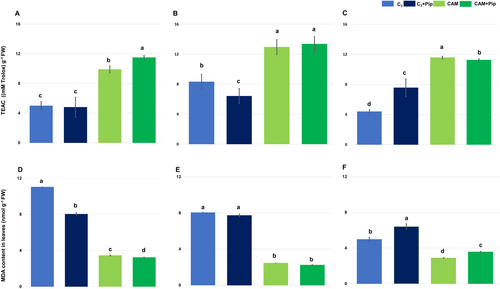
The level of MDA (malondialdehyde), a product formed from lipid peroxidation in response to increased levels of reactive oxygen species due to stress, correlated with the results of measuring the total antioxidant capacity of the examined plants. The MDA level in C3 plants was higher than in CAM plants, and supplementation with Pip caused a decrease in its level in both C3 and CAM plants (Figure 2D). C3 plants not supplemented with Pip exhibited a decrease in MDA level in the days following bacterial treatment, and a similar tendency was noted in plants after Pip supplementation (Figure 2E). However, when comparing the level of this molecule on the third day after infestation, a significantly higher concentration was noted in supplemented plants (Figure 2F). In CAM plants, regardless of Pip supplementation, a low content of MDA was maintained after bacterial treatment. However, a slight increase in MDA concentration was observed on the third day after infestation in supplemented plants, and it was significantly higher than in non-supplemented ones.
3.4 Antioxidant enzyme activities
The visualization of superoxide dismutase (SOD) activity on polyacrylamide gels revealed three isoforms: MnSOD, FeSOD, and two forms of Cu/ZnSOD (see Appendix S4 in Supporting Information). Densitometric analysis indicated that MnSOD activity was higher in CAM plants than in C3 plants, with Pip supplementation further increasing its activity in both. After bacterial treatment, MnSOD activity increased in both C3 and CAM plants, with Pip-treated plants showing significantly higher activity.
FeSOD activity was higher in C3 plants compared to CAM plants, with no changes observed after Pip supplementation in either group. Bacterial treatment did not affect FeSOD activity in C3 plants but led to a significant decrease in CAM plants by the 3rd day post-treatment.
Cu/ZnSOD was represented by two forms. The heavier form was more active in C3 plants than in CAM plants but increased slightly in both groups after Pip supplementation. Bacterial treatment decreased the heavier form's activity in C3 plants regardless of Pip supplementation. In CAM plants, the activity of this form remained stable in non-supplemented plants but significantly decreased after Pip supplementation following bacterial treatment. The lighter form of Cu/ZnSOD was more active in CAM plants and increased in C3 plants with Pip supplementation. Bacterial treatment caused a slight decrease in the lighter form's activity in Pip-supplemented C3 plants, while in CAM plants, its activity initially increased before returning to baseline levels.
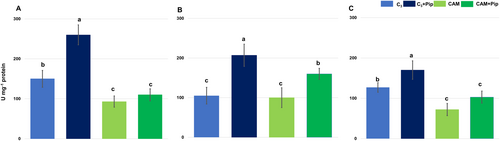
Results from spectrophotometric measurements of total SOD activity revealed higher enzyme activity in C3 plants compared to CAM plants, with Pip supplementation positively influencing SOD activity in both metabolic groups. The most significant increase was observed in Pip-supplemented C3 plants (Figure 3). Bacterial treatment caused a slight decrease in total SOD activity across all experimental groups, but the enhancement due to Pip supplementation remained unchanged.
Activity staining of gels for catalase (CAT) visualization revealed a single band corresponding with enzyme activity (see Appendix S4 in Supporting Information). The intensity of this band differed significantly between the analyzed samples. Densitometric analysis of visualized bands, as well as results of spectrophotometric measurement, confirmed that catalase activity was lower in CAM plants compared to C3 (Figure 4). However, Pip supplementation led to a significant increase in the activity of this enzyme in CAM plants but not in C3 plants. Interestingly, after bacterial treatment, both non-supplemented with Pip C3 and CAM plants exhibited a similar increase in catalase activity. On the contrary, in C3 plants supplemented with Pip, a stable level of catalase activity was maintained throughout the measurement days after bacterial treatment, while in CAM plants treated with Pip, a gradual decrease in CAT activity was observed in the following days after bacterial treatment. Pip seems to play a more complex role in CAT activity depending on the plant type (C3 vs. CAM) and the presence of bacteria in leaf tissue. In CAM plants, Pip supplementation might help to increase the baseline of CAT activity, but on the other hand, it also suppresses the already high activity of this enzyme caused by bacteria presence in the plant tissue.
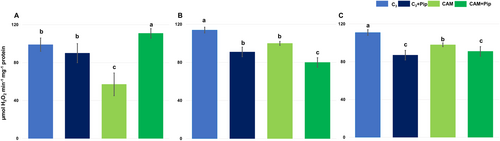
Several bands corresponding with peroxidase activity were visualized on gels after activity staining using DAB (see Appendix S4 in Supporting Information) and their total activity after densitometric analysis was presented on the plot. Spectrophotometric measurement confirmed the activity of total peroxidases visualized on the gels (Figure 5). It was found that total peroxidase activity was significantly higher in CAM plants than in C3 plants. Pip supplementation led to a decrease in peroxidase activity in both metabolic groups. In C3 plants, both those supplemented and those not supplemented with Pip, a similar tendency was observed to increase the activity of peroxidase during the days after bacterial treatment. In CAM plants, there was a difference in peroxidase activity after bacterial treatment between plants supplemented and not supplemented. CAM plants without Pip supplementation exhibited a stable level of peroxidase activity during the following day's post-bacterial treatment, while in Pip-supplemented plants, a significant increase in peroxidase activity was noted on 3rd day pbt.
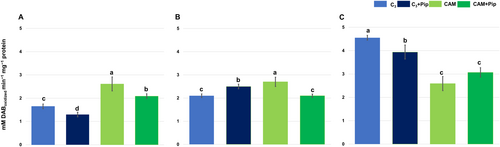
In conclusion, it can be inferred that Pip supplementation of plants from both metabolic groups had a stimulating effect on the activity of Mn and Cu/Zn isoform superoxide dismutase, while in the case of catalase and peroxidase, the more complex interplay between Pip supplementation, enzymes activity, and plant responses to stressors occurs, indicating the importance of considering plant type and environmental factors in understanding these interactions.
3.5 Low-molecular antioxidants
3.5.1 Glutathione and glutathione disulfide levels
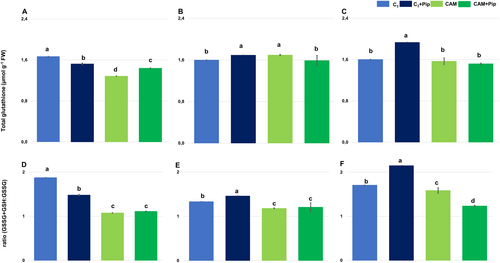
The studies revealed that C3 plants exhibited a higher level of total glutathione than CAM plants. Pip supplementation of C3 plants led to a decrease in the total glutathione pool (Figure 6A) and the ratio of total glutathione to its oxidized form (Figure 6D). On the contrary, the level of total glutathione increased in CAM plants after Pip supplementation, but the ratio of total glutathione to GSSG did not change. Pathogen treatment of non-supplemented C3 plants did not cause significant changes in the total glutathione pool (Figure 6B, C); however, the ratio of total glutathione to its oxidized form slightly decreased (Figure 6E, F). Bacterial treatment of Pip-supplemented C3 plants led to an increase in total glutathione as well as in the ratio of total glutathione to GSSG. CAM plants non-supplemented with Pip exhibited a slight increase in total glutathione (Figure 6B, C) and the ratio of total glutathione to its oxidized form after bacterial treatment (Figure 6E, F); however, in Pip-supplemented CAM plants, significant changes in total glutathione as well as in the ratio of total glutathione to GSSG were not visible. The results indicate that Pip supplementation had a reverse effect on the glutathione pool in C3 and CAM plants. It decreased total glutathione in C3 plants and increased it in CAM plants.
3.5.2 Ascorbate and dehydroascorbate levels
The results of ascorbate measurements showed that C3 plants had higher levels of total ascorbate compared to CAM plants (Figure 7A). Pip supplementation increased the total ascorbate pool in both C3 and CAM plants, but it also reduced the ratio of total ascorbate to its oxidized form (Figure 7D).
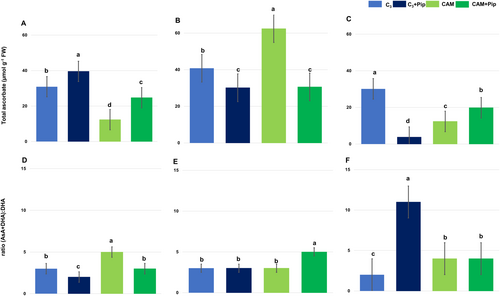
In C3 plants not supplemented with Pip, bacterial treatment did not significantly affect the total ascorbate pool (Figure 7B-C) or the ratio of total ascorbate to its oxidized form (Figure 7E, F). However, in Pip-supplemented C3 plants, a significant decrease in the total ascorbate pool, coupled with an increase in the ratio of total ascorbate to its oxidized form, was observed by the third-day post-infestation (Figure 7C, F).
For CAM plants without Pip supplementation, the bacterial treatment led to a significant increase in the total ascorbate pool (Figure 7B) and a slight decrease in the ratio of total ascorbate to its oxidized form by the first-day post-infestation (Figure 7E). Conversely, in Pip-supplemented CAM plants, only minor changes were observed in both the total ascorbate level and the ratio of total ascorbate to its oxidized form after the first day of bacterial treatment. Overall, Pip supplementation significantly altered ascorbate dynamics in both C3 and CAM plants under bacterial stress. Specifically, C3 plants showed a decrease in total ascorbate and an increase in its oxidized form, while CAM plants exhibited minimal changes in response to the same conditions.
3.5.3 Endogenous level of proline
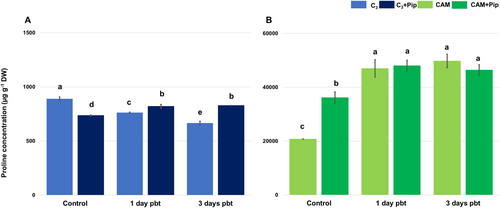
C3 plants exhibited significantly lower proline content compared to CAM plants, and their levels decreased after supplementation with Pip (Figure 8). In contrast, in CAM plants, Pip supplementation led to an increase in proline content. After bacterial treatment, the level of proline gradually decreased in non-supplemented C3 plants, but in those supplemented with Pip, an increase in proline content was noted and it remained at a similar level for 3 days pbt. Bacterial infestation of CAM plants resulted in a significant increase in proline content in both supplemented and non-supplemented plants, and it remained at the same high level until the end of the measurement period.
3.6 Correlation analysis
Pearson correlation analysis (Figure 9) confirms that Pip affects antioxidant systems differently in C3 and CAM plants, revealing distinct regulatory mechanisms. In C3 plants, endogenous Pip levels positively correlate with proline, total glutathione, and SOD activity during a bacterial infestation, indicating Pip enhances antioxidant defences. Pip supplementation further increases SOD and CAT activities but negatively correlates with proline concentration, suggesting a complex interaction between Pip and proline.
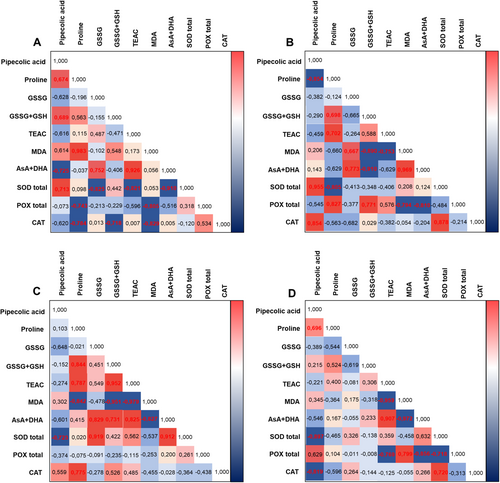
In CAM plants, high endogenous Pip levels during bacterial infestation negatively correlate with most antioxidant system components but statistically significantly only with total SOD activity. There is only one minor positive correlation with CAT activity, which indicates stable antioxidant defences. Pip supplementation significantly increases Pip concentration, with its fluctuations negatively correlating with CAT and SOD activity.
4 DISCUSSION
During biotic stress, plants activate defence mechanisms by rapidly producing ROS. While ROS have direct antimicrobial effects, they can also harm the host by causing lipid peroxidation, protein oxidation, and DNA damage, leading to cell death. At optimal levels, ROS serve as signalling molecules, activating defence genes, reinforcing cell walls, and inducing systemic acquired resistance (SAR) (Liu et al., 2024). To counteract ROS damage, plants activate antioxidant systems using enzymes and compounds like ascorbate (vitamin C), glutathione, tocopherols (vitamin E), and phenolic compounds to neutralize ROS and protect cells (Khan et al., 2020; Rosa et al., 2009; Sharma et al., 2019). Research is ongoing to explore how external supplementation with antioxidant-enhancing substances influences plant defence. These substances improve the regulation of ROS levels by boosting antioxidant enzyme activity and increasing non-enzymatic antioxidants, which help mitigate oxidative damage and support stress tolerance.
Exogenously supplied Pip plays a key role in pathogen resistance in plants activating salicylic acid (SA) signalling and inducing SAR (Bernsdorff et al., 2016; Návarová et al., 2012; Vogel-Adghough et al., 2013). Recent studies show Pip also contributes to drought tolerance by modifying the antioxidant system (Wang et al., 2021), with Pip and ROS mutually promoting each other's accumulation (Li et al., 2023; Wang et al., 2014; Wang et al., 2018). While most Pip research has focused on glycophytes, its role in halophytes is equally important, especially as climate change favours drought-tolerant plants. Our research on M. crystallinum, a facultative halophyte that switches from C3 to CAM photosynthesis under salinity stress, highlights Pip's potential to enhance plant defences against both biotic and abiotic stresses, including salinity and pathogen attacks. As soil salinization increases, leveraging natural compounds like Pip to boost crop resilience is critical. Pip could enhance crop defences, improving resistance and productivity, which is vital for food security and agriculture. These findings offer practical strategies for improving crop resilience with significant socio-economic benefits.
Mesembryanthemum crystallinum, known for its photosynthetic plasticity in response to salinity and drought (Bohnert and Cushman, 2000), has been widely studied for abiotic stress tolerance, but its biotic stress responses remain underexplored (Libik-Konieczny et al., 2011; Moog et al., 2023; You et al., 2015). Research on M. crystallinum-pathogen interactions provides insights into metabolism, redox homeostasis, and defence mechanisms (Libik-Konieczny et al., 2019). Stress-induced CAM metabolism leads to gene, protein, and metabolite changes that may inhibit pathogens (Barkla and Vera-Estrella, 2015; Guan et al., 2021; Winter and Holtum 2024). Prior stress exposure enhances plant tolerance, and studies suggest that CAM and antioxidant responses are linked, offering strategies to improve stress tolerance (Borland et al., 2006; Cushman et al., 2008; Sunagawa et al., 2010). Research also shows that salt stress upregulates antioxidant enzyme activity, further linking CAM and antioxidant defences (Achuo et al., 2006; Broetto et al., 2002; Miszalski et al., 1998; Ślesak et al., 2003; Nosek et al., 2018; Pastori and Foyer, 2002).
In our research, we found that Mesembryanthemum crystallinum plants exhibit higher endogenous Pip levels in CAM plants compared to C3 plants (Figure 1). This aligns with Barkla and Vera-Estrella (2015), who reported a significant increase in Pip concentration in salinity-induced CAM plants. The ability of halophytes to maintain high Pip levels suggests an efficient mechanism for its synthesis and utilization. Given that Pip is involved in osmoregulation, examining its external supplementation could provide valuable insights into how halophytes manage osmotic stress. This knowledge may lead to new approaches for enhancing stress tolerance in other plants through biotechnological methods. Through tests conducted on the cell sap from bacterial-treated leaves of Pip-supplemented plants and morphological observations, we have noted differences in the symptoms of infestation and variations in the bacteria growth abilities within host tissues. Our findings indicate that prior Pip supplementation affects both parameters, potentially aiding in the inhibition of bacterial spread in plant tissues (Appendix S2 and S3 in Supporting Information). It should also be noted that in C3 plants supplemented with Pip, an increase in the concentration of this molecule appears to reduce the number of bacterial colonies in the days following infestation. However, in CAM plants after supplementation, an increase in the number of bacterial colonies was observed in the days following treatment with Pseudomonas syringae. Interestingly, despite this increase, the morphological symptoms of infestation were ameliorated, suggesting that high Pip concentration may not directly affect bacterial vitality but rather influence the induction of different defence strategies.
The results of total antioxidant capacity also revealed differences between C3 plants and salt-induced CAM plants in favor of the latter (Figure 2A-C), confirming the hypothesis of increased defensive readiness in plants pretreated with stress. It can be concluded that the high antioxidant potential of CAM plants remains unchanged under Pip supplementation and prevents lipid peroxidation, as indicated by the low level of MDA. Meanwhile, significant fluctuations in antioxidant activity and MDA levels are observed in C3 plants under the influence of Pip supplementation, indicating their higher susceptibility to the action of this molecule. The MDA level is considered a marker of oxidative stress, causing an increase in membrane permeability due to lipid peroxidation. M. crystallinum C3 plants have an overall lower content of MDA each day after bacterial treatment, regardless of Pip supplementation. At the same time, CAM plants, despite slight fluctuations in MDA content, maintain a similar low level of this molecule (Figure 2D-F). These results confirm previously described data regarding the tolerance of M. crystallinum plants to other types of stress, such as heavy metal stress, where it has also been observed that the low level of MDA in M. crystallinum compared to other studied plants in response to stress is associated with their predisposition related to the adaptation to saline conditions (Amari et al., 2020). It was surprising to observe a decrease in MDA levels in the presence of bacteria in the tissue of the investigated plants, especially those with C3 metabolism. Typically, pathogen attack in plants leads to an overproduction of ROS, which causes lipid peroxidation and subsequently increases MDA production. However, recent studies have demonstrated the significant role of tocopherols in protecting membrane lipids from damage (Stahl et al., 2019). Based on earlier findings by Gabara et al. (2012), which showed a significant increase in tocopherols during infection of M. crystallinum with Botrytis cinerea in C3 metabolism, it can be inferred that the observed reduction in MDA levels in our studies might be caused by elevated tocopherol levels. Nevertheless, further studies are necessary to confirm this hypothesis.
To evaluate the role of specific antioxidant components in the overall antioxidant capacity of Pip-supplemented plants and their response to pathogens, we measured the activity of various antioxidant enzymes and quantified glutathione, ascorbate, and proline levels. Superoxide dismutase (SOD) is a crucial antioxidant enzyme, responsible for dismutation of superoxide radicals into hydrogen peroxide and oxygen, thus mitigating oxidative stress, a key factor in plant defence against pathogens (Nadarajah, 2020). The results of our study, focusing on total superoxide dismutase (SOD) activity, underscore significant differences between C3 and CAM plants in response to Pip supplementation and bacterial infection (Figure 3). Our findings revealed that CAM plants had lower baseline total SOD activity compared to C3 plants. However, Pip supplementation positively influenced SOD activity in both groups, with the most significant increase observed in Pip-supplemented C3 plants. This suggests that Pip enhances the antioxidant defence mechanisms in C3 plants more robustly than in CAM plants, possibly due to their different metabolic pathways. Importantly, even after bacterial treatment, the enhanced SOD activity resulting from Pip supplementation remained stable, indicating Pip's protective role against infection-induced oxidative stress. Total SOD activity is the sum of the activities of individual isoforms, each contributing based on its expression and function in different cellular compartments. Variations in the activity of these isoforms, in response to stress or signalling molecules, will directly influence the total SOD activity observed in a plant. In Mesembryanthemum crystallinum leaves, three main isoforms have been identified: MnSOD in mitochondria, FeSOD in chloroplasts, and Cu/ZnSOD in the cytoplasm (Emig et al., 1998; Miszalski et al., 1998; Surówka et al., 2007). Previous studies from our group confirmed their subcellular localization using selective staining and fractionation, with MnSOD showing the highest molecular mass and Cu/ZnSOD the lowest Miszalski et al., 1998. When examining specific SOD isoforms (Appendix S4 in Supporting Information), our data show that MnSOD, located in mitochondria, exhibited higher activity in CAM plants compared to C3 plants, with Pip further increasing its activity in both. This is consistent with the well-established role of mitochondrial metabolism in CAM plants, which have been shown to rely on enhanced mitochondrial activity for stress tolerance, particularly during the transition from C3 to CAM photosynthesis under salinity stress (Broetto et al., 2002). Pip's influence on MnSOD activity, especially in bacteria-treated CAM plants, supports the idea that Pip primes mitochondrial defences, leading to greater resilience against oxidative damage caused by pathogens. This increase in MnSOD activity aligns with studies showing that mitochondrial ROS) plays a dual role as both damaging agents and signalling molecules, triggering defence responses and programmed cell death in infected cells (Amirsadeghi et al., 2007).
Catalase and peroxidases regulate ROS levels by modulating hydrogen peroxide (H₂O₂). Catalase, present mainly in peroxisomes, mitochondria, and the cytoplasm, breaks down H₂O₂ into water and oxygen, crucial for scavenging H₂O₂ from photorespiration under normal and stress conditions (Baker et al., 2023). CAM plants, with their unique adaptations, exhibit lower catalase activity compared to C3 plants (Figure 4 and Appendix S4 in Supporting Information), suggesting they have evolved to reduce oxidative stress, possibly through decreased photorespiration or enhanced scavenging. Pip supplementation affects catalase activity differently in C3 and CAM plants. It increases baseline catalase activity in untreated plants but suppresses it in the presence of bacteria, potentially influencing photorespiration and the plant's oxidative damage management, impacting overall health and stress tolerance.
Peroxidases reduce H₂O₂ by oxidizing various electron donors and are classified into three classes, with Class III peroxidases being crucial for cell wall integrity and defence (Czégény and Rácz, 2023). Our study found significantly higher total peroxidase activity in CAM plants compared to C3 plants (Figure 5 and Appendix S4 in Supporting Information), supporting previous research on peroxidase activity in halophytic plants and its role in salinity tolerance (Ghanem et al., 2021; Homayouni et al., 2024; Kumar et al., 2021; Mann et al., 2023). The decrease in peroxidase activity after Pip supplementation may be due to SA-induced inhibition of peroxidase activity, leading to increased H₂O₂ levels that signal defence mechanisms (Kidwai et al., 2020). However, high peroxidase activity in the presence of bacteria and its increase in CAM plants after Pip supplementation confirm its crucial role in local defence and pathogen containment (Almagro et al., 2009).
Key non-enzymatic antioxidants in plant cells include ascorbate and glutathione, whose reduced and oxidized forms are linked through the ascorbate-glutathione cycle (Foyer and Kunert, 2024). This cycle is essential for managing oxidative stress in plants. Our research reveals distinct responses in C3 and CAM plants when supplemented with Pip, especially under bacterial stress. C3 plants, which naturally have higher levels of glutathione, showed a decrease in its concentration after Pip treatment, while CAM plants experienced an increase (Figure 6A). This suggests that Pip modulates the cycle according to the plant's metabolic type. Additionally, Pip raised ascorbate levels in both types of plants but increased the oxidized form, particularly in C3 plants under bacterial stress, which may lead to oxidative stress (Figure 7D-F). In contrast, CAM plants maintained a stable redox state with Pip, enhancing their defence mechanisms without significant disruption. These findings suggest that Pip could be used to improve stress resistance in crops, with C3 plants benefiting from a more dynamic response and CAM plants gaining consistent protection.
Proline accumulation is closely associated with stress tolerance, particularly under salinity, and tends to be higher in salt-tolerant plants (Tada et al., 2023). Our findings confirm this, showing that M. crystallinum, a salinity-induced CAM plant, has higher proline levels than C3 plants (Figure 8). Proline plays a key role in stress response by maintaining cell turgor, stabilizing membranes, and regulating ROS levels (Hayat et al., 2012; Bauduin et al., 2022; Ghosh et al., 2022). The involvement of proline in plant defence against pathogens has been studied in several plant species (Rogan et al., 2024; Verslues and Sharma, 2010). Although it is suggested that proline does not directly contribute to protection against biotic stress per se, but rather its metabolic pathway is enhanced during bacterial infection (Cecchini et al., 2011). Pathogen presence in plant tissue led to a decrease in proline content in C3 plants, which typically have lower basal proline levels than CAM plants. However, Pip supplementation resulted in an increase in proline concentration in C3 plants as early as the first day after infestation. Conversely, bacterial treatment led to increased proline concentration in CAM plants with higher basal proline levels.
Studies on stress-responsive plant species like M. crystallinum reveal that Pip supplementation has contrasting effects on antioxidant systems in C3 and CAM plants under bacterial treatment. Pip enhances antioxidant activity in C3 plants during bacterial exposure, while CAM plants maintain consistently high antioxidant levels for continuous protection. Enhancing stress tolerance in C3 plants through Pip could improve crop yield and resilience, which is crucial for global food security. Utilizing the naturally high antioxidant levels in CAM plants such as M. crystallinum can also promote the cultivation of drought- and salt-resistant crops, mitigating agricultural losses in climate-affected regions and supporting local economies and food security.
AUTHOR CONTRIBUTIONS
Emilia Gula conducted the experiments and performed the measurements of Total Antioxidant Capacity, MDA content, Antioxidant enzyme activity, Glutathione, and Ascorbate content. Emilia Gula also analyzed the data, prepared figures, and wrote the manuscript. Marta Libik-Konieczny designed the experiments, helped in writing the conclusions, and contributed to the final version of the manuscript. Michał Dziurka carried out measurements of the amount of Pip and proline. Natalia Hordyńska cultivated plants and performed statistical tests of correlation analyses.
The corresponding authors certify that all authors read and approved the final version of the manuscript and warrant that the article is the author's original work, has not received prior publication, and is not under consideration for publication elsewhere.
ACKNOWLEDGEMENTS
We extend our thanks to Professor Jürgen Zeier from Heinrich Heine University in Düsseldorf, Germany, for his valuable substantive guidance.
Open Research
DATA AVAILABILITY STATEMENT
All authors agreed that the data contained in this publication were collected based on laboratory experiments without the use of databases and websites used to analyze “omics” data, which are not included in the work and were not part of the research. The authors included all the results in this work, therefore there is no basis for redirecting to other sources of data. The physical data that support the findings of this study are available from the corresponding authors upon reasonable request.




