Comparative analysis of defensive secondary metabolites in wild teosinte and cultivated maize under flooding and herbivory stress
Abstract
Climate change is driving an alarming increase in the frequency and intensity of abiotic and biotic stress factors, negatively impacting plant development and agricultural productivity. To survive, plants respond by inducing changes in below and aboveground metabolism with concomitant alterations in defensive secondary metabolites. While plant responses to the isolated stresses of flooding and insect herbivory have been extensively studied, much less is known about their response in combination. Wild relatives of cultivated plants with robust stress tolerance traits provide an excellent system for comparing how diverse plant species respond to combinatorial stress, and provide insight into potential germplasms for stress-tolerant hybrids. In this study, we compared the below and aboveground changes in the secondary metabolites of maize (Zea mays) and a flood-tolerant wild relative Nicaraguan teosinte (Zea nicaraguensis) in response to flooding, insect herbivory, and their combination. Root tissue was analyzed for changes in belowground metabolism. Leaf total phenolic content and headspace volatile organic compound emission were analyzed for changes in aboveground secondary metabolism. Results revealed significant differences in the root metabolome profiles of teosinte and maize. Notably, the accumulation of the flavonoids apigenin, naringenin, and luteolin during flooding and herbivory differentiated teosinte from maize. Aboveground, terpenes, including trans-α-bergamotene and (E)-4,8-dimethylnona-1,3,7-triene, shaped compositional differences in their volatile profiles between flooding, herbivory, and their combination. Taken together, these results suggest teosinte may be more tolerant than maize due to dynamic metabolic changes during flooding and herbivory that help relieve stress and influence plant–insect interactions.
1 INTRODUCTION
As a product of climate change, extremes in water availability have become more severe and unpredictable (Tabari, 2020; Hirabayashi et al., 2021). Record-breaking flooding events are among the most damaging to crop production in North America, ranked second only after drought (Bailey-Serres et al., 2012; Li et al., 2018). For instance, in 2019, major crop-producing U.S. states recorded precipitation totals ranking in the top 10 of the last 127 years, which led to severe production losses totaling US$1.3 billion (NCEI, 2021; Bundy et al., 2022). Globally, an estimated 10-12% of the total cultivated land area is affected by flooding or severe soil drainage constraints (Shabala, 2011; Kaur et al., 2020). Despite the increasing severity of flooding, our understanding of how it affects plants in the context of plant–insect interactions is limited.
The term “flooding” is used to describe when all (submergence) or part of a plant (waterlogging) is underwater (Sasidharan et al., 2017). In either case, flooding inundates the soil with water and displaces oxygen, inducing hypoxic conditions for plant roots (Kozlowski, 1984; Grinieva, 1991; Kaur et al., 2020). These conditions are immediately linked to reductions in plant growth and photosynthesis, as well as dynamic changes in root metabolism associated with survival (Voesenek & Bailey-Serres, 2015; Nakamura & Noguchi, 2020; Sasidharan et al., 2021).
Belowground flooding-induced metabolic changes have been evaluated in a wide range of plant species, including economically important crops such as maize (Zea mays L.) (Subbaiah & Sachs, 2003; Ren et al., 2014; Sasidharan et al., 2021). For instance, maize roots experiencing hypoxia accumulate metabolites associated with oxidative stress relief, such as the flavonoids naringenin and apigenin (Mekawy et al., 2018; Yildiztugay et al., 2020). In addition, metabolites with hormonal functions, including jasmonic acid and ethylene, may also accumulate during flooding to coordinate root developmental responses (Raya-González et al., 2012; Cai et al., 2014; Borrego & Kolomiets, 2016; Mekawy et al., 2018; Yildiztugay et al., 2020; Xu et al., 2020). To evaluate changes in the metabolic profile of plant tissues experiencing environmental stress, untargeted LC-MS-based metabolomics remains a powerful tool (Khan et al., 2019; Christensen et al., 2021; Kumar et al., 2021; Singh et al., 2023).
Complex root-to-shoot signaling brings about the potential for belowground flooding-induced changes to alter aboveground defensive secondary metabolites, including phenolic compounds and volatile organic compounds (VOCs) that mitigate cellular damage and mediate ecological interactions (DeLucia et al., 2001; Jackson, 2002; van Geem et al., 2013; Lothier et al., 2020).
Phenolic compounds are a diverse group of secondary metabolites that contain one or more hydroxyl groups attached to an aromatic ring (Ainsworth and Gillespie, 2007; Kumar et al., 2020). As such, phenolic compounds have high antioxidant activities (Bors et al., 1990; Grace, 2005; Foyer, 2018). During flooding, declines in photosynthesis and respiration lead to the accumulation of harmful reactive oxygen species that induce oxidative stress in plant tissues (Yan et al., 1996; Yordanova & Popova, 2007; Lukić et al., 2021). There is evidence that phenolic compounds help alleviate oxidative stress by scavenging reactive oxygen molecules, such as H2O2, that accumulate during flooding (Grace & Logan, 2000; Yordanova & Popova, 2007; Lukić et al., 2021). For instance, Lukić et al. (2021) reported that an increase in H2O2 concentration in flooded maize was accompanied by an increase in phenolic content to cope with oxidative damage. Phenolic compounds also serve ecological functions by acting as feeding deterrents to herbivores (Singh et al., 2021). For example, Mao et al. (2007) showed a transgenic maize that expressed increased phenolic content, and decreased leaf consumption and stalk tunneling of Ostrinia nubilalis (Lepidoptera: Crambidae) larvae.
Complementing phenolic compounds within the secondary metabolite defense arsenal of plants are volatile organic compounds (VOCs), which are particularly important in mediating ecological interactions between plants and insects (Loreto & Schnitzler, 2010; Dudareva et al., 2013; Possell & Loreto, 2013). VOCs form a diverse blend emitted in response to abiotic stress, such as drought (Jin et al., 2021), heat (Werner et al., 2020), or flooding (Ngumbi & Ugarte, 2021); and biotic stress, such as insect herbivory (Takabayashi, 2022). Herbivore-induced VOCs have been studied extensively in maize, where emitted compounds can be utilized by natural enemies to locate their herbivore host with high specificity (Turlings et al., 1998; Schnee et al., 2006; War et al., 2011). For example, Turlings et al. (1990) demonstrated that maize plants experiencing herbivory by Spodoptera exigua (Lepidoptera: Noctuidae) emitted a blend of terpenes, including trans-α-bergamotene and (E)-4,8-dimethylnona-1,3,7-triene, which recruited the parasitic wasp Cotesia marginiventris that oviposited into the larvae. In general, stress-induced VOCs can differ in quantity and quality depending on the stress factor, plant identity, and environmental conditions (Gouinguené & Turlings, 2002; Dicke & Baldwin, 2010; Degen et al., 2012). For instance, Degen et al. (2012) demonstrated that six maize inbred lines experiencing herbivory by Spodoptera frugiperda (Lepidoptera: Noctuidae) emitted distinctly different blends of VOCs with a near 15-fold difference in total emission. Overall, much of the information available on stress-induced secondary metabolites is from studies investigating isolated stress factors; yet, under field conditions, plants can experience stress sequentially or simultaneously.
Although limited, the studies available suggest that plant responses to combinatorial abiotic and/or biotic stresses are unique, and this uniqueness is reflected in changes in secondary metabolites (Holopainen & Gershenzon, 2010; Rasmussen et al., 2013 Suzuki et al., 2014; Pandey et al., 2015; Nguyen et al., 2016). For example, Ngumbi and Ugarte (2021) reported that the combined stress of flooding and insect herbivory on two maize hybrids significantly increased their total volatile emissions compared to each stress applied in isolation. In addition, Obata et al. (2015) observed that the combined stress of heat and drought on maize significantly changed the accumulation of leaf metabolites, with fewer significant effects when each stress was applied separately. Combined stress factors may also influence secondary metabolism, such that one factor dominates the overall response (Holopainen & Gershenzon, 2010; Suzuki et al., 2014; Nguyen et al., 2016). For instance, in combination with ozone and salinity, heat had a dominant effect on the global metabolite patterns of Arabidopsis thaliana (Sewelam et al., 2020). Together, these studies demonstrate unique metabolite responses of plants to combinatorial stress, which may not be extrapolated from their response to each stress independently. However, there is still a need for empirical evidence from diverse plant species, including wild relatives of modern crops.
Wild relatives of cultivated plants are often revered as treasure troves of stress-tolerance traits that may strengthen modern germplasms with broad-spectrum tolerance and improve food security in an unpredictable world (Ford-Lloyd et al., 2011; Mammadov et al., 2018; Razzaq et al., 2021; Sahoo et al., 2021; Cockel et al., 2022; Hossain et al., 2022; Toulotte et al., 2022). The wild relatives of modern maize (Zea mays L.) within the genus Zea are collectively termed the teosintes (Piperno et al., 2009; Xu et al., 2022). Of these, Nicaraguan teosinte (Zea nicaraguensis Iltis and Benz) is noteworthy because it is considered the most flood-tolerant species in the genus (Bird, 2000; Iltis & Benz, 2000). In particular, this teosinte has traits for constitutive aerenchyma formation and oxygen loss barriers, which are enhanced compared to maize (Abiko et al., 2012; Mano & Omori, 2013, 2015). These root structures confer flood-tolerance by improving the internal movement of oxygen and restricting oxygen leakage, which helps maintain aerobic metabolism and root health in flooded soil (Watanabe et al., 2017; Gong et al., 2019). While the morphological traits that make this teosinte flood-tolerant are well studied, how its belowground root metabolism or aboveground defensive secondary metabolism responds to flooding and herbivory stress, and if these responses also confer tolerance, is unknown.
Accordingly, in this study, we compared the effects of flooding, herbivory, and their combination on the belowground root metabolites and aboveground defense-related metabolites (phenolic and volatile organic compounds) of teosinte (Zea nicaraguensis) and maize (Zea mays). For herbivory, the fall armyworm Spodoptera frugiperda was chosen because it shares a long evolutionary history with both plant species and is responsible for over US$300 million in damages per annum to maize production in the United States (de Lange et al., 2018; Overton et al., 2021). The objective of this study was to elucidate how the defensive secondary metabolisms of teosinte and maize respond to flooding and herbivory stress and highlight differences in metabolic content.
2 MATERIALS AND METHODS
2.1 Plant material and growth conditions
Teosinte and maize seeds were obtained from the USDA North Central Regional Plant Introduction Station (Ames, IA, USA). Zea nicaraguensis accession Iltis 30919 was selected for its tolerance to flood stress and its use as germplasm for breeding flood-tolerant maize (Mano & Omori, 2013, 2015; Watanabe et al., 2017; Gong et al., 2019). Zea mays inbred accession B73 was selected for its prevalence as a model organism and its genetic stability (Jia et al., 2016; Lu et al., 2018; Woodhouse et al., 2021).
Teosinte and maize seeds were sown in individual pots (12 cm x 14 cm dia.) filled with field-collected and steamed-sterilized topsoil (Drummer silty clay loam) obtained from the University of Illinois Urbana-Champaign Plant Care Facility (USA). This soil was mixed with torpedo sand in a ratio of 80:20 (soil:sand) to increase drainage. Plants were grown in greenhouse conditions with 25 ± 5°C, 50 ± 5% relative humidity, and 16L:8D photoperiod. Fertilizer (20–20–20 NPK) was applied biweekly until the experimental treatments were applied.
2.2 Experimental treatments
Flooding (waterlogging) was imposed by placing individual 14-day-old plants in larger one-gallon plastic containers (16.5 cm x 21.5 cm dia; Consolidated Plastics), filled with tap water 5 cm above the soil surface, and maintained until the end of the experiment. Plants in no flood treatments were watered daily to maintain field capacity.
Insect herbivory was imposed seven days after flooding using third instar Spodoptera frugiperda J.E. Smith (Lepidoptera: Noctuidae) larvae. Preliminary experiments showed third instar S. frugiperda acclimate and feed within 12 to 18 hours after placement on plants. Larvae were purchased from Benzon Research, placed individually in sterilized nylon mesh bags (11.5 cm x 9 cm), and tied around the end of the last fully expanded leaf of each plant receiving herbivory treatment. The mesh bags were moved daily down the leaves to provide sufficient food supply and ensure feeding until the end of volatile collection. Herbivore feeding was maintained for 12 hours to initiate metabolic response without negatively impacting plant biomass.
2.3 Belowground defensive secondary metabolite analysis
2.3.1 Root tissue sampling
To assess the influence of flooding, herbivory, and their combination on belowground metabolites, the root systems of teosinte and maize (n = 4 each) from each treatment were removed from their pots, carefully rinsed under tap water to remove soil, and rinsed with deionized water for additional cleaning. Immediately after cleaning, 1 g of tissue was collectively sampled from brace roots, crown roots, seminal roots, and primary roots of each plant and placed in 2-mL tubes. Any necrotic tissue in flooded treatments was avoided. Tubes with root tissue were immediately snap-frozen in liquid nitrogen and stored at −80°C until the day of analysis.
2.3.2 Root metabolite extraction and liquid chromatography-mass spectrometry analysis
Root tissue was extracted and analyzed by the Metabolomics Unit of the Roy J. Carver Biotechnology Center (University of Illinois Urbana-Champaign, Urbana, IL, USA), following the protocol of Elolimy et al. (2019). In summary, 100 mg of root tissue was homogenized with a bead mill (Fisherbrand Bead Mill 4), extracted with 1 mL of acetonitrile:isopropanol:water (3:3:2 v/v) solution, spiked with chlorophenylalanine as the internal standard, and centrifuged at 18,000 rpm (39,846 g) for 10 min. Then, 100 μL of extract was auto-injected into a Dionex UltiMate 3000 series HPLC system (Thermo Fisher Scientific Inc.) equipped with a Phenomenex Kinetex C18 column (4.6 x 100 mm, 2.6 μm) with mobile phase A (water with 0.1% formic acid) and mobile phase B (acetonitrile with 0.1% formic acid) at a flow rate of 0.25 mL/min. The linear gradient was programmed for 100% phase A at 0-3 min, 0% A at 20-30 min, and 100% A at 31-36 min. Mass spectra were analyzed with a Q Exactive Plus Hybrid Quadrupole-Orbitrap MS system (Thermo Fisher Scientific Inc.) under positive (3.5 kV) and negative electrospray ionization mode (−2.5 kV), both with capillary and auxiliary gas heater temperatures set to 250°C and 415°C, respectively. [M-H]-, [2M-H]- and [M+H]+, [2M+H]+, [M + NH4] + , [M + Na] + were included in the adduct ion settings for negative and positive mode, respectively. The sheath, auxiliary, and sweep gas flow rates were set to 45, 11, and 2, respectively, for both modes. Scans were obtained in the mass spectrum range of 67 to 1000 m/z with a resolution of 70,000.
Identification of peaks and chromatographic alignment was performed using Thermo Compound Discoverer software version 2.1 SP1 (Thermo Fisher Scientific). The workflow was untargeted metabolomics using online databases. Mass spectra were selected using a precursor mass between 65 and 5000 Da, a maximum retention time shift of 1 min, and a mass tolerance of 5 ppm. Unknown compounds were detected using a minimum peak intensity of 1e6, an intensity tolerance of 30%, and a mass tolerance of 5 ppm. To identify compounds, the mzLogic algorithm was used with the mzCloud (ddMS2) and ChemSpider libraries to rank results. Compounds were annotated by m/z and MS/MS spectra using a match factor threshold of 75. Additional manual inspection of data was performed to avoid misidentification of compounds during database matching. Those compounds not confidently identified are labelled as unknown. Data were normalized to the internal standard and sample weight.
2.4 Aboveground defensive secondary metabolite analysis
2.4.1 Leaf tissue sampling
To assess the stress-induced production of phenolic compounds during flooding, herbivory, and their combination, fresh leaf tissue was sampled from teosinte and maize (n = 8 each) from each treatment nine days after flooding was imposed and 48 hours after larvae were introduced. Using a 7-mm cork borer, leaf tissue was cut from the last fully expanded leaf of each plant and from areas that displayed evidence of larval feeding (in herbivory treatments). Leaf tissue was immediately placed in 2 mL tubes, snap-frozen in liquid nitrogen, and stored at −80°C until the day of analysis.
2.4.2 Determination of total phenolic content
The total non-structural phenolic content of teosinte and maize leaf tissue was determined colorimetrically using Folin-Ciocalteu reagent, as described in Ainsworth and Gillespie (2007). In summary, 20 mg of snap-frozen leaf tissue from teosinte and maize (n = 8 each) from each treatment was homogenized with a bead mill (TissueLyser, Qiagen Sciences Inc.) and extracted with 95% methanol in the dark for 48 h. Samples were centrifuged at 13,000 g for 5 min and 100 μL of supernatant was collected in fresh tubes. Then, 200 μL of 10% Folin-Cioclateu reagent and 800 μL of 700 mM Na2CO3 were added, in order, to the sample supernatant and left in the dark to incubate at room temperature for 2 h. After incubation, 200 μL of each sample was transferred to a clear microplate and the absorbance of each well was read with a microplate reader (Synergy HT Multi-Detection, BioTek Instruments Inc.) at 765 nm. Total non-structural phenolic content was calculated using the linear regression equation between gallic acid standards (3.91–250 μM) and the methanol blank-corrected absorbance of each well. Total phenolic content is expressed as mg of gallic acid equivalents per g of fresh weight.
2.4.3 Volatile organic compound collection and gas chromatography-mass spectrometry analysis
Volatile organic compound emissions were determined by sampling the headspaces of teosinte and maize (n = 6 each) from each treatment starting nine days after flooding and 48 hours after larvae were introduced, as described in Ngumbi and Ugarte (2021). In summary, the plant headspace was enclosed in an odor-blocking oven bag (Arcadia Intl. Inc.) for one hour to allow for the concentration of volatile compounds. Preliminary experiments showed these bags are not strong volatile emitters and thus do not contaminate the experimental headspace. Volatile collection was performed by inserting a solid-phase micro-extraction (SPME) fiber (polydimethylsiloxane/divinylbenzene (PDMS/DVB), 65 μm) (Supelco Inc.) into the bag and exposing the fiber to the plant headspace for 60 minutes. After collection, the SPME fiber was manually inserted into the injection port of an Agilent 7890B GC system in splitless mode, equipped with a fused silica DB-5ms phase (30 m x 0.250 mm x 0.25 μm) column, and interfaced to an Agilent 5977A MSD with helium carrier gas (Agilent Technologies Inc.). The injection chamber and transfer lines were set to 200°C, and the column was programed from 40°C/2 min, increasing 5°C/min to 200°C for a 40-minute run time. As this system had a single injection port (for one SPME fiber), volatile collection and analysis were performed individually for each plant and thus required 7 days to complete.
Identification of peaks was performed using the Wiley Registry 12th Edition/NIST 2020 MS library (Wiley Science Solutions; National Institute of Standards and Technology) and the Biologically and Environmentally Important Organic Compounds: GC-MS Library (Isidorov) (Wiley Science Solutions).
2.5 Determination of plant growth parameters
After volatile collection, 30-day-old teosinte and maize (n = 8 each) from each treatment were destructively harvested by removing the entire root system from their pots. Soil was rinsed from the root system and the below and aboveground biomass was separated. Root and shoot lengths were measured from their base to their fully extended length using a ruler. Root and shoot fresh weights were measured using a precision balance (Pioneer PX, OHAUS Scale Inc.). Dry weights were measured using the same scale after drying the plants in individual paper bags at 65°C for 3 days.
2.6 Statistical analysis
Statistical analysis and data normalization for the root metabolome data were performed using MetaboAnalyst 5.0 (Pang et al., 2022). Peak area data were imported to MetaboAnalyst's statistical analysis [metadata table] module. Identified peaks detected in the negative and positive ion modes were joined into a combined dataset for analysis. Data were filtered by interquartile range (IQR) and normalized by sum, log10 transformed, and auto-scaled (Figure S1) to reduce any problematic variance and improve downstream analysis (Hackstadt & Hess, 2009; Khan et al., 2019; Pang et al., 2022). To assess the influence of Zea species (Z. nicaraguensis or Z. mays), flooding, and herbivory on the accumulation of metabolites, univariate two-way ANOVAs were generated, using false discovery rate (FDR) for testing correction and adjusted P-values. To assess the compositional dissimilarity between the metabolomes of teosinte and maize, principal component analysis (PCA) was performed. A heat map with hierarchical clustering was generated using Pearson distance and Ward clustering to show the accumulation patterns of the top 30 metabolites selected by the linear model (Ritchie et al., 2015). The linear model used a significance cutoff value of P < 0.05, species as the blocking factor, and the no flooding control treatment as the reference group to obtain a list of metabolites significantly contributing to treatment differences.
Pathway analysis was also performed in MetaboAnalyst 5.0 to identify which pathways contributed to significant differences between the root metabolomes of teosinte and maize during flooding. Because the two-way interactions of species and herbivory, and flooding and herbivory were not significant for all metabolites (Tables S3 and S4), only metabolites identified by the interaction of species and flooding (Table S2) were imported into MetaboAnalyst. Pathway analysis parameters included Fisher's exact test and relative-betweenness centrality. The reference metabolome was the Oryza sativa japonica Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database (Kanehisa et al., 2023). Of the three KEGG reference metabolomes available, Oryza sativa japonica (Japanese rice, Poales: Poaceae) was selected over Arabidopsis thaliana (thale cress, Brassicales: Brassicaceae) and Chlorella variabilis (green alga, Chlorellales: Chlorellaceae) because rice is a poaceous monocot and therefore more evolutionarily—and likely metabolically—related to teosinte and maize (Poales: Poaceae) than the other plant species.
Statistical analyses for (1) total phenolic content, (2) total volatile organic compound emissions, and (3) plant growth parameters were performed using R studio statistical software version 4.2.0 (R Core Team, 2022). To assess the influence of Zea species, flooding, and herbivory on these measurements, three-way analyses of variance (ANOVAs) were used. Tukey's honestly significant difference tests established mean separation grouping labels for their respective figures using the HSD.test function (package ‘agricolae’ version 1.3, de Mendiburu, 2021). To assess the compositional dissimilarity between the volatile profiles of teosinte and maize, non-metric dimensional scaling (NMDS) was used on a Bray-Curtis dissimilarity matrix of the volatile profiles of both species, using the metaMDS function (package ‘vegan’ version 2.6.4, Oksanen et al., 2022). Volatile organic compounds that most strongly influenced differences between teosinte and maize were identified and ranked by the Random Forest model (Breiman, 2001) using the randomForest function (package ‘randomForest’ version 4.7.1.1, Liaw & Wiener, 2022). Models ran for 1000 iterations to obtain variable selection. Compound importance was ranked by percent increase in mean square error, where higher values indicate higher importance. Figures were produced using the ggplot function (package ‘ggplot2’ version 3.4.0, Wickham et al., 2016).
3 RESULTS
3.1 Belowground defensive secondary metabolites
3.1.1 Root metabolite profiles
To compare the root metabolic responses of teosinte (Zea nicaraguensis) and maize (Zea mays) exposed to flooding, herbivory, and their combined stress, root tissue was collected from plants in each treatment and analyzed by LC-MS. A total of 405 annotated metabolites were identified and cross-referenced with MetaboAnalyst 5.0. These consisted of organic acids, fatty acyls, polyketides, benzenoids, lipids, organoheterocylic compounds, nucleic acids, carbohydrates, organic nitrogen compounds, organic oxygen compounds, and alkaloids (Table S1).
The root metabolomes of teosinte and maize were compositionally different, evident by separate clustering in the principal component analysis (PCA) (Figure 1A), with 40.3% of the total variance accounted for in the first two principal components. Additional clustering by flooding was observed for teosinte but not for maize. To elucidate which metabolites contributed to this difference, heat mapping and hierarchical clustering revealed the accumulation patterns of root metabolites by treatment (Figure 1B). Two major clusters were identified with distinct patterns of metabolite abundance: metabolites that accumulated in roots during flooding and metabolites that accumulated during no flooding conditions. Among the 53 metabolites influenced by the interaction of species and flooding (Figure 1C) was the oxylipin jasmonic acid (F = 34.13, P = 0.00014) and the flavanone naringenin (F = 9.13, P = 0.022) (Table S2). No metabolites were significantly influenced by the interaction of species and herbivory (Figure 1D), nor the interaction of flooding and herbivory (Figure 1E, Tables S3 and S4). These data suggest the root metabolome of teosinte may be differentially regulated during flooding compared to maize, and flooding is a more significant driver of root metabolic changes than herbivory or combined flooding and herbivory.
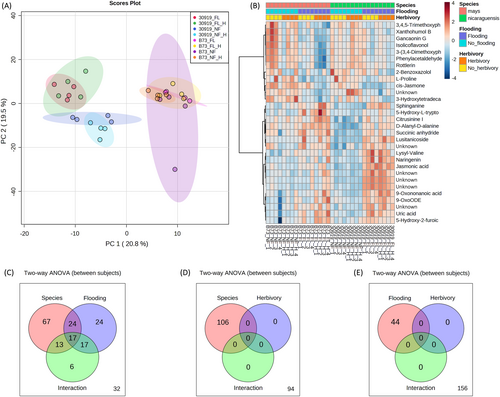
3.1.2 Root metabolite pathways
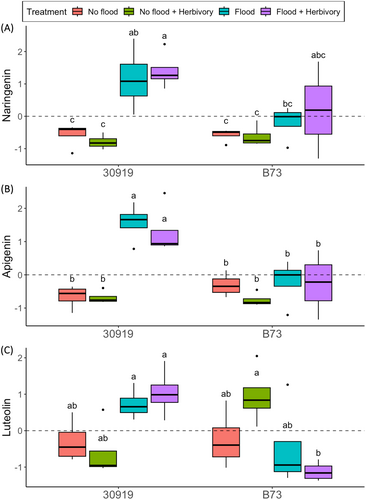
To assess which biosynthetic pathways contributed to significant differences between the root metabolic responses of teosinte and maize during flooding, pathway analysis was performed using Oryza sativa as the reference metabolome (Kanehisa et al., 2023). Of the 13 pathways identified, only the flavone and flavonol biosynthesis pathway was confidently attributed to significant differences between teosinte and maize (Holm P = 0.012) (Table 1). The three metabolites in this pathway were the flavanone naringenin and flavones apigenin and luteolin, which accumulated during flooding in only teosinte (Figure 2).
| Pathway | Total | Hits | Holm P | FDR |
|---|---|---|---|---|
| Flavone and flavonol biosynthesis | 12 | 3 | 0.012 | 0.012 |
| Flavonoid biosynthesis | 47 | 3 | 0.74 | 0.37 |
| Arginine and proline metabolism | 28 | 2 | 1.0 | 0.82 |
| Phenylpropanoid biosynthesis | 35 | 2 | 1.0 | 0.94 |
| Nicotinate and nicotinamide metabolism | 13 | 1 | 1.0 | 1 |
| Butanoate metabolism | 17 | 1 | 1.0 | 1 |
| Tyrosine metabolism | 18 | 1 | 1.0 | 1 |
| Arginine biosynthesis | 18 | 1 | 1.0 | 1 |
| Citrate cycle (TCA cycle) | 20 | 1 | 1.0 | 1 |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 22 | 1 | 1.0 | 1 |
| Alanine, aspartate, and glutamate metabolism | 22 | 1 | 1.0 | 1 |
| alpha-Linolenic acid metabolism | 27 | 1 | 1.0 | 1 |
| Aminoacyl-tRNA biosynthesis | 46 | 1 | 1.0 | 1 |
3.2 Aboveground defensive secondary metabolites
3.2.1 Total phenolic content
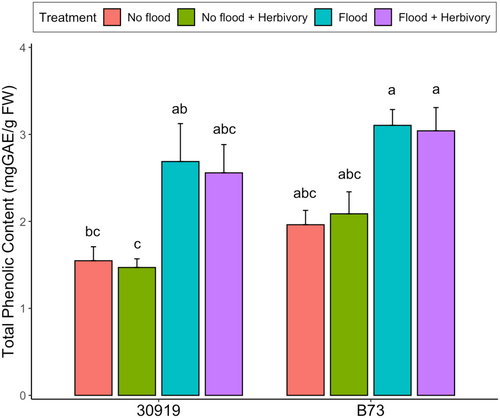
To compare the total phenolic content of teosinte and maize challenged with flooding, herbivory, and their combination, leaf tissue was collected and analyzed from plants in each treatment. Significant differences in the total phenolic content were observed between teosinte and maize (F = 7.070, P = 0.010) (Table 2), where teosinte maintained a lower phenolic content than maize across all treatment combinations (Figure 3). Flooding in isolation increased the total phenolic content of both species (F = 35.48, P < 0.0001), while herbivory in isolation had no effect (Table 2). The combination of flooding and herbivory increased the phenolic content of both species, but no more or less than flooding in isolation. These results suggest flooding is the most significant driver of phenolic accumulation in both teosinte and maize.
| Factors | Degrees of freedom | Sum of squares | R2 | F-value | P-value |
|---|---|---|---|---|---|
| Species | 1 | 3.725 | 3.725 | 7.070 | 0.010 |
| Flooding | 1 | 18.69 | 18.69 | 35.48 | <0.0001 |
| Herbivory | 1 | 0.021 | 0.021 | 0.040 | 0.84 |
| Sp*F | 1 | 0.018 | 0.018 | 0.033 | 0.85 |
| Sp*H | 1 | 0.073 | 0.073 | 0.139 | 0.71 |
| F*H | 1 | 0.058 | 0.058 | 0.110 | 0.74 |
| Sp*F*H | 1 | 0.019 | 0.019 | 0.035 | 0.85 |
| Residual | 56 | 29.50 | 0.527 |
3.2.2 Volatile organic compound profiles
To compare the emissions of volatile organic compounds (VOCs) from teosinte and maize exposed to flooding, herbivory, and their combination, headspace volatiles were collected from plants in each treatment. A total of 45 VOCs were detected from their aboveground headspaces (Tables S5 and S6). These consisted of sesquiterpenes (trans-α-bergamotene, trans-g-cadinene, and 19 others), monoterpenes (β-myrcene, linalool, and 6 others), ketones (2-ethylcyclopentanone, [1,1’-bicyclopentyl]-2-one, and 1 other), aldehydes (nonanal, and 2 others), hydrocarbons (pentadecane, and 2 others), an alcohol (3-hexen-1-ol), an ester (Z-3-hexen-1-ol,acetate), a homoterpene (E-4,8-dimethylnona-1,3,7-triene), an alkene, a benzoate ester, an aromatic amino acid derivative, and a diterpene alcohol.
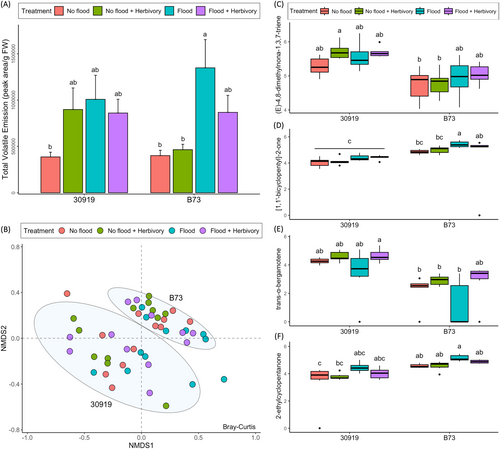
Quantitatively, flooding in isolation significantly increased the total VOC emissions of both teosinte and maize (F = 13.38, P = 0.0007), while herbivory in isolation increased the emissions of only teosinte (Figure 4A, Table 3). The combination of flooding and herbivory significantly increased the total VOC emissions of both species (F = 5.19, P = 0.028), but less than flooding in isolation (Table 3). These data suggest that the influence of flooding dominates the volatile emission response of teosinte and maize.
| Factors | Degrees of freedom | Sum of squares | R2 | F-value | P-value |
|---|---|---|---|---|---|
| Species | 1 | 3.92e + 09 | 3.92e + 09 | 0.019 | 0.89 |
| Flooding | 1 | 2.80e + 12 | 2.80e + 12 | 13.38 | 0.0007 |
| Herbivory | 1 | 2.06e + 09 | 2.06e + 09 | 0.010 | 0.92 |
| Sp*F | 1 | 4.40e + 11 | 4.40e + 11 | 2.10 | 0.15 |
| Sp*H | 1 | 4.58e + 11 | 4.58e + 11 | 2.18 | 0.14 |
| F*H | 1 | 1.08e + 12 | 1.08e + 12 | 5.19 | 0.028 |
| Sp*F*H | 1 | 9.91e + 09 | 9.91e + 09 | 0.047 | 0.82 |
| Residual | 40 | 8.38e + 12 | 2.09e + 11 |
Qualitatively, the volatile profiles of teosinte and maize were compositionally different, evident by separate clustering in the non-metric dimensional scaling (NMDS) analysis (Figure 4B). To elucidate which VOCs contributed most to this compositional difference, the Random Forest model ranked the compounds of each species by their importance (Table 4). Within the teosinte volatile profile, the homoterpene (E)-4,8-dimethylnona-1,3,7-triene (DMNT) and the sesquiterpene trans-α-bergamotene ranked highest. Conversely, within the maize volatile profile, the ketones [1,1’-bicyclopentyl]-2-one and 2-ethylcyclopentanone ranked highest. Flooding in isolation significantly increased the emissions of only 2-ethylcyclopentone (F = 9.49, P = 0.0037) (Figure 4F), while herbivory in isolation increased the emissions of only trans-α-bergamotene (F = 3.13, P = 0.084) (Figure 4E) in both species (Table 5). These data suggest the important volatile compounds contributing to compositional differences between teosinte and maize are differentially emitted during flooding or herbivory, but not during their combined stress.
| Rank | Teosinte – volatile compounds | % increase in MSE | Maize – volatile compounds | % increase in MSE |
|---|---|---|---|---|
| 1 | (E)-4,8-dimethylnona-1,3,7-triene | 23.51 | [1,1’-bicyclopentyl]-2-one | 6.79 |
| 2 | trans-α-bergamotene | 21.48 | 2-ethylcyclopentanone | 5.85 |
| 3 | trans-γ-cadinene | 17.50 | pentadecane | 5.49 |
| 4 | α-sesquiphellandrene | 17.38 | bicyclopentyl-1,1’-diene | 5.19 |
| 5 | (E)-β-farnesene | 17.11 | indole | 4.89 |
| 6 | bicyclopentyl-1,1’-diene | 15.30 | β-ocimene | 4.79 |
| 7 | 3-hexen-1-ol,acetate, (Z) | 13.89 | trans-γ-cadinene | 4.28 |
| 8 | σ-cadinene | 12.18 | cyclopentanone | 4.20 |
| 9 | linalool | 9.92 | D-limonene | 3.26 |
| 10 | trans-β-ocimene | 9.10 | α-sesquiphellandrene | 2.57 |
| 11 | α-terpinolene | 8.53 | β-myrcene | 2.48 |
| 12 | α-muurolene | 7.56 | heptadecane | 2.42 |
| 13 | β-copaene | 7.24 | α-copaene | 2.20 |
| 14 | α-copaene | 7.13 | caryophyllene | 2.15 |
| 15 | caryophyllene | 6.65 | α-terpinolene | 2.06 |
| 16 | cyclopentanone | 6.38 | γ-terpinene | 1.86 |
| 17 | trans-geranylgeraniol | 6.30 | 1-pentadecane | 1.76 |
| 18 | α-myrcene | 4.95 | linalool | 1.55 |
| 19 | β-bisabolene | 4.73 | α-myrcene | 1.33 |
| 20 | α-cubebene | 4.55 | germacrene-D | 1.16 |
| 21 | γ-terpinene | 4.52 | 3-hexen-1-ol,acetate, (Z) | 0.89 |
| 22 | humulene | 3.98 | σ-cadinene | 0.86 |
| 23 | sesquisabinene | 3.57 | trans-β-ocimene | 0.74 |
| 24 | ylangene | 3.45 | α-muurolene | 0.72 |
| 25 | β-ocimene | 3.19 | β-copaene | 0.67 |
| 26 | gamma-himachalene | 2.99 | (E)-4,8-dimethylnona-1,3,7-triene | 0.62 |
| 27 | heptadecane | 2.94 | γ-muurolene | 0.55 |
| 28 | [1,1’-bicyclopentyl]-2-one | 2.83 | humulene | 0.05 |
| 29 | pentadecane | 2.74 | trans-α-bergamotene | 0.03 |
| 30 | 3-hexen-1-ol | 1.99 | gamma-himachalene | 0.02 |
| Factors | Degrees of freedom | Sum of squares | R2 | F-value | P-value |
|---|---|---|---|---|---|
| (E)-4,8-dimethylnona-1,3,7-triene | |||||
| Species | 1 | 1.59e+12 | 1.59e+12 | 19.78 | <0.0001 |
| Flooding | 1 | 1.02e+11 | 1.02e+11 | 1.26 | 0.26 |
| Herbivory | 1 | 8.91e+10 | 8.91e+10 | 1.10 | 0.29 |
| Sp*F | 1 | 1.12e+10 | 1.12e+10 | 0.14 | 0.71 |
| Sp*H | 1 | 1.07e+11 | 1.07e+11 | 1.32 | 0.25 |
| F*H | 1 | 1.33e+11 | 1.33e+11 | 1.65 | 0.20 |
| Sp*F*H | 1 | 1.13e+11 | 1.13e+11 | 1.40 | 0.24 |
| Residuals | 40 | 3.22e+12 | 8.06e+10 | ||
| [1,1’-bicyclopentyl]-2-one† | |||||
| Species | 1 | 5.50 | 5.50 | 8.44 | 0.0059 |
| Flooding | 1 | 0.33 | 0.33 | 0.50 | 0.48 |
| Herbivory | 1 | 0.39 | 0.39 | 0.60 | 0.44 |
| Sp*F | 1 | 0.29 | 0.29 | 0.45 | 0.50 |
| Sp*H | 1 | 0.65 | 0.65 | 1.00 | 0.32 |
| F*H | 1 | 1.096 | 1.096 | 1.68 | 0.20 |
| Sp*F*H | 1 | 0.85 | 0.85 | 1.30 | 0.26 |
| Residuals | 40 | 26.066 | 0.65 | ||
| trans-a-bergamotene | |||||
| Species | 1 | 2.16e+10 | 2.16e+10 | 14.95 | 0.0003 |
| Flooding | 1 | 7.02e+08 | 7.02e+08 | 0.48 | 0.49 |
| Herbivory | 1 | 4.54e+09 | 4.54e+09 | 3.13 | 0.084 |
| Sp*F | 1 | 5.60e+08 | 5.60e+08 | 0.38 | 0.53 |
| Sp*H | 1 | 4.05e+09 | 4.05e+09 | 2.80 | 0.10 |
| F*H | 1 | 1.01e+08 | 1.01e+08 | 0.070 | 0.79 |
| Sp*F*H | 1 | 7.47e+07 | 7.47e+07 | 0.052 | 0.82 |
| Residuals | 40 | 5.79e+10 | 1.44e+09 | ||
| 2-ethylcylcopentanone† | |||||
| Species | 1 | 9.78 | 9.78 | 24.02 | <0.0001 |
| Flooding | 1 | 3.87 | 3.87 | 9.49 | 0.0037 |
| Herbivory | 1 | 0.032 | 0.032 | 0.079 | 0.77 |
| Sp*F | 1 | 0.18 | 0.18 | 0.45 | 0.50 |
| Sp*H | 1 | 0.074 | 0.074 | 0.18 | 0.67 |
| F*H | 1 | 1.029 | 1.029 | 2.52 | 0.11 |
| Sp*F*H | 1 | 0.31 | 0.31 | 0.77 | 0.38 |
| Residuals | 40 | 16.29 | 0.40 |
3.3 Treatment influence on plant growth parameters
The growth of teosinte and maize significantly differed when exposed to flooding, herbivory, and their combination (Figure 5). Teosinte maintained a higher shoot and root dry weight than maize (Figure 5A,B). Flooding in isolation decreased all growth parameters of both species, while herbivory in isolation had no significant impact on plant growth (Table 6). The combination of flooding and herbivory decreased the growth of both species, but less than flooding alone. Unexpectedly, the root dry weight of teosinte under combined flooding and herbivory did not differ from the control (Figure 5B). In addition, no difference in the root length of teosinte was recorded, while the root length of maize was significantly affected by flooding (Figure 5D). These results together suggest flooding in isolation is more detrimental to the growth of both species than flooding in combination with herbivory, yet the belowground growth of teosinte is flood-tolerant compared to maize.
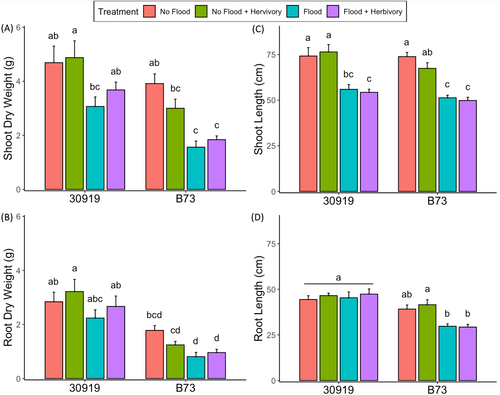
| Sources of Variation | Species | Flooding | Herbivory | Sp*F | Sp*H | F*H | Sp*F*H |
|---|---|---|---|---|---|---|---|
| Degrees of Freedom | 1, 56 | 1, 56 | 1, 56 | 1, 56 | 1, 56 | 1, 56 | 1, 56 |
| (Num, Den) | |||||||
| Growth Parameter | F-value with P-value in parenthesis | ||||||
| Shoot Length | 5.073 (0.028) | 96.86 (<0.0001) | 0.79 (0.37) | 0.001 (0.98) | 1.09 (0.30) | 0.017 (0.89) | 1.15 (0.28) |
| Shoot Fresh Weight | 1.15 (0.28) | 43.11 (<0.0001) | 0.48 (0.48) | 1.87 (0.17) | 1.42 (0.23) | 3.40 (0.07) | 0.40 (0.52) |
| Shoot Dry Weight | 28.08 (<0.0001) | 31.31 (<0.0001) | 0.02 (0.88) | 0.38 (0.53) | 1.61 (0.20) | 2.03 (0.15) | 0.45 (0.50) |
| Root Length | 47.48 (<0.0001) | 9.77 (0.0028) | 0.90 (0.34) | 13.51 (0.0005) | 0.11 (0.73) | 0.21 (0.64) | 0.17 (0.67) |
| Root Fresh Weight | 30.36 (<0.0001) | 7.38 (0.0087) | 0.013 (0.90) | 0.003 (0.95) | 0.44 (0.50) | 1.78 (0.18) | 0.056 (0.81) |
| Root Dry Weight | 57.89 (<0.0001) | 8.80 (0.0044) | 0.27 (0.60) | 0.015 (0.90) | 2.16 (0.14) | 0.83 (0.36) | 0.61 (0.43) |
4 DISCUSSION
This study investigated the effects of flooding, insect herbivory, and their combination on the below and aboveground defense-related metabolites of a wild teosinte (Zea nicaraguensis) and cultivated maize (Zea mays). Belowground, our results revealed distinct differences in the root metabolite profiles of teosinte and maize. Notably, the flavanone naringenin and flavones apigenin and luteolin contributed to compositional differences between the responses of these plants. During flooding, because of hypoxic conditions and imbalances in critical physiological and metabolic processes, such as photosynthesis, respiration, and accumulation of reactive oxygen species, plant tissues experience oxidative stress (Shabala et al., 2014). Moreover, oxidative stress may be stronger in waterlogged than in fully submerged plants (Alves et al., 2013). Flavone and flavonol-derived metabolites have been reported to play roles in alleviating oxidative stress by scavenging reactive oxygen species such as H2O2 (Shabala et al., 2014; Mekawy et al., 2018; Yildiztugay et al., 2020). That our results show a significant accumulation of naringenin and apigenin in teosinte but not maize root tissue during flooding (waterlogging) may indicate ongoing oxidative stress in teosinte root tissue. But whether teosinte differentially accumulates flavones as a mechanism to mitigate oxidative stress during flooding, thus providing this wild relative with improved tolerance to flooding compared to maize, warrants further investigation.
Surprisingly, the oxylipin jasmonic acid further contributed to the differences in root-produced metabolites in teosinte and maize exposed to flooding, herbivory, and their combination. This result is intriguing as the accumulation of jasmonic acid in hypoxic conditions is often documented during reoxygenation after flooding rather than during flooding itself (Yuan et al., 2017; Yeung et al., 2019; Pérez-Llorca et al., 2023). However, previous studies have reported increases in jasmonic acid in maize roots experiencing hypoxia, where preferential expression of factors regulated the synthesis and signaling of jasmonic acid (Youseff et al., 2019). Jasmonic acid works in concert with ethylene and auxin to regulate root morphological changes during flooding, including lateral and adventitious root formation (Raya-González et al., 2012; Cai et al., 2014; Borrego & Kolomiets, 2016; Xu et al., 2020). Indeed, teosinte (Zea nicaraguensis) forms long lateral roots (Pedersen et al., 2020) to allow the root system to grow toward oxygen-rich surfaces (Karlova et al., 2021). This behavior was observed in the present study (Figure S2). Therefore, the role of jasmonic acid in mediating developmental changes in roots may explain the accumulation of jasmonic acid during flooding.
Aboveground, the phenolic content of teosinte and maize increased during flooding but not herbivory. Secondary metabolites such as phenolic compounds play numerous defensive roles in plant tissues, including functioning as antioxidants and feeding deterrents to herbivorous insects. This result is consistent with previous studies investigating flooding-induced increases in the phenolic content of maize (Grace & Logan, 2000; Yordanova & Popova, 2007; Lukić et al., 2021). Such responses may be a mechanism to mitigate oxidative stress (Bors et al., 1990; Grace, 2005). Flooding impairs photosynthesis and respiration, leading to the accumulation of reactive oxygen species that induce oxidative damage in plant leaves (Yan et al., 1996; Yordanova & Popova, 2007; Lukić et al., 2021). There is mounting evidence that phenolic compounds can scavenge these reactive oxygen species (Foyer, 2018; Šola et al., 2021). To this end, the flooding-induced increase in the phenolic content of teosinte and maize may be due to the antioxidant capacity of phenolic compounds. However, the absence of herbivore-induced change in phenolic content may indicate that a single herbivore elicited a subdued response. There is evidence that herbivore identity and intensity are important factors in eliciting herbivore-induced responses, including changes in phenolic content (Quintero & Bowers, 2011; Pan et al., 2021). For instance, Block et al. (2020) showed infestation by five (rather than one) S. frugiperda larvae enhanced the production of soluble phenolic compounds in maize. Therefore, herbivore intensity may be an important factor driving herbivore-inducible changes in phenolic content and may explain why a single herbivore influenced no change in the total phenolic content of teosinte and maize.
Volatile organic compounds (VOCs) are a diverse group of secondary metabolites emitted by plants that play vital roles in mitigating cellular damage and mediating ecological interactions, yet our understanding of how VOC blends are altered quantitatively and qualitatively by stress combinations remains limited (Loreto & Schnitzler, 2010; Dudareva et al., 2013; Possell & Loreto, 2013).
Quantitatively, the total VOC emissions of teosinte and maize increased during combined flooding and herbivory stress, but less than during flooding alone. These results contrast with previous studies investigating the influence of flooding and herbivory on maize VOC emissions (Block et al., 2020; Ngumbi & Ugarte, 2021; Ngumbi et al., 2022). For instance, Block et al. (2020) reported that the combined stress of flooding and herbivory by S. frugiperda enhanced the emissions of (3E,7E)-4,8,12-trimethyl-1,3,7,11-tridecatetraene (TMTT), linalool, and volatile phenolics in B73 maize. Similarly, Ngumbi and Ugarte (2021) showed that flooding and herbivory also increased emissions of maize, but of β-myrcene, β-ocimene, undecane, ylangene, trans-α-bergamotene, and germacrene-D. The differences between these studies and our results may be due to different methods for volatile collection, herbivory, and flooding. Block et al. (2020) collected VOC emissions using dynamic collection and SuperQ columns after removing plants from flooded conditions and using five neonate larvae for herbivory. Similar to Ngumbi and Ugarte (2021), our study maintained flooding conditions while collecting VOC emissions using static collection and solid-phase micro-extraction (SPME) from plants with a single, third instar larvae. Indeed, several factors, including the method of volatile collection, condition of flooding, and growth stage of herbivorous larvae can alter secondary metabolites qualitatively and quantitatively (Holopainen & Gershenzon, 2010; Degen et al., 2012; De Lange et al., 2020). These variabilities in volatile collection from disparate flooding and herbivory studies highlight the importance of harmonizing experimental methods and performing more combinatorial stress studies.
Qualitatively, teosinte and maize emitted different volatile profiles. The compounds contributing most to these differences were the homoterpene (E)-4,8-dimethylnona-1,3,7-triene (DMNT) and the sesquiterpene trans-α-bergamotene, which were influenced by herbivory in both species. These terpenes have been detected in maize and other teosintes experiencing herbivory and are associated with the recruitment of natural enemies, such as the parasitic wasp Cotesia marginiventris (Turlings et al., 1990; Gouinguené et al., 2001; Tholl & Lee, 2011; de Lange et al., 2016, 2018). Furthermore, the primary enzyme associated with DMNT production in maize was induced upon feeding damage by Spodoptera littoralis, identifying DMNT as an herbivore-induced compound (Degenhardt & Gershenzon, 2000). That DMNT and trans-α-bergamotene were important contributors to the volatile profile of teosinte and were influenced by herbivory may explain why the total volatile emission of teosinte increased during herbivory compared to maize (Figure 4A). Whether teosinte prioritizes the production of these terpenes during herbivory and may therefore be better defended than maize through the recruitment of natural enemies warrants further investigation.
Our study showed decreased biomass of teosinte and maize during flooding, consistent with previous studies (Kozlowski, 1984; Perata et al., 2011; Sasidharan et al., 2020; Jia et al., 2021; Lukić et al., 2021; Jia et al., 2021). However, flooding combined with herbivory resulted in less decrease in biomass when compared to flooding alone. This result suggests herbivory abated the flooding-induced decrease in growth. Previous studies have reported that aboveground herbivory can stimulate biomass allocation, even in roots, in part due to mobilization of resources away from herbivore-damaged sites (Xue et al., 2012; Robert et al., 2014; McNickle & Evans, 2018). Moreover, Block et al. (2020) demonstrated flooding-induced resistance of maize to S. frugiperda larvae, which may alleviate the impact of herbivory on growth during flooding. Flooded teosinte displayed this phenotype more prominently than maize (Figure 5A) by maintaining growth during combined flooding and herbivory, suggesting a greater flooding-induced resistance to herbivory than maize. In addition, Zea nicaraguensis is native to the seasonally flooded northwest coastal plain of Nicaragua and has morphological traits, such as aerenchyma and oxygen loss barriers, that allow flooding tolerance (Iltis & Benz, 2000). The maintenance of belowground growth during combined flooding and herbivory in teosinte may be due to flood-adapted root architecture and herbivory-induced compensatory growth.
Taken together, our study showed differential responses of teosinte and maize to flooding, insect herbivory, and their combination. Root-derived flavonoids (associated with antioxidative functions) and jasmonic acid (associated with root development) were differentially accumulated in teosinte during flooding. Volatile terpenes stimulated by herbivory dominated the volatile profile of teosinte, which may provide an ecological advantage over maize. Moreover, teosinte maintained growth during flooding compared to maize, likely due to enhanced flood-tolerant root architecture. These results suggest the secondary metabolite responses of teosinte to flooding and herbivory impart some tolerance to these stresses over maize through a combination of cellular and ecological functions. While it is clear that wild relatives of cultivated plants can have improved tolerance to climate-linked stressors through changes in defensive secondary metabolism, there is a need for more comparative studies to understand the molecular and metabolic mechanisms underpinning such superior tolerance to individual and combinatorial stress.
AUTHOR CONTRIBUTIONS
Aaron D. Mleziva and Esther N. Ngumbi conceived and designed the project. Aaron D. Mleziva carried out the project and analyses. Aaron D. Mleziva wrote the manuscript and Esther N. Ngumbi provided significant editorial comments. Both authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We sincerely thank Alex Lozano from St. Charles Community College for assistance with all greenhouse and laboratory work. We also thank Erinn Dady, Miles Arceneaux, and Michael Sommerville for assistance with experiment takedown and greenhouse work. We thank Dr. Elizabeth Ainsworth, Dr. May Berenbaum, and Dr. Larry Hanks for graciously allowing the use of lab equipment and materials. We also thank Alex Ulanov and Michael La Frano of the Roy J. Carver Metabolomics Unit for performing the root tissue extraction and LC-MS metabolite analysis. We lastly acknowledge the plants and animals that were sacrificed for this study.
FUNDING INFORMATION
This work was supported by funding to Dr. Esther Ngumbi from the University of Illinois Urbana-Champaign and by funding to the Phenotypic Plasticity Research Experience for Community College Students (PRECS) from the National Science Foundation (1950819).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




