Seed pretreatment with brassinosteroids stimulates sunflower immunity against parasitic weed (Orobanche cumana) infection
Abstract
Broomrape (Orobanche cumana) negatively affects sunflower, causing severe yield losses, and thus, there is a need to control O. cumana infestation. Brassinosteroids (BRs) play key roles in plant growth and provide resilience to weed infection. This study aims to evaluate the mechanisms by which BRs ameliorate O. cumana infection in sunflower (Helianthus annuus). Seeds were pretreated with BRs (1, 10, and 100 nM) and O. cumana inoculation for 4 weeks under soil conditions. O. cumana infection significantly reduced plant growth traits, photosynthesis, endogenous BRs and regulated the plant defence (POX, GST), BRs signalling (BAK1, BSK1 to BSK4) and synthesis (BRI1, BR6OX2) genes. O. cumana also elevated the levels of malondialdehyde (MDA), hydroxyl radical (OH−), hydrogen peroxide (H2O2) and superoxide (O2•–) in leaves/roots by 77/112, 63/103, 56/97 and 54/89%, as well as caused ultrastructural cellular damages in both leaves and roots. In response, plants activated a few enzymes, superoxide dismutase (SOD), peroxidase (POD) and reduced glutathione but were unable to stimulate the activity of ascorbate peroxidase (APX) and catalase (CAT) enzymes. The addition of BRs (especially at 10 nM) notably recovered the ultrastructural cellular damages, lowered the production of oxidative stress, activated the key enzymatic antioxidants and induced the phenolic and lignin contents. The downregulation in the particular genes by BRs is attributed to the increased resilience of sunflower via a susceptible reaction. In a nutshell, BRs notably enhanced the sunflower resistance to O. cumana infection by escalating the plant immunity responses, inducing systemic acquired resistance, reducing oxidative or cellular damages, and modulating the expression of BR synthesis or signalling genes.
1 INTRODUCTION
Sunflower (Helianthus annuus L.) is a popular oilseed crop grown worldwide as a major contributor to livestock feed. In some countries, such as Asia and Southern Africa, the cultivation of sunflower may pose a greater challenge compared to other crops, such as maize, soybeans, and sorghum (Adeleke et al., 2020). Sunflower, along with soybean and rapeseed, are the three major oilseed crops farmed today. It is widely renowned as a primary supplier of premium edible oil. The requirement for obtaining sunflower seeds (edible oil) and by-products has grown due to the continual expansion of the human population. To meet demand, it is necessary to step up efforts to increase sunflower production (Taher et al., 2017).
Broomrape species (Orobanche and Phelipanche spp., Orobanchaceae) are root parasitic weeds which are particularly harmful to crops, including tobacco, tomato, and sunflower. These weeds drastically reduced the quality and production of crops (Sisou et al., 2021). Broomrape (Orobanche cumana) is a significant obstruction to sunflower cultivation, mainly in the Middle East, Southeast Europe, and Asia (Parker, 2013). The invasion of O. cumana has the potential to reduce the sunflower yield by up to 80% (Duca et al., 2015). The life cycle of O. cumana consists of four stages. In the soil, O. cumana seeds remain in an inactive state until activated by germination cues produced by host plant roots (stage 1) (Brun et al., 2017). Right after germination, O. cumana initiates contact with host plant roots through haustoria, and root exudates enhance its development. O. cumana releases a number of enzymes (protease, cellulase, and xylanase, etc.) involved in cell wall degradation and establishes a haustorial attachment to ease its penetration into the host roots (stage 2). Upon reaching the vascular bundles of the host plant roots, O. cumana (via haustoria) draws water, essential nutrients and other macromolecules, resulting in host weakening. During Stage 3, a nutrient storage site (tubercle) rapidly develops at the attachment point, a place of underground shoot emergence. The 4th and last stage begins with the above-ground emergence of O. cumana, characterized by the development of flowering spikes and the production of seeds (Krupp et al., 2019). The basic infestation mechanism is that O. cumana interferes with plant metabolism, restricts the uptake of essential mineral nutrients, impairs the photosynthetic performance, causes extra generation of reactive oxygen species, lipids peroxidation, imposes the ultrastructural cellular damages and synchronizes the antioxidants defence system (Louarn et al., 2016; Li et al., 2019; Yang et al., 2020). Various approaches have been utilized for the management of O. cumana. For instance, some herbicides, such as imazamox and glyphosate, displayed herbicidal effects on O. cumana (Nadler–Hassar et al., 2009). However, sometimes, they cause adverse effects on crop productivity (Hosni et al., 2020). Therefore, effective management of O. cumana infection is required. Recent reports have documented the involvement of different plant hormones in plant growth promotion and management of O. cumana infestation (Li et al., 2019; Yoneyama et al., 2019).
Brassinosteroids (BRs), a plant steroid hormone, play crucial roles in regulating the physiological and biochemical processes involved in plant growth and development (Jiang et al., 2013). As a nontoxic steroid compound, BRs have the potential to modulate plant responses against abiotic and biotic stresses, thereby aiding in the enhancement of plant immunity (Jiang et al., 2013; Yao et al., 2023). In addition, BRs possess antiviral and anti-ecdysteroids properties, rendering them an ideal alternative to chemical herbicides and pesticides (Yao et al., 2023). Being a signalling hormone, BRs induce a hypersensitive response, the expression of genes associated with pathogenesis, and ensure the acquisition of systemic resistance by plants (Loake & Grant, 2007; Kim et al., 2022). However, it is not clear how O. cumana infestation modulated the growth performances of sunflower at physiological, biochemical, cellular and genetic levels. Furthermore, the potential roles of BRs in enhancing the plant immunity against O. cumana invasion in sunflower plants remain largely uninvestigated. Hence, our main objective was to evaluate the protective efficacy of seed-pretreated BRs in counteracting the O. cumana invasion on sunflower plants. For this purpose, we targeted plant growth and biomass production, photosynthetic machinery, oxidative stress, phenolic compounds, antioxidants defence (enzymatic and non-enzymatic), expression of BRs signalling, homeostasis or defence related genes, and cellular ultrastructural changes in sunflower seedlings.
2 MATERIALS AND METHODS
2.1 Experimental design and growth conditions
The seeds of sunflower (cv. TK0409) and Orobanche cumana (race G) were acquired from the Inner Mongolia Academy of Agricultural and Animal Husbandry Sciences (Hohhot, China). Before treatment, seed sterilization was carried out with 1% KMnO4 for 2 min, and then 95% ethanol was applied for 1 min, followed by distilled water washing. The sterilized seeds were incubated for 8 h, followed by treatment with different concentrations of BRs (0, 1, 10, and 100 nM). Then, the seeds were shifted into pots consisting of a substrate of peat and vermiculite in 1:1 v/v. 0.1 g of O. cumana seeds were added with 0.5 kg substrate for inoculation. All the plants were kept under 14/10 h photoperiod, relative humidity at 65% and 200 μM m−2 s−1 light intensity. A factorial design was employed to create eight treatment groups with four levels of BRs (0, 1, 10, and 100 nM) combined with two levels of O. cumana (with and without). Each treatment group consisted of at least three replicates. Four weeks after inoculation, sunflower plants (leaves and roots) were individually separated and harvested to carry out morphological, physio-biochemical, cellular alterations and transcription profiling.
2.2 Measurement of agronomic parameters and photosynthesis traits
Immediately following harvesting, the number of O. cumana, fresh biomass of stem and roots, and plant height were measured manually. Dry analysis was performed after oven-drying the samples at 70°C until constant weight. Chlorophyll a, chlorophyll b, and carotenoids were estimated according to earlier protocols (Lichtenthaler, 1987). Gas exchange parameters were determined from the fully stretched leaves using the LiCor-6400 portable photosynthesis system during periods of maximum light intensity (between 10:00 and 12:00 am) (Ulhassan et al., 2023).
2.3 Estimation of lipid peroxidation and reactive oxygen species
Lipid peroxidation, denoted as MDA, was estimated by the 2-thiobarbituric (TBA) method, as reported in an earlier study (Krupp et al., 2019). Superoxide (O2•–) was estimated using an earlier described method with slight changes (Jiang et al., 2001). Fresh plant samples (0.5 g) were intermixed in 3 mL of 70 mM potassium phosphate buffer (PBS) (pH 7.8) and later centrifuged for 10 min at 5000 g in iced-cold conditions. Then, 1 mL supernatant was mixed with 0.9 mL of 70 mM PBS and 0.1 mL of 10 mM hydroxylaminde hydrochloride. After 24 h of incubation under 25°C, 1 mL of 17 mM sulphanilamide and 1 mL of 7 mM α-naphthylamine were mixed with 1 mL solution for 20 min at 25°C. After this, the same volume of n-butanol was affixed and centrifuged at 15000 g for 5 min. A standard curve determined the O2•– generation, and absorbance was measured at 530 nm. An established method was used to determine H2O2 contents in leaves and roots (Velikova et al., 2000). In addition, the accumulation of H2O2 and O2•– was confirmed by leaf staining with NBT (nitroblue tetrazolium) and DAB (diaminobenzidine tetrahydrochloride) dyes, respectively.
2.4 Determination of antioxidant and non-enzymatic antioxidants
0.5 g fresh leaf and root samples were separately intermixed in 50 mM PBS (8 mL) of pH 7.8 at iced-cold conditions. To evaluate the enzyme activities, the homogenized supernatant was undergone centrifugation for 15 min at 10,000 g under 4°C. Catalase (CAT, EC 1.11.1.6) activity was determined by estimating the degradation of H2O2 (extinction coefficient of 39.4 mM−1 cm−1) for 1 min at 240 nm (Aebi, 1984). Superoxide dismutase (SOD, EC 1.15.1.1) activity was established as nitro blue tetrazolium (NBT) mediated photochemical inhibition (Zhang et al., 2008). To estimate peroxidase (POD, EC 1.11.1.7) activity, guaiacol-mediated variation in absorbance was measured at 480 nm, as reported previously (Zhou et al., 1999). Ascorbate peroxidase (APX, EC 1.11.1.11) was estimated following the method described by Nakano and Asada (1981). Regarding the non-enzymatic antioxidants, the measurements of reduced glutathione (GSH) and oxidized glutathione (GSSG) were carried out following previously described methods (Hasanuzzaman et al., 2011).
2.5 Gene expression analysis
Total RNA was extracted from 0.1 g of leaf and root samples (each) using the Plant RNA Extraction Kit (TakaRa MiniBEST). The quality of the total RNA was determined using the Nano Drop-1000 Spectrophotometer (Thermo Scientific). Then, 200 ng of RNA was reversed transcripted using the TaKaRa PrimeScript™ RT Master Mix reagent kit with a gDNA eraser. The CFX96TM Real-Time System (Bio-Rad) was utilized to conduct polymerase chain reactions (PCRs) using the SYBR Premix Ex Taq II (Ti RNaseH Plus, TakaRa) reagent, according to manufacturer protocol. To verify the relative expression of the particular genes, the primers listed in Table S1 were used. For the analysis of relative gene expression, the Ct method with four replicates was performed (Livak and Schmittgen, 2001), and the EF-1a gene was taken as a reference gene.
2.6 Estimation of phenolics, lignin and endogenous brassinosteroids levels
Total phenolic content was determined using the previous methodology (Kofalvi & Nassuth, 1995). At a wavelength of 725 nm, phenolic concentrations were determined using p-coumaric acid as a standard. Lignin content was quantified according to the earlier method, which involved measuring the absorbance of lignin-like thioglycolic acid (LTGA) derivatives at 280 nm (Cahill & McComb, 1992). In detail, plant tissues were extracted in 2 mL methanol extracted for overnight. The obtained solids were oven-dried for 48 hr at room temperature, and dry weights were estimated. After this, samples were ground with 2 mL of 0.5 M NaOH, followed by extraction for 24 hr and neutralization with 0.5 mL of 2 M HCl. Then, the collected residue was centrifuged 1000 g, washed with distilled water, reintegrated in 5 mL mathanol and centrifuged the collected solids. The obtained dried pellets were resuspended in 5 mL of 2 M HCl, supplemented with 0.5 mL thioglycolic acid. The tubes were tightly closed and placed in water bath at 95°C for 4 hr, allowed to cool before being centrifuged. Then, solids were suspended in 0.5 M NaOH, incubated for 24 hr at 4°C and washed with distilled water. Later, samples were added to NaOH supernatant, 1 mL HCl and placed at 4°C for 24 hr to precipitate the LTGA. The final pellet was dissolved in 0.3 mL of 0.5 M NaOH, and residues were discarded with centrifugation. At the end, the absorbance of 1 mL LTGA was determined at 280 nm. The content of different BRs was determined through their extraction, purification, and subsequent analysis using ultra-high performance liquid chromatography (UHPLC) followed by mass spectrometry. This analytical approach followed the established methodology (Tarkowská & Strnad, 2017).
2.7 Transmission electron microscopy
Leaf fragments (1 mm2) without veins and root tips (2–4 mm) were placed in 2.5% (v/v) glutaraldehyde in 0.1 M phosphate-buffered saline (PBS, pH 7.4) for 24 hr and then washed with the same PBS buffer. Afterwards, samples were fixed with 1% osmium tetraoxide (for 1 hr) in PBS solution and washed again with 0.1 M PBS (pH 7.4) and with 10-min time intervals. Later, samples were dehydrated twice with 30, 50, 70, 80, 90, 95, and 100% ethanol for 15 min and then washed with acetone for 20 min. The samples were filtered and kept overnight in pure resin. Following 8 hr exposure to 65°C, ultra-thin sections were examined using transmission electron microscopy (TEM, JOEL-30EX instrument Ltd.) (Yang et al., 2020).
2.8 Statistical analysis
The statistical tool SPSS version 20.0 (SPSS) was employed to analyze the data. Two-way analysis of variance (ANOVA) was performed. Fisher's least significant test (LSD) was used for multiple comparisons to calculate the significant difference (P < 0.05) between individuals. All reported values in results are the mean of at least three replicates ± standard deviation (SD).
3 RESULTS
3.1 Exogenous BRs improved the plant phenotype and growth attributes against O. cumana infection
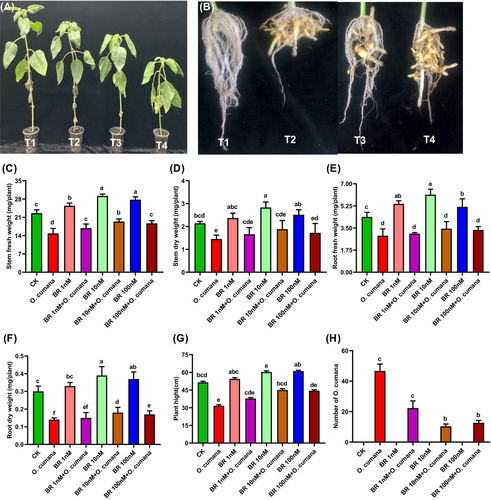
To evaluate the protective roles of BRs in controlling the O. cumana infestation on plant growth attributes, we supplied different levels of BRs on the plant phenotype, height, fresh and dry biomasses of stems and roots, and the number of O. cumana flowering spikes that had emerged from sunflower roots in response to the infection (Figure 1A–H). Distinct phytotoxic effects in the form of chlorosis, yellowing of leaves, plant growth inhibition (Figure 1A), and a higher number of O. cumana flowering spikes (Figure 1B) were observed after inoculation. While BRs treatments minimized these visual symptoms, as seen by the improved plant growth performance and fewer O. cumana flowering spikes attached to sunflower roots (Figure 1A, B). The sole O. cumana infection significantly reduced the stem fresh weight (SFW) (34.4%), stem dry weight (SDW) (32.2%), root fresh weight (RFW) (34%), root dry weight (RDW) (53.3%) and plant height (PH) (38.6%) and increased the number of O. cumana flowering spikes compared to controls. In opposite, the exogenous applications of BRs (1, 10, 100 nM) alleviated the O. cumana infection and notably improved the SFW (13, 30.7, 24%), SDW (14.5, 29.7, 18.4%), RFW (8.8, 19.4, 15.9%), RDW (7.1, 28.6, 21.4%), PH (19.8, 42.5, 36.2%), and reduced the O. cumana flowering spikes attached with roots as compared to alone O. cumana infection (Figure 1C–H). These findings demonstrated that the exogenous supply of BRs (especially at 10 nM) attenuated the harmful effects of O. cumana infection on sunflower growth characteristics. While the BRs-mediated reduction in O. cumana spikes in sunflower roots indicated the involvement of BRs in planta defence responses.
3.2 Exogenous BRs improved the chlorophyll content and photosynthetic apparatus after O. cumana infection
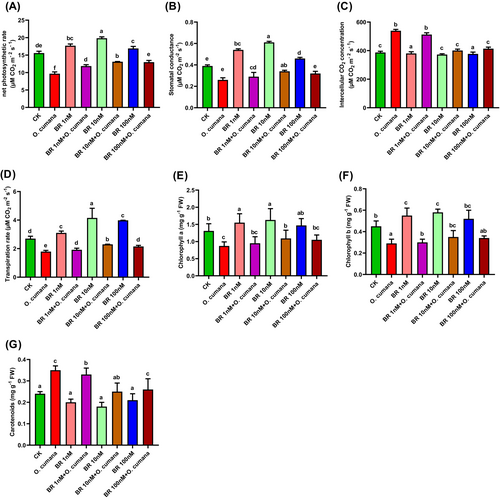
To investigate the influence of O. cumana infection on photosynthetic characteristics traits and the potential preventive effects of BRs. We examined the effects of varying concentrations of BRs on chlorophyll and gas exchange parameters (stomatal conductance, net photosynthetic rate, transpiration rate and intercellular CO2 concentration) in sunflower leaves infected with O. cumana (Figure 2A–G). The alone O. cumana inoculation greatly reduced the levels of Chl a (33.5%), Chl b (35.8%), carotenoids (43.1%), stomatal conductance (32.9%), net photosynthetic rate (37.9%), transpiration rate (33.6%), and intercellular CO2 concentration (39.4%). In contrast, applications of BRs (1, 10, 100 nM) improved the photosynthetic attributes including stomatal conductance (11.5, 30.8, 23.07%), net photosynthetic rate (22.5, 35.4, 34.1%), transpiration rate (7.3, 28.7, 20.1%), intercellular CO2 concentration (4.9, 25.6, 23.3%), Chl a (8.7, 24.7, 20.4%), Chl b (3.5, 21, 17.2%) and carotenoids (5.7,28.6, 25.7%) under O. cumana infection (Figure 2A–G). These findings suggested the protective roles of BRs in alleviating the photosynthetic inhibition caused by O. cumana in sunflower.
3.3 Exogenous BRs preserve membrane integrity and reduce the oxidative stress induced by O. cumana infection
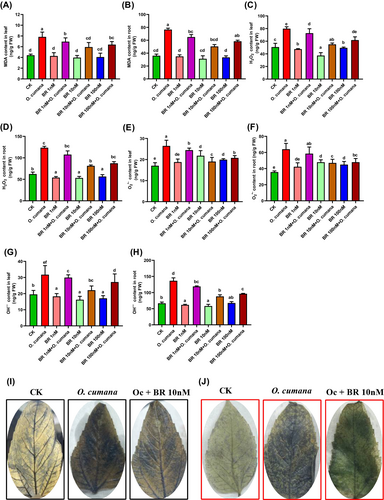
To evaluate whether BRs can preserve the integrity of the plasma membrane (lipids peroxidation as MDA) and minimize the oxidative damages induced by O. cumana infection in sunflower tissues, we estimated the MDA, OH−, H2O2 and O2•– levels under control and O. cumana exposure. Under infection of O. cumana alone, MDA, OH−, H2O2 and O2•– levels were dramatically increased in both leaves/roots by 77.2/112.8, 62.5/104.4, 57.6/98.3 and 55/89.4%, respectively compared with untreated control (Figure 3A–H). The significantly higher levels of MDA, OH−, H2O2 and O2•– were noted in roots relative to leaves, signifying that O. cumana triggered a stronger oxidative stress response in roots than leaves. In responding to alone O. cumana inoculation, the treatments of BR (1, 10 and 100 nM) notably reduce the accumulation of MDA (12.2, 24.1, 18.3%/16.1, 34.1, 23.6%), OH− (8.6,30.3,14.4%/12.7,35.4,29.4%), H2O2 (9.9,31.1,22.6%/12.9,34.2,29.2%) and O2•– (8.4,28,21.5%/9.6,36.5,25.1%) both in leaf/root cells. Markedly, the supplementation of BRs at 10 nM enormously restricted the extra accumulation of MDA, OH−, H2O2 and O2•– levels in leaves/roots against O. cumana infection (Figure 3A–H). To further confirm the O. cumana inoculation elicited lipids peroxidation and oxidative stress via extra generation of MDA, H2O2 and O2•–, and their alleviation effects by BR, sunflower leaves were treated with 3,3-diaminobenzidine (DAB) and nitro-blue tetrazolium (NBT), respectively (Figure 3I, J). The DAB staining showed a dark brown colour (an indicator of H2O2), and NBT staining displayed a dark blue (an indicator of O2•–) in leaf tissues at the exposure of O. cumana infection compared to the bright leaves seen in the control treatments. In response, the exogenous supplementation of BR substantially minimized the intensity of dark brown and dark blue colours, indicating the reduction in oxidative stress induced by H2O2 and O2•–. These findings verified that BRs greatly minimized the O. cumana-induced lipids peroxidation and oxidative stress in leaf and root tissues of sunflower seedlings.
3.4 Influence of BRs on GSH, GSSG and GSH/GSSG ratios against O. cumana infection
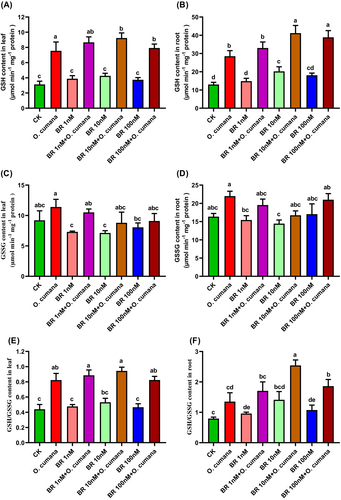
To assess the efficacy of BRs in regulating the non-enzymatic antioxidants against O. cumana infection, we determined the GSH and GSSG contents, as well as the GSH/GSSG ratio in the leaves and roots of sunflower plants. In comparison to uninfected controls, sole O. cumana infection dramatically increased the contents of GSSG (82/65%), GSH (109/97%), GSH/GSSG ratio (65/57%) in both leaves/roots of sunflower (Figure 4A–F). The addition of BRs (1, 10 and 100 nM) improved the GSH (14.8, 22.4, 4.9/15.8, 44.4, 36.4%), GSSG (7.9, 22.2, 20.2/11.1, 23.9, 4.5%) contents and GSH/GSSG ratio (7.6, 24.6, 11.1/26.7, 88.1, 37.6%) in leaves/roots, respectively under O. cumana infection. The increase of BRs levels (from 1 to 10 nM) enhanced the GSH contents and the GSH/GSSG ratio, while there was a decreasing trend from 10 to 100 nM BRs. With the increase in BRs levels (1, 10 and 100 nM), GSSG exhibited a decreasing trend (especially in leaves) irrespective of O. cumana infection. These findings revealed that BRs (particularly at 10 nM) were most effective in enhancing the GSH/GSSG ratio and GSH levels in sunflower tissues against O. cumana infection.
3.5 Exogenous BR activate the antioxidant defence system against O. cumana infection
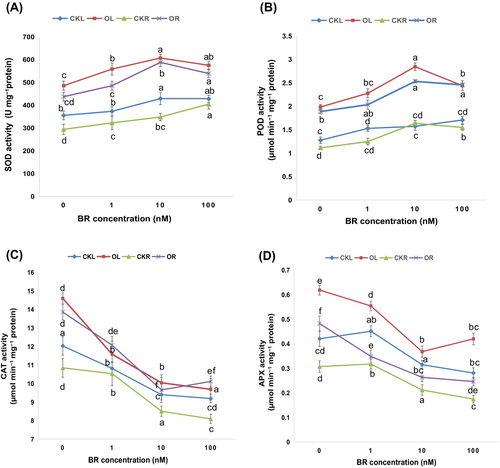
To appraise the potency of BRs in regulating the enzymatic antioxidants against the oxidative stress induced by O. cumana infection, we estimated the activities of SOD, POD, CAT, and APX enzymes in sunflower plants (Figure 5A–D). In response to O. cumana inoculation alone, the activities of SOD and POD enzymes were elevated by 26.84/32.77, 38.72/41.03%, while the activities of CAT and APX enzymes were decreased by 27.56/31.72% and 32.04/36.28% in leaves/roots, respectively compared to their corresponding controls. Upon the addition of BR at different concentrations (1, 10 and 100 nM), it was observed that the activities of SOD and POD were increased to the maximum level at BR concentrations from 1 to 10 nM and then decreased from 10 to 100 nM of BR application. In parallel to this, the CAT and APX activities were substantially decreased (from 1 to 10 nM), and the maximum decrease was recorded under 10 nM BR by 31.19/30.33% and 40.55/45.41%, respectively and then started increasing at the exposure of 100 nM BRs (Figure 5C, D). These observations indicated that SOD and POD activities participate more in the plant defence system to manage the O. cumana infection. While the decreasing trends in CAT and APX activities illustrated that these enzymes could not cope with the excessive oxidative stress indulged by O. cumana infection, thus severely hampering the sunflower defence system.
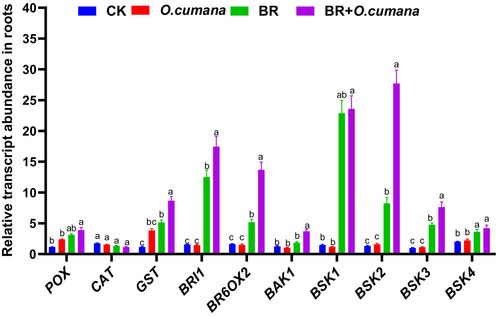
3.6 Influence of BR in regulating different transcript levels against O. cumana infection
To analyse the effects of BRs treatments on the expression profiles of various genes in the roots of infected or non-infected plants, we examined the antioxidant defence (POX and GST), BRs signalling (BAK1, BSK1, BSK2, BSK3 and BSK4) and BRs synthesis (BRI1, BR6OX2) genes (Figure 6). In response to O. cumana infection treatment alone, transcript levels of the genes involved in antioxidant defence, signalling and synthesis were downregulated compared to non-stressed control, except POX, GST and BSK3. The supply of BRs alone significantly enhanced the expression profile of GST, BR6OX2 and BSK1, indicating the crucial involvement of these genes in plant defence, and BRs signaling and synthesis. The co-treatments of BRs and O. cumana infection further escalated the expression profiles of most of the above-reported genes. This suggested the involvement of BRs in plant defence and signalling transduction and endogenous BR synthesis to control the O. cumana infection (Figure 6).
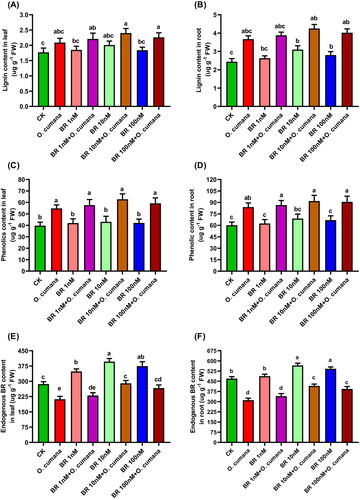
3.7 Effects of BRs and O. cumana on lignin, phenolics and endogenous BRs contents
Phenolic compounds, including phenolics and lignin, not only enhance plant growth but also offer protection against environmental stresses. To validate the defensive roles and cell wall thickening by BRs in response to O. cumana infection, we quantified the endogenous production of phenolics, lignin and BRs in sunflower plants. In response to O. cumana infection alone, lignin contents exhibited a significant increase in both leaves/roots (23.6/47.4%) compared to uninfected controls (Figure 7A, B). Under O. cumana infection, BRs (1, 10, 100 nM) increased lignin content (7.7, 16.8, 9.1/7.9, 17.8, 12.0%). Compared with controls, phenolic contents were increased by 34.1/39.2% in leaves/roots, respectively (Figure 7C, D). While the application of BRs (1, 10, 100 nM) further increased the phenolic content (8.2, 15.7, 9.3/7.8, 12.5, 9.7%) irrespective of O. cumana infection. Quantitative analysis revealed that O. cumana infection alone significantly decreased the endogenous levels of BRs (27.8/35.6) (Figure 7E, F). However, the application of BRs (1, 10, 100 nM) to seeds led to an increase in the contents of endogenous BRs (13.5, 34.8, 25.9/11.5, 31.7, 28.7) compared to O. cumana infection alone (Figure 7E, F). These findings suggested that an exogenous supply of BRs (particularly at 10 nM) significantly improved the plant defence against O. cumana infection.
3.8 Exogenous BRs minimize the O. cumana-induced cellular ultrastructural damages
To observe the protective effects of BRs against O. cumana infection-induced cellular variations, we performed transmission electron microscopy (TEM) analysis on the leaves and roots of sunflower plants (Figure 8A–H). In non-infected controls, all the observed cellular ultrastructures were well-developed and structured. Leaf mesophyll cells displayed eminent cell wall (CW), cell membrane (CM), healthy mitochondria (MC), numerous starch grains (SG), regular arrangement of thylakoids membrane (Thy) in the chloroplast (Chl) and plastoglubuli (P) within the cytoplasm (Figure 8A). In response to O. cumana infection alone, visible toxic symptoms in the form of scattered cell wall (CW), immature plastoglubuli (P), smaller starch grains (SG), invisible nucleus (N) and nucleolus (Nue), swollen granum thylakoids (Thy), cracked cell membrane (CM) and broken nuclear membrane (NM) were noticed (Figure 8B). The treatments with BRs greatly rescued the sunflower cells from O. cumana infection and its induced cellular damage as noticed by well-developed cell wall (CW) and cell membrane (CM), clear nucleus (N) with nuclear membrane (NM), dense granum thylakoids (Thy) organization in chloroplast (Chl), clustering of higher number of plastoglubuli (P), and large sizes of starch grains (SG) (Figure 8C, D).
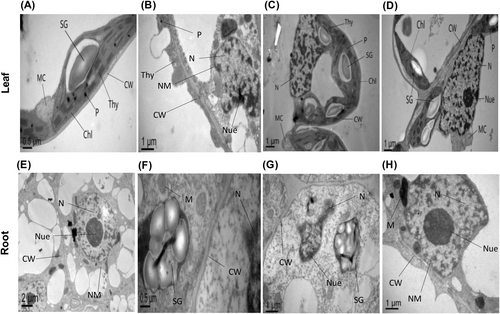
TEM micrograph of root tip cells of sunflower under control treatments showed visible and well-sized nucleus (N), nucleolus (Nue), nuclear membrane (NM) and cell wall (CW) (Figure 8E). Under O. cumana infection, the TEM micrograph displayed dispersion in the nucleus (N), nucleolus (Nue), broken cell wall (CW), immature mitochondria (M) and small sizes of starch grains (SG) (Figure 8F). The applications of BRs greatly recovered the damaged root cells as observed by the clear nucleus (N) and nucleolus (Nue), large sizes of starch grains (SG), well-developed cell wall (CW), thick nuclear membrane (NM) and matured mitochondria (M) (Figure 8G, H). These ultrastructural damages in leaf and root cells were effectively recovered by BRs, especially at 10 nM. These observations verified that BRs have great potential to minimize the O. cumana infection-induced cellular ultra-structural damage in sunflower tissues.
4 DISCUSSION
Our study aimed to evaluate the protective effectiveness of BRs in reducing the damaging impacts of O. cumana infection on sunflower plants. We mainly focused on the alterations in plant growth and biomass, photosynthetic apparatus, cellular ultrastructures, oxidative stress, phenolic compounds and the multifaceted antioxidant defence system (enzymatic and non-enzymatic components). Until now, there have been few studies on the effectiveness of seed-treated BRs in protecting sunflower plants against O. cumana infestation. Plant immunity is inversely associated with the plant growth. Our research shows that O. cumana infection in sunflower plants resulted in detrimental impacts on plant growth and fresh and dry biomass (Figure 1C–G). The decrease in plant growth could partially be attributed to the reshuffling of photosynthates and mineral or water absorption for the growth of parasites, which lowers the photosynthetic efficiency of host plants. In this scenario, the presence of root parasite could have a negative effect on the biomasses of host plants, as it competes as a sink for assimilates, potentially undermining the efficacy of carbon assimilation (Mauromicale et al., 2008). Plant growth and biomass reduction can also be linked to protein inhibition and chlorophyll degradation (Yang et al., 2016). In accordance with our results, a reduction in plant growth and biomass production by O. cumana infection was observed in tomato plants (Madany et al., 2020). In contrast, the negative effects of O. cumana infection on these growth characteristics are greatly diminished by adding BRs. Consistent with our findings, it was reported that BRs alleviate the deficiencies of protein synthesis, boost chlorophyll levels and ultimately enhance plant growth attributes (Kaya et al., 2019). Previous investigations have also shown that the exogenous applications of BRs mitigated the biotic and abiotic toxicity by enhancing the growth and biomass production in Leymus chinensis (Yang et al., 2018), Zea mays (Anjum et al., 2011).
The number of O. cumana spikes attached to the host roots significantly increased after the O. cumana infection. The exogenous treatments with BRs reduced the number of O. cumana spikes in sunflower roots (Figure 1H). It was obvious that BRs may delay the formation of O. cumana tubercles and restrict the attachment of O. cumana with sunflower roots. Similarly, a recent study reported that salicylic and indole acetic acid treatments retarded the development of tubercles in O. crenata on Vicia faba roots (Briache et al., 2020). This indicated a specific defence response to these hormones. The O. cumana inoculation limited the functionality of the photosynthetic machinery (Figure 2A–G), as noticed in earlier studies (Li et al., 2019; Yang et al., 2020). There is a strong possibility that O. cumana infection degrades the photosynthetic pigments, which may impair the electron transport chain and photochemical efficiency (PS I and PS II) (Krupp, 2019). In the current study, the applications of BRs markedly reduced the deterioration of chlorophyll induced by O. cumana infection and augmented the capacity for photosynthesis under conditions with and without O. cumana infection (Figure 2A–G). Prior research has shown a substantial increment in photosynthetic efficiency and concurrent suppression of chlorophyll degradation in Cucumis sativus (Xia et al., 2006) and cereal crops (Anjum et al., 2011) through the applications of BRs.
Leaf gas exchange parameters significantly decreased under O. cumana infection. While BRs treatments effectively restored the photosynthetic damages (Figure 2A–D). The inhibitory effects of O. cumana infection on the gas exchange parameters were directly correlated with a reduction in photosynthetic pigments. It seems that O. cumana undermines the efficiency of carbon assimilation and diminishes chlorophyll synthesis under severe environmental conditions. While BRs might enhance both respiration and photosynthetic rates, thereby helping to improve the photosynthetic pigments (Kothari & Lachowiec, 2021; Ennami et al., 2022). A significant generation of MDA, H2O2, O2•– and OH− levels by O. cumana infection caused lipids peroxidation and oxidative stress. While, BRs notably repaired the oxidative and cellular membrane damages (Figure 3A–F). The overproduction of these oxidative stress markers could be the reason behind the severe growth retardation of sunflower plants under O. cumana infection, as observed earlier in tomato (Madany et al., 2020). The extra generation of ROS has been associated with growth reduction and lower crop yields at the exposure to environmental stressors (Gill & Tuteja, 2010; Ulhassan et al., 2019). Given the involvement of ROS in acclimatization pathways in response to plant stress, we suggested that ROS signalling from leaves to roots play a crucial role in limiting the accumulation of MDA and ROS (H2O2, O2•– and OH−) contents in leaves compared to roots. It can be linked to the impact of ROS on modulating the expression of genes involved in antioxidant defence (POX and GST), BRs signalling (BAK1, BSK1, BSK2, BSK3 and BSK4) (Figure 6) as reported in earlier studies (Dos Santos et al., 2003).
To regulate the excessive accumulation of ROS and the consequent cellular damage, sunflower plants activated their antioxidant defence system, manifested through enzyme activities (mainly SOD and POD) (Figure 4A, B). In accordance with our current study, a pronounced upsurge in the activities of antioxidant enzymes was observed under O. cumana infection. Generally, the activation of antioxidant signals indicates increased production of oxidative stress. The introduction of BRs resulted in increased activities of SOD and POD enzymes compared to untreated infected plants. This indicates that BRs effectively mitigated the overproduction of ROS. While BRs exposure reduced the activities of CAT and APX enzymes compared to untreated O. cumana infected plants (Figure 5A–D). This suggests that BRs disturb the antioxidant defence and limit their involvement in the plant defence system. Similar antioxidant resistance mechanisms were observed under O. cumana and O. ramosa infestation by ALA and SA in sunflower (Li et al., 2019) and tomato (Madany et al., 2020) plants. Thus, BRs possess the potential to function as a signalling molecule, facilitating the enhancement of the antioxidant defence system and reducing the oxidative damages caused by O. cumana infection. On the other hand, plants enhance their non-enzymatic free-radical scavengers (GSH and GSH/GSSG ratio) to tackle the extra generation of ROS. In the current study, the higher levels of GSH and lower GSSG levels in response to O. cumana infection (Figure 4A–F) caused a reduction in ascorbic acid levels, indicating the induction of oxidative stress. Under O. cumana infection, the utilization of BRs resulted in increased GSH and GSH/GSSG ratio. It could be inferred that BRs may be effective in regulating redox homeostasis, decreasing oxidative damages and enhancing sunflower tolerance capacity against O. cumana infection. It was noticed that BRs regulated the antioxidant defence (POX, GST), BRs signalling (BAK1, BSK1, BSK2, BSK3 and BSK4) and synthesis (BRI1, BR6OX2) related genes under O. cumana infection (Figure 6). Under O. cumana infection alone, a downregulation in the expression levels of most of the genes associated with plant defence, infection signalling or BRs synthesis were noticed. While, the applications of BRs alone significantly enhanced the gene expression profiles and further elevated the expression levels of most of the above-reported genes was found during O. cumana infection. This indicates the crucial participation of BRs in the plant defence system and signalling. In accordance with current findings, earlier studies reported that salicylic acid significantly enhanced the pathogenesis-related gene expression in sunflower plants grown under O. cumana infection. This indicated a salicylic acid-induced systemic acquired resistance in sunflower plants (Yang et al., 2016). Like the current outcomes, earlier studies have documented that defence-associated genes such as GST and POX detoxify oxidative stress (Dos Santos et al., 2003). In our study, higher GST and POX levels (Figure 6) indicated the induction of detoxification potential that leads to better plant growth.
The upregulation of genes linked to endogenous BRs synthesis was observed both in BRs treatment alone and O. cumana infection) (Figure 6), suggesting a relatively elevated synthesis, indicating a potential connection between BRs production and sunflower defence against O. cumana invasion. The induction in BRs-responsive genes under O. cumana and further escalation under BRs treatments and O. cumana infection (Figure 6) revealed the BRs-related increase in the defence mechanisms of sunflower plants under O. cumana infection. The current investigations revealed that an exogenous supply of BRs enhanced lignin accumulation in O.cumana-infected sunflower seedlings (Figure 7A, B). These findings suggested that BRs help in reducing the attachment of O. cumana via lignification, as reported in earlier findings (Kusumoto et al., 2007). Previous findings revealed that SA enhances the resistance of host plants by inducing lignification in the roots during O. minor infection in red clover. These outcomes revealed that BRs mediated lignification to host resistance by serving as a mechanical barrier that strengthens the plant cell wall. This also indicates the crucial roles of BRs in enhancing the thickness and defence capabilities of the cell wall.
Phenylalanine ammonia lyase (PAL) is a crucial enzyme in the synthesis of phenolic compounds. The induction in phenolic contents in O. cumana-infected sunflower plants and further escalation by BRs treatment suggested the activation in the transcript levels of PAL synthesis genes, directly correlated with endogenous BRs synthesis (Figure 7C–F). These results indicated that the systemic acquired resistance by BRs may partially participate in BRs-mediated synthesis or activation of PAL genes. An earlier study also noticed the induction of PAL genes in grape berry by salicylic acid (Wen et al., 2005). A significant accumulation of endogenous BRs in sunflower tissues, especially under O. cumana infection, indicated the potential involvement of BRs in plant defence system against O. cumana infestation (Figure 7E, F). This indicated the crucial importance of BRs in plant-weed interactions. Consistent with our findings, previous reports have validated the endogenous synthesis of salicylic acid in sunflower plants infected with O. cumana and suggested its role in sunflower defence mechanisms (Yang et al., 2016). In the present study, significant structural changes in the leaf mesophyll cells and root tip cells were noticed under O. cumana infection and/or BRs treatments (Figure 8A–H). When compared with controls, the severe structural damages in key cellular organs (cell wall, chloroplast, mitochondria, plastoglubuli, starch grains, nucleus, nucleolus, granum thylakoids, cellular and nuclear membrane) were noticed in the leaves and roots of sunflower plants under O. cumana infection (Figure 8B, F). These cellular damages were directly correlated with the elevated reactive oxygen species and oxidative damages. Importantly, BRs supply alleviated the damage to cellular components and provided cellular protection against O. cumana infection (Figure 8C, D, G, and H). It is conceivable that BRs diminished the elevated reactive oxygen species within cellular compartments, thereby substantially rescuing the cellular structures and preserving the cellular integrity. These cellular changes suggested that proper concentration of exogenous BRs (particularly at 10 nM) can recover the leaf mesophyll and root tips-related cellular structures against O. cumana infection. Earlier studies illustrated that the exogenous supplementation of phytohormones (ALA) and herbicide (imazapic) could diminish the O. cumana-infection induced cellular damage and enhanced the sunflower tolerance (Li et al., 2019; Pincovici et al., 2018). However, deeper cellular and molecular investigations are required to further validate the protective roles of BRs in improving the sunflower tolerance to O. cumana pathogen infection.
5 CONCLUSIONS
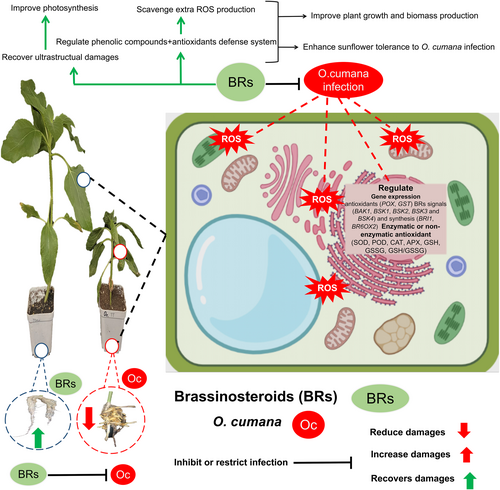
Our findings revealed that exogenous supplementation of BRs provides tolerance to sunflower plants against O. cumana infection, as explained in the schematic diagram (Figure 9). In detail, the alone O. cumana inoculation drastically inhibited the plant growth traits, fresh or dry biomass production, disturbed the photosynthesis-related parameters, induced the oxidative stress, cellular damages and desynchronized the overall antioxidant defence system in sunflower tissues. In response, BRs treatments (especially at 10 nM) significantly minimized the O. cumana infection indulged photosynthetic inhibition, plant growth and biomass inhibition, and extra production of oxidative stress and cellular damages. Moreover, BRs applications regulated both enzymatic and/or not-enzymatic antioxidants defence systems and accumulating specific metabolites (phenolics and lignin). Moreover, BRs recovered the O. cumana infection mediated damages to cellular ultra-structures (leaf mesophyll cells and root tip cells) and activated the genes linked to plant defence, and BRs signalling or synthesis indicates a high response to plant immunity. These observations provide novel approaches to minimize the O. cumana infestation on sunflower or other agricultural crops. Further studies should be extended at metabolic and molecular levels to achieve a deeper understandings of the BRs mediated signalling pathways and molecular mechanisms that underlie these beneficial effects.
AUTHOR CONTRIBUTIONS
Na Zhang, Skhawat Ali, Zaid Ulhassan, Chong Yang and Weijun Zhou designed the study. Na Zhang, Skhawat Ali, Zaid Ulhassan, Qian Huang, Weiqi Chen and Kangni Zhang performed the experiments. Na Zhang, Skhawat Ali, Zaid Ulhassan, and Sharafat Ali analyzed the data. Na Zhang, Skhawat Ali, Zaid Ulhassan, and Basharat Ali wrote the original draft. Basharat Ali, Zaid Ulhassan and Weijun Zhou reviewed and edited the manuscript.
ACKNOWLEDGMENTS
This work was supported by the Science and Technology Department of Zhejiang Province (2023C02002-3), the National Natural Science Foundation of China (32172429, 32372566), Collaborative Innovation Center for Modern Crop Production co-sponsored by Province and Ministry (CIC-MCP), and the Agriculture and Rural Affairs Department of Zhejiang Province (2022SNJF010). We acknowledge the Zhejiang Key Laboratory of Crop Germplasm, and Rui Sun and Weizhen Hu from Agricultural Experiment Station of Zhejiang University for their assistance.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.




