Iron status and root cell morphology of Arabidopsis thaliana as modified by a bacterial ferri-siderophore
Abstract
We previously provided evidence for the contribution of pyoverdine to the iron nutrition of Arabidopsis. In the present article, we further analyze the mechanisms and physiology of the adaptations underlying plant iron nutrition through Fe(III)-pyoverdine (Fe(III)-pvd). An integrated approach combining microscopy and nanoscale secondary ion mass spectrometry (NanoSIMS) on plant samples was adopted to localize pyoverdine in planta and assess the impact of this siderophore on the plant iron status and root cellular morphology. The results support a possible plant uptake mechanism of the Fe(III)-pvd complex by epidermal root cells via a non-reductive process associated with the presence of more vesicles. Pyoverdine was transported to the central cylinder via the symplastic and/or trans-cellular pathway(s), suggesting a possible root-to-shoot translocation. All these processes led to enhanced plant iron nutrition, as previously shown. Overall, these findings suggest that bacterial siderophores contribute to plant iron uptake and homeostasis.
1 INTRODUCTION
Iron is an essential microelement for almost all living organisms. A significant proportion of their energy metabolism is used for Fe acquisition from the environment and its safe utilization inside the cell. Due to its redox properties, iron is a central cofactor for enzymatic reactions that involve electron transfer in essential metabolic pathways such as respiration or photosynthesis (Balk & Schaedler, 2014). However, being a redox-active element, iron can also be toxic. Free Fe(II) catalyzes the Fenton reaction and generates reactive oxygen species (ROS) that may cause irreparable damage to cellular components when present in excess (Mittler, 2017; Winterbourn, 1995). Therefore, the intracellular concentrations and forms of iron must be tightly regulated at the cellular level.
In soils, iron is largely present but mostly in forms non-available to plants and microorganisms, which have developed active strategies for iron uptake (Lemanceau et al., 2009; Robin et al., 2008). Two active strategies of root iron uptake have been described for a long time (Römheld & Marschner, 1986)—strategy I is used by dicots and non-graminaceous monocots. Acidification of the rhizosphere increases Fe(III) solubility, and Fe(III) is reduced to the more soluble Fe(II) prior to root cell uptake through a membrane transporter. Strategy II is used by Graminaceae. It is a chelation-based mechanism mediated by externalized phytosiderophores (PS) that chelate Fe(III) for further internalization of the corresponding complexes by root cells. Major progress has been achieved in the knowledge of the regulation mechanisms and key molecular components that mediate these uptake and internal transport strategies (Curie and Briat, 2003; Kobayashi & Nishizawa, 2012; Álvarez-Fernández et al., 2014; Liang, 2022).
However, it is now widely accepted that this binary paradigm is probably oversimplified (Li et al., 2023). The exclusive use of either strategy for iron uptake by plants has already been questioned by Marschner and Römheld (1994), who proposed the possible contribution of additional mechanisms based on the synthesis of (1) low-molecular-weight root exudates, such as phenolics, whose secretion is enhanced under iron-deficient conditions and (2) microbial siderophores. Since then, this proposal has been corroborated by several other studies: (1) incorporation of Fe(II) by rice using the strategy-I mechanism in addition to its regular Fe-PS (strategy II) (Ishimaru et al., 2006), (2) incorporation by a strategy-I plant (peanut) of Fe-PS from a strategy-II plant (maize) (Xiong et al., 2013) via a membrane transporter belonging to the Yellow Stripe-like (YSL) family of YS1 transporters of Fe-PS identified in maize (Curie et al., 2001; Curie et al., 2009). Thus, these reports indicate that both iron-uptake strategies I and II may occur in some plant species.
Moreover, enhanced secretion of phenolic compounds under iron-deficient conditions may also contribute to iron uptake in strategy-I plants via a chelation-based mechanism, as described in strategy II with phytosiderophores (Fourcroy et al., 2014; Jin et al., 2007; Lefèvre et al., 2018). Arabidopsis thaliana secretes coumarin-derived compounds under iron stress conditions (Fourcroy et al., 2014; Robe et al., 2021; Tsai et al., 2018), which chelate Fe(III) (Schmid et al., 2014; Tsai et al., 2018). This Fe(III)-chelating step may further be coupled with the strategy-I reduction mechanism for ultimate iron uptake by root cells (Fourcroy et al., 2016). In addition, phenolic compounds released by red clover roots are involved in the reutilization of apoplastic iron (e.g., iron adsorbed on cell walls) (Jin et al., 2007). This could represent a possible Fe source under iron stress conditions (Bienfait et al., 1985; Lei et al., 2014; Curie & Mari, 2017).
Additionally, many studies described the beneficial effects of siderophore-producing microorganisms and microbial siderophores (MS), including pyoverdines produced by fluorescent Pseudomonas spp., on plant iron nutrition (e.g. reviewed in Lurthy et al., 2021). Some of them even demonstrated that pyoverdine chelated to iron contributes to plant iron nutrition of both dicots (strategy I) and graminaceous monocots (strategy II) more efficiently than the synthetic ferric chelate (Fe(III)-EDTA) does (Jin et al., 2010; Shirley et al., 2011; Vansuyt et al., 2007). However, whether this enhanced iron nutrition relies on iron reduction-based uptake, ligand exchange between microbial and plant siderophores, and/or internalization of ferri-siderophore is still under debate. The stability constant (pH 7) of the complex formed by Fe(III) and the MS pyoverdine (pvd), (1024) (Meyer & Abdallah, 1978), is significantly higher than that of Fe-PS (1018) (Raymond et al., 1984). This suggests that dissociation and reduction-based uptake, or ligand exchange between Fe(III)-pyoverdine (Fe(III)-pvd) and Fe-PS is unlikely to account for the enhanced iron nutrition by Fe-MS. This was confirmed by measurements of 15N-labeled Fe(III)-pvd in planta and by immunodetection using anti-pvd antibodies (Vansuyt et al., 2007). These authors further demonstrated that the membrane transporter of Fe(II) in strategy-I plants (IRT1, IRON-REGULATED TRANSPORTER-1) was not involved in iron uptake from Fe(III)-pvd suggesting that reduction-based uptake might not be implied in this process. Taken together, the methods used so far have demonstrated that (1) iron from Fe(III)-pvd can be used by plants, and (2) the pvd is internalized. However, a better understanding of pvd uptake, fate and transport in plants remains necessary to optimize the beneficial effects of rhizosphere microbiota on plant nutrition and develop environmentally friendly innovative cropping systems.
We applied an integrated approach combining microscopy and NanoSIMS to localize pvd in planta and assess the impact of this siderophore on the plant iron status and root cellular morphology in order to advance knowledge of processes implied in uptake and utilization of pvd by plants. The results open up new prospects in our understanding of the role of microbial siderophores in plant iron uptake and homeostasis.
2 MATERIALS AND METHODS
2.1 Pyoverdine production and purification
Pseudomonas fluorescens C7R12 – an efficient biocontrol agent against pathogenic Fusarium oxysporum – was isolated from a soil naturally suppressive to soil-borne disease (Lemanceau & Alabouvette, 1991; Eparvier et al., 1991). P. fluorescens C7R12 was grown in succinate medium (Meyer & Abdallah, 1978) at 25°C with shaking at 180 rpm for 72 h. Pyoverdine (pvd) was obtained from the supernatant of bacterial cultures and purified as described by Hartney et al. (2013). The production of pyoverdine was estimated by measuring the absorbance at 400 nm (ε = 19 000 M−1 cm−1 at pH 7; Meyer & Abdallah, 1978) on the supernatants of three independent cultures after 72 h. Pvd labelled with 15N isotope was obtained using 99% (15NH4)2SO4. Pvd or 15N-labelled pvd (pvd*) were mixed with a solution of inorganic FeCl3 at a molar ratio of 1:1. Fe(III)-pvd, Fe(III)-pvd* and Fe(III)-EDTA stock solutions were filter-sterilized, adjusted to pH 4.5 and stored at 4°C in the dark.
2.2 Plant material, growth conditions and sampling
Seeds of Arabidopsis thaliana ecotype Columbia 0 (Col-0) were sterilized for five min with a mixture of sodium hypochlorite (1.2%, v/v) and ethanol (50%, v/v), washed three times with sterile ethanol/water (96%, v/v), and dried in a flow chamber.
The sterilized seeds were sown under sterile conditions as follows: 25 seeds per vertical 12 × 12-cm square Petri dish sown on a gel made with Hoagland nutrient solution (pH 6.3) and 10 g l−1 of Sigma A1296 agar (Sigma-Aldrich). The nutrient solution contained 5 mM KNO3 and Ca(NO3)2, 2 mM MgSO4, 1 mM KH2PO4, 50 μM H3BO3, 5 μM MnSO4, 15 μM ZnSO4 7 H2O, 3 μM Na2MoO4 2 H2O, 2.5 μM KI(H2O)7 and CuSO4 5 H2O. The residual Fe concentration evaluated in triplicate by HR ICP-MS was 8.68 μM ±0.58. The plates were kept at 4°C in the dark for 2 days and then placed in a growth chamber under controlled conditions of temperature (21°C), light (16/8 h light/dark cycle; 300 μmol m−1 s−1) and relative humidity (70%) for 14 days.
- To analyse the impact of the iron source on the plant iron content, three replicates of 50 plantlets were treated with water, Fe(III)-pvd or Fe(III)-EDTA;
- To analyse ferric reductase activity, four replicates of five plantlets were treated with water, Fe(III)-pvd or Fe(III)-EDTA;
- For transmission electron microscopy (TEM) analyses, three replicates of 25 plantlets were treated with Fe(III)-pvd or Fe(III)-EDTA;
- For nanoscale secondary ion mass spectrometry (NanoSIMS) analyses, three replicates of 50 plantlets were treated with Fe(III)-pvd*;
- For immunoelectron microscopy (IEM) analyses, three replicates of 50 plantlets were treated with Fe(III)-pvd.
The plantlets were cultivated in a growth chamber for seven additional days after iron supplementation. The details of the experimental design and a block diagram of the analytical setup are presented in the supplementary materials (Figure S1).
2.3 Plant iron content
The plant iron content was determined in the roots and the shoots by spectrophotometry at 535 nm, using bathophenantrolin as a Fe(II) chelator, after mineralization of the samples as detailed in Trapet et al. (2016). Measurements were performed in triplicate. The whole plant iron content was determined by summing up the root and shoot iron contents.
2.4 Root ferric reductase activity
Plantlets were incubated under shaking (70 rpm) in the dark at room temperature for 30 min in 2 mL of a solution containing 0.1 mM Fe(III)-EDTA and 0.3 mM Ferrozine (Sigma-Aldrich). Ferric reductase activity was quantified by measuring the formation of purple-coloured Fe(II)-ferrozine complexes spectrophotometrically at 562 nm (Beckman-Coulter DU800) using a molar extinction coefficient of 28.6 mM−1 cm−1 (Gibbs, 1976) with 1 mL of the same assay solution used as a blank.
2.5 Transmission electron microscopy
Chemical fixation was performed on two-millimetre-long root sections sampled from 16-day-old A. thaliana grown in the presence of Fe(III)-pvd or Fe(III)-EDTA for 168 h. The samples were fixed with 2% glutaraldehyde and 3% paraformaldehyde in phosphate buffer saline (PBS) 0.1 M pH 7.2, followed by 1% osmium tetroxide in PBS at 4°C for 1 h. Then, the samples were dehydrated through graded baths of ethanol and propylene oxide and finally embedded in Epon (Spi-Chem) according to the standard procedure for conventional TEM (Luft, 1961). Ultrathin sections (90 nm) were cut with a diamond knife blade on a Reichert Ultracut E ultramicrotome (Leica). They were collected on 300-mesh copper-palladium grids, counterstained with 5% aqueous uranyl acetate (w/v) and 0.5% aqueous lead citrate (Reynolds, 1963) for conventional TEM, and examined with a transmission electron microscope (HITACHI H7500) operating at 80 kV and equipped with an AMT camera driven by AMT software (AMT).
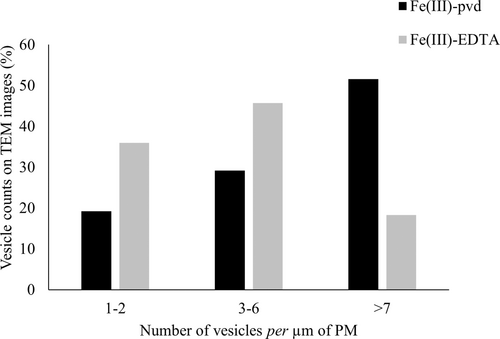
The vesicles present in between the plasma membranes (PM) and cell walls (CW) were counted on a cumulated length of 1200-μm of PM observed in 40 sections. Three classes were defined based on the number of vesicles per μm of PM (low: 1–2, medium: 3–6, high: >7).
2.6 Sample preparation for nanoscale secondary ion mass spectrometry (NanoSIMS) analysis
Cryofixation was performed on two-millimetre-long pieces of 16-day-old A. thaliana roots grown for 168 h in the presence of Fe(III)-pvd*. The samples were placed in a 3-mm-diameter aluminium specimen carrier with a cavity of 200 μm filled with hexadecene as a cryoprotectant and immediately frozen in a High-Pressure Machine (HPM 100, Leica). The samples were stored in liquid nitrogen and freeze-substituted with Spurr® resin (ultra-low viscosity embedding media kit, Polysciences Europe) using an automatic freeze substitution unit (AFS, Leica). The detailed procedures of freeze substitution and embedding are presented in supplementary material S2.
NanoSIMS analyses were carried out on ultrathin sections of 100–120 nm collected on a solid 7 × 7 mm silicon square. SIMS analysis images were obtained with a Nano SIMS-50™ ion microprobe (Cameca). Dynamic SIMS imaging was operated in scanning mode. The primary ion beam was generated from a caesium source (Cs+) for negative (12C14N−, 12C15N− and 31P−) secondary ion analyses. Images were processed using ImageJ software (https://imagej.nih.gov/ij/; Guerquin-Kern et al., 2005).
2.7 Immunoelectron microscopy (IEM)
Cryofixation was performed on the roots of 16-day-old plantlets cultivated in the presence of Fe(III)-pvd for 168 h. Briefly, the samples were immediately high-pressure frozen with a Leica HPM 100 and freeze-substituted with Lowicryl® HM20 resin (Biovalley) in an AFS. Ultrathin sections (90 nm) were cut as described above and collected on Formvar–carbon-coated 300-mesh nickel grids. Solutions of 1/800-diluted pvd antibody (Vansuyt et al., 2007) and of 1/25 diluted anti-rabbit secondary antibody conjugated with 15-nm colloidal gold were successively applied prior to TEM observations carried out using an electron microscope (HITACHI H7500) as described above. The detailed procedures for freeze substitution, embedding and IEM are presented in supplementary material S3. The nanogold particles were counted on thirty-one 15 μm2-sized IEM images and ascribed to the epidermis, cortex or central cylinder, depending on their localization. The results were subjected to one-way ANOVA followed by pair-wise comparisons with Fisher's least significant test.
2.8 Statistical analyses
Pearson's chi-square test and one-way ANOVA, followed by pair-wise comparisons with Fisher's least significant test, were performed using XLSTAT (XLSTAT 19.02, Addinsoft).
3 RESULTS
3.1 Effect of Fe(III)-pvd and Fe(III)-EDTA on the plant iron content and root ferric reductase activity
The iron concentration in the roots and whole plants supplemented with Fe(III)-pvd was significantly higher than in the no-Fe-added control (Table 1), while no significant difference was observed in the shoots of the plants supplemented or not with iron (Table 1). The increased iron concentration in the roots of the Fe(III)-pvd-supplemented plants was not associated with a significant increase of the root ferric reductase activity (p = 0.091): 29.7 ± 6.1 μM Fe(II) h−1 g−1 of root fresh weight in the control plants, 26.2 ± 6.1 μM Fe(II) h−1 g−1 in the with Fe(III)-pvd-supplemented plants, and 19.9 ± 4.2 μM Fe(II) h−1 g−1 in the Fe(III)-EDTA-supplemented plants.
| Roots | Shoots | Whole plant | |
|---|---|---|---|
| Fe(III)-pvd | 98.7 ± 13.8a | 76.9 ± 2.0a | 80.8 ± 3.9a |
| Fe(III)-EDTA | 85.4 ± 15.6ab | 77.4 ± 8.7a | 80.9 ± 4.7a |
| No-Fe-added | 73.5 ± 11.7b | 64.1 ± 8.7a | 68.4 ± 1.8b |
3.2 Effect of Fe(III)-pvd and Fe(III)-EDTA on the root ultrastructure and the presence of vesicles
At low magnification (tissue level), the TEM images of root cell sections from plantlets supplemented with Fe(III)-pvd and Fe(III)-EDTA showed the usual shape with large central nuclei, many small vacuoles, and a fine unbroken plasma membrane with adherence to the cell wall (Figure 1A and B, respectively). The cell wall thickness was uniform. The cells showed a classic pattern of cell division or elongation, with dense cytoplasm and numerous active organelles (mitochondria, Golgi complex, endoplasmic reticulum) regardless of the treatment. At higher magnification (cellular level), the TEM images from plants supplemented with Fe(III)-pvd showed numerous vesicles located in the cell wall, the intercellular spaces (Figure 1C, E), the cytoplasm (Figure 1E), and closely associated with the plasma membrane (Figure 1F). Vesicles were also detected in plants supplemented with Fe(III)-EDTA (Figure 1D), but at a significantly lower frequency (Figure 2).

3.3 Localization of pyoverdine in plants supplemented with Fe(III)-pvd
NanoSIMS and IEM were applied to monitor the fate of pvd and Fe(III)-pvd in planta and to localize pvd from Fe(III)-pvd chelate within the root cells of A. thaliana.
3.3.1 Tracing 15N-labeled pvd by NanoSIMS analysis
15N from 15N-labeled pvd (hereafter pvd*) was detected in all three root zones (mature tissue, meristem tissue and root cap) of 16-day-old plantlets supplemented with Fe(III)-pvd* (Figure 3A). NanoSIMS mapping and the corresponding amounts of isotope in various areas within these three root zones are showed in Figure 3B and C. In all these zones, the highest 15N enrichments (>50%) were observed in areas predominantly composed of cytoplasm, as compared to the background 15N/14N ratio measured in the embedding resin on the sides of the preparations. No significant 15N enrichment was recorded in the vacuoles (Figure 3B, C).
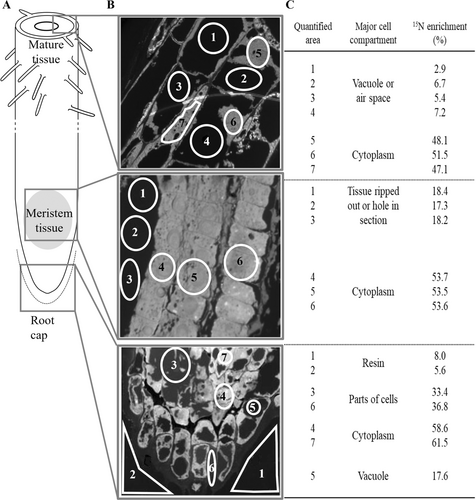
3.3.2 Tracing of pvd by IEM
Immunogold labelling of pvd and TEM analyses were carried out on the three root zones (mature tissue, meristem tissue and root cap as defined in Figure 3A) of 16-day-old plantlets supplemented with Fe(III)-pvd. The results showed the presence of pvd in the mature tissue zone, more specifically in the conductive vessels and epidermis (Figure 4), but not in the meristem tissue and root cap zones (data not shown).
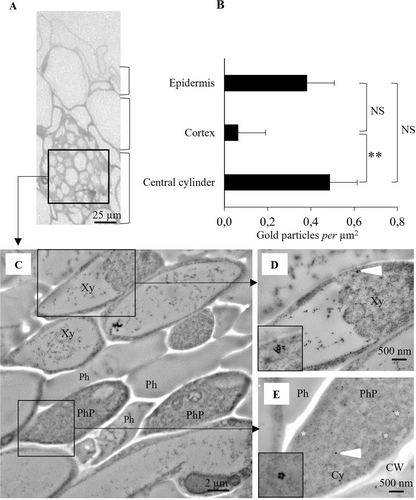
The gold particles staining pvd per μm2 of root area in the epidermis, cortex and central cylinder were counted on IEM images (Figure 4B). Gold particles were detected in all three types of root tissues. Their densities were higher in the central cylinder and epidermal cells than in the cortex cells. The most intense labelling in the central cylinder was observed in the xylem and phloem tissues (Figure 4B -E).
4 DISCUSSION
After a previous report on the contribution of pvd to iron nutrition of Arabidopsis (Vansuyt et al., 2007), the present work localizes pyoverdine in planta and describes its impact on the plant iron status and root cellular morphology by combining microscopy observations and NanoSIMS to provide insights into the physiological mechanisms underlying Fe(III)-pvd-mediated plant iron nutrition.
We evidenced the occurrence of pvd in plant roots and localized it for the first time by NanoSIMS based on 15N detection and by IEM analyses. In agreement with Vansuyt et al. (2007) and Trapet et al. (2016), Fe(III)-pvd supplementation promoted both iron and pvd uptake by plant roots, as indicated by measurements of the plant iron content and pvd labelling. In addition, pvd was found in the cytoplasm of root epidermal cells of A. thaliana. On the basis of pvd detection in the central cylinder zone (i.e., beyond the Casparian strip) by immunogold labelling, we hypothesize that the Fe(III)-pvd internalization is mediated by the symplastic pathway or by a coupled trans-cellular pathway involving both the apoplastic and symplastic pathways (Barberon & Geldner, 2014). The description made here by immunogold labelling of the presence of pvd in xylem and phloem tissues specifies better its localisation in shoots which was previously reported by Vansuyt et al. (2007) and Trapet et al. (2016) and indicates that pvd was not metabolized. Pvd presence in vascular tissues is in favour of its possible contribution to long-distance iron transport and iron homeostasis in planta (Curie et al., 2009; Schuler et al., 2012; Álvarez-Fernández et al., 2014; Kobayashi et al., 2019).
Fe(III)-pvd contributed to plant iron uptake and homeostasis even when other iron sources were available, as indicated by the comparison with non-iron-deficient plants: the no-Fe-added control plants were still able to take up iron traces from the medium, as indicated by their iron concentration and ferric-reductase activity. This observation supports the increasing acknowledgement that discriminating the two strategies (I and II) of iron uptake is probably an oversimplification (Li et al., 2023; Robe et al., 2021). In A. thaliana, as in other dicots and non-graminaceous monocots, strategy I – which involves an extracellular reduction process of Fe(III) to Fe(II) followed by Fe(II) transport across the cell membrane of root epidermal cells – is an important pathway for plant iron uptake. Vansuyt et al. (2007) observed iron acquisition from Fe(III)-pvd and the presence of pvd inside the roots of an A. thaliana mutant with an impaired Fe(II) membrane transporter (IRT1). This led to the hypothesis of the existence of an additional pathway for iron uptake based on the internalization of the Fe(III)-pvd chelate. Our results strengthen this hypothesis by providing circumstantial evidence of the internalization of the microbial Fe(III)-pvd chelate through a non-reductive process. This hypothesis does not contradict the one proposed by Yehuda et al. (1996), Nagata et al. (2013) and Radzki et al. (2013), according to which iron from MS could be internalized via the strategy I pathway. MS are highly diverse, and their highly variable stability (Guerinot & Yi, 1994) is totally dependent on the rhizosphere conditions (Robin et al., 2008). The efficiency of plant iron promotion differs depending on pyoverdines (Lurthy et al., 2020). Therefore, different mechanisms could be involved in MS-mediated plant iron acquisition. This is supported by Trapet et al. (2016), who reported an increased level of expression of the root surface ferric-reductase (FRO2) and of the Fe(II) membrane transporter (IRT1) under iron-deficient conditions in the presence of pvd. They further evidence that the induction of the strategy-I pathway and the internalization of microbial chelates could occur together.
We observed significantly more vesicles in the epidermal cells of the mature zones of the roots of the plants supplied with Fe(III)-pvd than in those of the plants supplied with Fe(III)-EDTA. This is in agreement with Chao et al. (2021), who described exocytosis synaptic-like images associated with nicotianamine iron chelation in A. thaliana. However, more vesicles could also result from endocytosis-mediated iron uptake by root cells. Kazamia et al. (2018) evidenced direct intracellular uptake of microbial ferri-siderophore by diatoms – unicellular photosynthetic algae –, the non-reductive uptake of ferri-siderophores being mediated by endocytosis through the formation of vesicles.
In the present study, the final concentration of pvd in the plant growth medium (1 μM) is within the range of those most commonly used when testing the influence of microbial siderophores on plant iron nutrition (1 to 10 μM; e.g., reviewed in Lurthy et al., 2021). However, one may wonder how these concentrations relate to those found in soil. Bacteria from the family Pseudomonadaceae are characteristic of the rhizosphere environment (Bulgarelli et al., 2012; Hacquard et al., 2015; Banerjee et al., 2018) and expression of siderophore genes is also a key feature of this environment (Bulgarelli et al., 2015). Pyoverdine synthesis was evidenced in the rhizosphere of cucumber (Loper & Henkels, 1999), and estimated to range between 500 and 800 pmol g of root−1 in the rhizosphere of white lupine and barley (Marschner & Crowley, 1997). High concentrations of siderophores, up to 300 μM, can be produced by pseudomonads cultured in iron stress conditions (Meyer et al., 1987; Soares, 2022). In the present work, the concentration of pvd-C7R12 reached 40 ± 1 μM. These values cannot be directly extrapolated to the rhizosphere, where iron, in its different forms, and many different microbial strains interact in a complex environment. However, they reveal a high potential for the synthesis of these compounds. In soil, microbial siderophores were detected at concentrations ranging from 0 to 32 pmol g−1 dry soil (Essen et al., 2006; Ahmed & Holmstrom, 2014; Boiteau et al., 2018), which corresponds to 0 to 0.32 μM based on 10% soil moisture (as converted by Crowley et al., 1991); the highest value having been observed in a grassland rhizosphere (Boiteau et al., 2018). In the soil environment, siderophores may be subjected to many processes of degradation and immobilization, which can lead to a significant underestimate (Harrington et al., 2015). According to Rai et al. (2020) extraction methods should be optimized for each siderophore type. Thanks to the optimization of extractions, these authors reported for the first time the presence in the soil of a given siderophore and estimated its concentration at 461 pmol g−1, which corresponds to 4.6 μM based on 10% soil moisture. Based on the current knowledge summarized above, one can consider that the present work and studies carried out at concentrations commonly used for siderophore supplementation stay close to concentrations expected to be found in the rhizosphere and suggest that microorganisms could impact plant nutrition in natural conditions. Plants might have adapted mechanisms to benefit from microbial siderophores rather than be challenged, which would make this iron uptake pathway an important feature in the functioning of the plant holobiont (a supra-organism made of the plant and its microbiota: Vandenkoornhuyse et al., 2015; Lyu et al., 2021). The long-standing co-evolution of plants and rhizosphere bacteria (encompassing pseudomonads) has led to reciprocal beneficial interactions (Lemanceau et al., 2017; Lemanceau et al., 2018). Additional research should shed light on the fate and impact of this form of iron supply in relation to pH and Eh (redox potential) in different compartments of the same plant, which can vary (Grillet et al., 2014; Krohling et al., 2016).
Taken together, our results based on an integrated approach combining microscopy observations and NanoSIMS show that Fe(III)-pvd – the MS of the model strain P. fluorescens C7R12 complexed to iron(III) – is taken up by epidermal root cells via a non-reductive process associated with the presence of more vesicles (hence a possible endocytosis pathway) and promotes plant iron nutrition. Our results also bring circumstantial evidence supporting pvd transport via the symplastic or trans-cellular pathways to the central cylinder and its further root-to-shoot translocation in A. thaliana.
Overall, these findings support the role of iron chelates of microbial origin in plant iron uptake and homeostasis.
AUTHOR CONTRIBUTIONS
LA, PL and SM designed the research and wrote the manuscript with the contribution of JLGK. BP critically revised the manuscript. LA, TL, JL, CA, PML, TDW, JLGK, JPL, performed the experimental work. LA, SM, PL, TDW, JLGK, JL, CA, and BP participated in the experimental results analysis. All authors contributed to the article and approved the submitted version.
ACKNOWLEDGEMENTS
This work was supported by the ‘Conseil Régional de Bourgogne-Franche-Comté’, by the POSiTiF project of the Plant2Pro Carnot Institute and by the AMETHYSTES project of the ANR-French National Research Agency. We are grateful to Gérard Vansuyt and Eric Bernaud for their support during the initial phase of the project. The authors acknowledge the DImaCell Imaging Facility for the use of transmission electron microscopy, sample preparation and for technical assistance. We acknowledge also the CurieCoreTech, the technological platforms at the Institut Curie, for the use of the Curie-NanoSIMS instrument. Finally, we are grateful to the CNRS GDR Pseudomonas for promoting scientific interactions and to Annie Buchwalter for editing the English text.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as all new created data is already contained within this article or in the corresponding supplementary material.




