Abiotic and biotic factors regulate the timing of floral induction: a review
Abstract
Time of flowering is an important phenomenon which ensures reproductive success and better adaptability to various environmental conditions. There are a few proteins that function as master regulators of flowering. The expression of these proteins highly depends on many extrinsic and intrinsic factors along with several biotic components. Further, alterations in these factors result in drastic changes in the transition from the vegetative to the reproductive phase that ultimately results in yield loss. Different abiotic stresses, such as drought, salinity, cold, heat, nutrient scarcity, sugar availability and exogenous chemicals, collectively affect the whole ambit of the floral induction process. Nevertheless, biotic stresses like bacterial (Pseudomonas syringae) and fungal (Fusarium oxysporum) infections promote the expression of the FT gene, which further accelerates the flowering phenomenon. Soil-dwelling microorganisms, herbivores, pollinators and a few endophytes are associated with the promotion of flowering. Although plants have adapted several strategies to cope with the stress conditions and manage to maintain their reproductive physiology, it is noteworthy that flowering in plants under stress conditions shows species-specific response patterns by accelerating or retarding the floral induction. Several transcription factors and some miRNAs were also involved in the regulation of flowering under stress conditions. In this review, we have summarised the different biotic and abiotic stresses that affect the flowering time and also include several strategies that plants adapt to escape the stress condition.
1 INTRODUCTION
Plants are sessile organisms, and unlike animals, they cannot migrate to different places due to various environmental changes. In this context, plants have developed several mechanisms, including the alterations in the floral induction pathway, which further help plants withstand stressful conditions. Flowering is an important phase in a plant's life cycle, governed by an intricate network of genetic and epigenetic reorganization (Capovilla et al., 2015, Cho et al., 2017). This transition from the vegetative to the reproductive phase is controlled by several abiotic as well as biotic factors. Therefore, the control of floral timing is crucial for successful reproduction and increased crop yield.
Large studies on floral transition under different stress conditions show that plants alter their flowering time in order to reduce the adverse effects. For example, under drought stress, the long-day (LD) plants accelerate flowering by activating the master floral integrator gene; contrary, short-day (SD) plants showed delayed flowering (Tun et al., 2021). Similarly, many reports have elucidated that the adverse effects of salt stress (Kim et al., 2007), cold stress (Knight et al., 1996), heat stress (Posé et al., 2013), as well as several biotic stresses like bacterial and fungal infection (Korves and Bergelson, 2003) alters flowering. Arabidopsis thaliana, when challenged with the Burkholderia phytofirmans endophyte, promotes early flowering through the expression of bioactive gibberellic acid (GA) (Poupin et al., 2013). Pollinators have a mutual relationship with plants and play a vital role in the fertilization process. However, environmental stresses alter the flowering time, resulting in a communication gap between the plant and pollinators, leading to ecological imbalance.
To understand the molecular mechanisms encompassing flowering, experiments have been carried out in Arabidopsis thaliana (Amasino, 2010, Song et al., 2013) and rice (Jeong et al., 2015, Lee and An, 2015). Previous literature highlights the involvement of the photoperiodic pathway as a master regulator of flowering. This pathway comprises three main important genes, specifically GIGANTEA (GI) (Fowler et al., 1999), FLOWERING LOCUS T (FT) (Corbesier et al., 2007, Jaeger and Wigge, 2007) and CONSTANS (CO) (Putterill et al., 1995). These three genes collectively or independently affect the photoperiodic induction of flowering in plants. Various studies have concluded that GI controls CO, which thereby regulates the expression of FT, and their association induces flowering. The autonomous pathway is another important pathway which promotes flowering by directly suppressing FLOWERING LOCUS C (FLC) (Michaels and Amasino, 1999). Additionally, mutations in this pathway result in delayed flowering. Further, vernalization is also an important process affecting flowering. This process suppresses the expression of the FLC gene and thereby leads to enhanced expression of the FT gene and results in blooming (Kim et al., 2009). Moreover, GA is another important factor that induces vernalization in non-inductive photoperiodic conditions (Huang et al., 2017).
The global food demand is increasing day by day with the increase in the world's population. Indeed, global climate change has also enhanced the problem by another degree. Appropriate timing of floral induction under stress conditions is essential in plants to mitigate the increasing food demand globally. Therefore, in this review, we have summarized the different abiotic and biotic stresses that alter the floral induction timing, and focused on the genetic and epigenetic modulation of the expression of flowering control genes.
2 TRANSCRIPTIONAL REGULATION OF FLORAL INDUCTION PATHWAY
Flowering is a light-dependent process that is controlled by different “floral integrator genes” involving GI, CO, FT, FLC, SUPRESSOR OF OVEREXPRESSION OF CONSTANS (SOC1), CRYPTOCHROMES (CRY) and several miRNAs (Kinoshita and Richter, 2020). The circadian clock and photoperiod regulate the expression of the CO protein. The level of the CO protein oscillates under LD conditions, which directly affects the expression of its immediate downstream target, the FT protein, the main component of florigen. The FT protein is synthesized in the leaves and moves to the shoot apical meristem and induces the transition from the vegetative to the reproductive phase in plants (Song et al., 2013, Golembeski and Imaizumi, 2015). Various reports in A. thaliana suggest that under LD conditions, CO expression is upregulated in the afternoon in the phloem of leaves that interact with the promoter region of the FT gene and positively regulate its expression, accelerating flowering. The low amount of CO protein observed in leaves in the morning is unable to induce FT-mediated floral induction (Mizoguchi et al., 2005). Further, the suppressor of the CO protein CYCLING DOF FACTOR (CDF1, CDF2, CDF3, CDF5) is a family of transcription factors whose expression peaks at night and in the early morning, resulting in suppression of CO transcription (Young Hun Song, 2012, Fornara et al., 2009). Additionally, CDF forms a complex with another co-repressor, TOPLESS (TPL), that further enhances their ability to suppress the expression of CO and FT (Goralogia et al., 2017). During the late afternoon, another component of the circadian clock, PSEUDO RESPONSE REGULATOR 9 (PRR9), PRR 1, PRR5 and PRR7 interacts with CDF and reduces its expression, which promotes CO protein accumulation (Hayama et al., 2017, Wang et al., 2023). Furthermore, the expression of the photoreceptor E3 ubiquitin ligase FLAVIN-BINDING, KELCH REPEAT, F-BOX 1 (FKF1), and GI increases simultaneously and form a FKF1-GI complex in the presence of blue light (Takato Imaizumi, 2003). The FKF1-GI complex then ubiquitinates and degrades the CDF transcription factor and promotes the expression of CO protein during the day, which induces the FT gene expression and promotes flowering (Mariko Sawa, 2007).
Photomorphogenesis is another crucial process governed by two photoreceptors, Phytochrome (phyA & phyB) and Cryptochrome (CRY1 & CRY2), which controls the floral induction. phyA protein is light labile, and phyB is stabilised by the light (Li et al., 2011). Skotomorphogenesis and photomorphogenesis completely depend on the light availability, which further affects the expression of the floral integrator gene CO. During dark conditions, CONSTITUTIVE PHOTOMORPHOGENESIS 1 (COP1), a RING-type E3 ubiquitin ligase form a COP1-SPA1(SUPRESSOR OF phyA 105) complex which degrades the positive regulators of photomorphogenesis like LONG HYPOCOTYL IN FAR RED 1 (HER1), ELONGATED HYPOCOTYL 5 (HY5) and LONG AFTER FAR RED1 (LAF1) and also degrades the CO protein (Sheerin et al., 2015) (Liu et al., 2011). In light conditions, phyA prevents the interaction between COP1 and SPA1 and thus results in CO protein stabilization (Figure 1) (Laubinger et al., 2006). Blue light photoreceptor cryptochrome (CRY1 & CRY2) stabilize the CO protein through the inactivation of the COP1-SPA1 complex. In the presence of blue light, the C-terminal domain of CRY1 interacts with the C-terminal domain of SPA1, which inhibits the complex formation between COP1 and SPA1, resulting in the stabilization of the CO protein. In the case of CRY2, the N-terminal domain binds to the N-terminal domain of SPA1 and forms a tripartite complex of CRY2-COP1-SPA1, which represses the activity of the COP1-SPA1 complex and maintains the stability of the CO protein (Zuo et al., 2011). In the morning, the CO protein is degraded by another RING-type E3 ubiquitin ligase HOS1 (HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENE 1) in a proteasome-dependent manner and acts as a negative regulator of floral induction (Figure 1) (Lazaro et al., 2012).
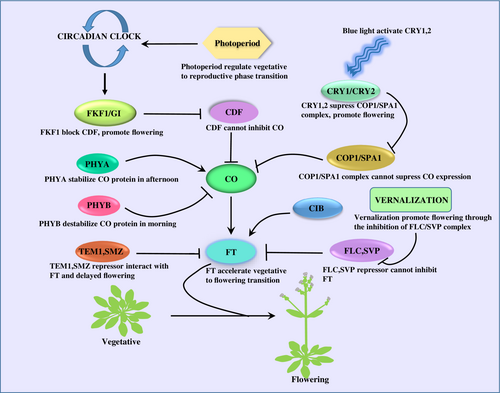
Flowering in plant highly depend on photoperiod and circadian clock. In the morning, CDF transcription factor represses CO expression. During afternoon, an E3 ubiquitin ligase FKF1 expression increases and form a FKF1/GI complex, further FKF1/GI complex promote CO accumulation through the degradation of CDF and accelerates FT mediated flowering. Phytochrome and cryptochrome were also involved CO accumulation in light dependent manner. In the morning phyB destabilize CO protein whereas, phyA stabilize CO protein in afternoon. Cryptochrome also increases CO accumulation in the presence of blue light. At night, COP1/SPA1 complex inhibits flowering through the degradation of CO protein. At day time, blue light activates CRY1, 2 which further accelerates flowering by the inhibition of COP1/SPA1 complex. Several transcription factors like CIB promotes flowering at afternoon. FLC, SVP, TEM1 and SMZ act as a repressor of FT gene and prevents flowering during unfavorable condition. Vernalization accelerates flowering through the inhibition of these transcription factors.
CYCLING DOF FACTOR-CDF, CONTANS- CO, FLAVIN-BINDING, KELCH REPEAT, F-BOX 1- FKF1, GIGANTEA- GI, FLOWERING LOCUS T- FT, PHYTOCHROME (phyA & phyB), CONSTITUTIVE PHOTOMORPHOGENESIS 1- COP1, SUPRESSOR OF phyA 105- SPA1, CRYPTOCHROMES (CRY), CRYPTOCHROME-INTERACTING BASIC HELIX–LOOP–HELIX- CIB, FLOWERING LOCUS C- FLC, SHORT VEGETATIVE PHASE- SVP, TEMPRANILLO- TEM1.
Several transcription factors, including CIB (CRYPTOCHROME-INTERACTING BASIC HELIX–LOOP–HELIX), FLC SVP (SHORT VEGETATIVE PHASE), TEM1 (TEMPRANILLO1 and SMZ also modulate FT gene expression in LD conditions (Castillejo and Pelaz, 2008). The CIB protein promotes FT expression in the afternoon under blue light exposure and directly interacts with CRY2 to bind to the FT promoter region, whereas FLC, SVP, TEM1 and SMZ are transcriptional repressors that bind to the promoter region of the FT gene and suppress its expression. During unfavourable conditions for flowering, these transcription factors interact and repress FT expression, which prevents precocious flowering of the plant. Additionally, a MADS-box type transcription factor, SVP, forms a heterodimeric complex with FLC. This complex remains higher in young leaves compared to older leaves, inhibiting FT expression and preventing flowering (Lee et al., 2007). FLC expression is repressed by prolonged exposure to cold, known as vernalization (Iain Searle, 2006). Further, the above mentioned process promotes FT expression by the removal of the FLC repressor to facilitate flowering (Figure 1) (Amasino, 2005). Expression of these floral integrator genes was regulated by various abiotic and biotic factors (Table 1), including drought, cold, heat, salinity, nutrients, bacteria, fungi, as well as photoperiod, which further results in altered floral induction.
| Sl no | Stresses | Causative agent | Target | Function | Reference |
|---|---|---|---|---|---|
| Abiotic Stress | |||||
| 1 | Drought | GIGANTIA↑ | CDF↓ | Early flowering | (Sawa & Kay, 2011) |
| 2 | Salinity | NTL8↑ | FLC↑ | Delayed flowering | (Kim et al., 2007) |
| 3 | Heat | miR172↑ | SVP-FLMꞵ↓ | Promotes flowering | (Posé et al., 2013) (Lee et al., 2010) |
| 4 | sugar | CO↑ | FT↑ | Promotes flowering | (Tognetti et al., 2013) |
| 5 | chemicals | CO2↑ | FLC↑ | Delayed flowering | (Springer & Ward, 2007) |
| 6 | nutrient | - | FT↑ | Early flowering | (Wada & Takeno, 2010) |
| 7 | Cold | HOS1↑ | FLC↑ | Delayed flowering | (Jung et al., 2013; Jung et al., 2012) |
| Biotic Stress | |||||
| 8 | Bacterial | Xanthomonas campestris | ethylene↑ | Early flowering | (Korves & Bergelson, 2003) |
| 9 | Fungi | Fusarium oxysporum | FLC↓ | Promote flowering | (Rebecca Lyons1*, 2015) |
| 10 | Herbivory | Pieris brassicae | glucobrassicanapin↑ | Early flowering | (Tobias Zu¨ st1, 2011) |
| 11 | Rhizosphere Microorganisms | Arthrobacter | IAA↑ | Delay flowering | (Tao Lu1†, 2018) |
| 12 | Endophyte |
Burkholderia phytofirmans | GA3ox1↑ | Promote flowering | (Poupin1* & 2013) |
3 DROUGHT STRESS
In general, plants face fluctuating environmental conditions throughout their lives. One such environmental stress is drought, which hampers the floral development, causing sterility (Su et al., 2013). Plants have evolved many adaptive strategies to cope with adverse conditions. Drought escape results in early flowering (Sherrard and Maherali, 2006), minimising the cost associated with drought stress and the production of high-quality seeds. Another popular approach used by plants is drought avoidance, which enhances water use efficiency and delays the process of flowering (Kenney et al., 2014 Kooyers, 2015). Extensive research in this field shows that the floral developmental pathways and drought stress are highly connected. GI, FT and CO being the master regulators of the floral developmental process, control the expression of other downstream genes for successful floral induction. GI activates the drought escape strategy in a photoperiod-dependent manner. Under LD conditions, GI degrades CDF, the repressor of the CO gene (Figure 2) (Sawa and Kay, 2011, Riboni et al., 2013). The activated CO gene thereby upregulates FT gene expression. Research on one of the most used cultivars of Arabidopsis thaliana (Ler-0) showed early flowering and shortened vegetative phase under drought conditions, which clearly depicts the drought escape behaviour. In another study, it was noticed that two transcription factors, namely ABF3 and ABF4, directly target one floral integrator, SOC1, and promote flowering under drought stress (Hwang et al., 2019). Similarly, in Curcuma kwangsiensis, some floral integrator genes were upregulated under stress conditions, which accelerated flowering (Feng et al., 2022). In Brassica rapa, early flowering was evident under drought stress (Franks et al., 2007). Under SD, many repressors of the FT gene delay the flowering process. Recent research shows that miRNAs are potential regulators of plant growth and development. Previous literature reveals that miR172e is activated by GI, which directly degrades the repressor AP2 of the FT gene, resulting in floral induction (Figure 2) (Han et al., 2013). Additionally, miR393 promotes early flowering but reduces the tolerance to drought in rice (Xia et al., 2012).
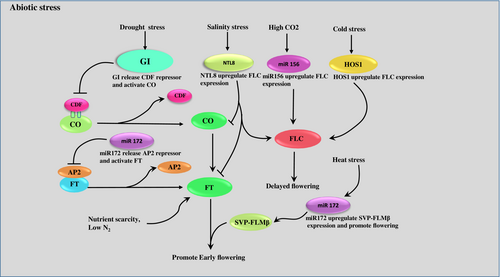
During drought condition, GI expression up regulates. GI releases CDF and activates CO further CO promote early flowering through the activation of FT. miR172 a key regulator that promote early flowering by preventing AP2 mediate FT repression and SVP-FLMꞵ up regulation under drought condition and heat stress respectively. NTL8, miR156 and HOS1 up regulates under salinity, high CO2 and cold stress respectively, which further promotes FLC expression and delayed flowering. Nutrient stress promotes early flowering through the up regulation of FT gene.
GIGANTEA- GI, CYCLING DOF FACTOR-CDF, CONTANS- CO, FLOWERING LOCUS T- FT, SHORT VEGETATIVE PHASE- SVP, FLOWERING LOCUS M- FLM, HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENE 1- HOS1, CARBON DI OXIDE- CO2, FLOWERING LOCUS C- FLC.
4 SALINITY STRESS
In Arabidopsis thaliana, under LD conditions, it was evident that salinity delays flowering, but the molecular mechanism behind it remained obscure until a group of researchers (Kim et al., 2007), revealed that NTL8 (a NAC family transcription factor) represses the expression of the FT gene when exposed to high salt condition (Figure 2). The DELLA protein, an antagonist of GA signalling, also plays a crucial role in this regard. Under high salinity, the DELLA protein gets stabilised and, in turn, extends the vegetative growth and delays flowering by promoting the expression of FLC and down-regulating the expression of the CO and FT transcripts (Achard et al., 2006). Studies have also shown that DELLA mutant plants experience early flowering under the stress condition. Further research on the floral repressor BROTHER OF FT (BFT) and TFL1 (TERMINAL FLOWER 1) revealed that these protein competes with the FT to bind with FLOWERING LOCUS D (FD) and thereby introduce a delay in the floral induction under salt stress conditions (Ryu et al., 2014). Moreover, CYCLIN DEPENDENT KINASE G2 (CDKG2), another suppressor of floral induction in Arabidopsis thaliana and its mutant form promotes flowering by increasing the transcript level of FT and LEAFY (LFY) under salt stress (Ma et al., 2015). Under normal conditions, GI, being the master regulator of the floral induction pathway, makes a complex with SOS2 that prevents the interaction between SOS2 and SOS3, however, under stress conditions, GI undergoes proteasomal degradation, making SOS2 free to interact with SOS3 and ultimately interact with the plant-specific Na+/H+ antiporter SOS1. This, in turn, helps the plant to export Na+ out of the cell, thereby making the plant salt tolerant (Kim et al., 2013). Recent studies in potato (Solanum tuberosum) revealed that a particular gene, StBBX24 (B box type transcription factor), in its overexpressed line delayed flowering, which caused tolerance to the salt stress, whereas exactly opposite phenomena were observed in its silenced line. (Kiełbowicz-Matuk et al., 2022). Another piece of evidence in an endangered dicotyledonous plant, Reaumuria trygyna, showed enhanced tolerance to salt stress and delayed flowering when overexpressing the RtWRKY23 gene in comparison to wild type (Du et al., 2019). These data portray a clear relation between salinity stress and flowering time. Studies of the roles of miRNA in floral induction under salt stress conditions have shown that miR169d is upregulated and, in turn, induces early flowering by controlling the expression of its target NF-YA in Arabidopsis (Xu et al., 2014). In rice (Oryza sativa), miR393 results in early flowering and increased sensitivity to salt stress by directly targeting two auxin receptors, namely TIR1 (Transport Inhibitor Response) and AFB2 (Auxin Signalling F-box2) (Xia et al., 2012). Overexpression of miR156 delays flowering by targeting SQAMOSA PROMOTER BINDING PROTEIN LIKE9 (SPL9) and promotes anthocyanin biosynthesis, thereby making the plant resistant to salt stress (Cui et al., 2014). A recent report revealed that the RAV TEMPRANILLO 1 (TEM1) gene negatively regulates the flowering process under salinity stress, whereas the double mutant of the TEM1 gene promotes early flowering (Osnato et al., 2021).
5 HEAT STRESS
Upon heat stress, altered membrane fluidity, increased misfolded proteins, and an accumulation of reactive oxygen species (ROS) help the plant understand the level of stress it is exposed to and alter the expression of the floral integrator genes (Zhou et al., 2012). These above conditions help the plant to alter the expression of mainly two components of the floral induction pathway, namely FLOWERING LOCUS M (FLM), with its two-temperature dependent splice variant FLMꞵ, FLMδ, and the floral repressor SVP. Under low temperatures, SVP-FLMꞵ prevents flowering, while under high temperatures, SVP-FLMδ promotes flowering (Posé et al., 2013). High temperatures during night time can have more detrimental effects than during day time, with massive yield losses as a result (Vargas et al., 2021). Many studies have shown that regulation of flowering during heat stress is species-specific as it can induce or delay flowering. Under an absolute non-inductive SD condition, Arabidopsis thaliana undergoes flowering when the temperature is slightly increased (Balasubramanian et al., 2006). On the other hand, studies on Boechera stricta (Anderson et al., 2011), and Chrysanthemum morifolium (Nakano et al., 2013) have shown a delay in floral induction by suppressing the expression of FT gene. Scientists have shown that chromosomal remodelling is also involved in floral induction under heat stress. In Arabidopsis thaliana, the PHYTOCHROME INTERACTING FACTOR 4 (PIF4) induces flowering under heat stress (Kumar et al., 2012) by removing the histone variant H2A.Z, thereby getting full access to the FT promoter, which otherwise remains blocked by the above said histone variant (Kumar and Wigge, 2010). High temperature due to global warming negatively affects the floral timing of apple (Malus domestica), hampering the industrial yield. Studies have shown that under heat stress conditions, a component of NUCLEAR PORE COMPLEX (NPC) Nup62 in its overexpression line results in heat injury and early flowering. On the other hand, the wild type plant shows heat-resistant behaviour under heat stress (Zhang et al., 2022). Furthermore, miR172 was suppressed by the SVP-FLMꞵ complex and introduced delayed flowering, whereas high temperature mediated the degradation of SVP and resulted in the upregulated expression of miR172, promoting flowering (Figure 2) (Lee et al., 2010). miR399 was also involved in floral induction under heat stress by targeting PHOSPHATE 2 (PHO2) transcript, however, the exact mechanism of action remains to be elucidated (Kim et al., 2011).
6 COLD STRESS AND VERNALIZATION
Plants sense the cold shock by a well-defined process, which ultimately increases the cytosolic Ca2+ concentration by both extracellular influx and vacuolar release (Knight et al., 1996). Continuous exposure to low temperatures leads to floral induction via the vernalization-dependent pathway, whereas brief exposure to low temperature hinders the process of flowering by inducing the FLC gene. One common pathway to achieve cold tolerance is the GI-dependent pathway, whereas the mutant form (gi) helps to accumulate CDF repressor, which represses CO and FT expression and delays flowering (Fornara et al., 2015). In this context, it was also reported that the loss of function mutation of GI confers increased sensitivity to cold stress (Cao et al., 2005). In Arabidopsis, under normal conditions, the MSI4 or FVE genes (homolog of human Rb) make a complex with HISTONE DEACETYLASE6 (HDA6) and directly bind with FLC, resulting in transcriptional inhibition (Gu et al., 2011). Under cold stress conditions, one important E3 ligase, HOS1, delays the floral induction by inhibiting the formation of the MSI4/FVE-HDA6 complex, which results in FLC activation. HOS1 also inactivate the CO-mediated FT gene activation (Figure 2) (Jung et al., 2012, Jung et al., 2013). The SOC1 gene is another master regulator of the flowering process, like the FT gene, whose expression promotes the transition from the vegetative to the floral stage during normal conditions by directly suppressing the GATA family transcription factors. Under cold stress, SOC1 expression is blocked by the two paralogs of the GATA transcription factor family, namely GNC (GATA, NITRATE-INDUCIBLE, CARBON METABOLISM INVOLVED) and GNL (GNC-LIKE), which delays the floral induction (Richter et al., 2010). Expression analysis through qRT-PCR has also shown that overexpression of these transcription factors results in the upregulation of two important cold-responsive genes (CBF2, COR15a,b) that confers tolerance to the stress conditions (Richter et al., 2013). Vernalization is an utmost important strategy for winter cereals whose flowering time coincides with long-term exposure to low temperature. The VERNALIZATION1 (VRN1) gene plays a crucial role in this regard by suppressing the FLC gene via an inhibitory histone modification H3K27me3 (Diallo et al., 2012, Sheldon et al., 2009) and only one functional copy of the VRN1 gene can produce a normal phenotype. Studies have also shown that VRN1 expression can hamper the cold tolerance capacity by inhibiting the expression of some cold-resistant marker genes such as CBFs, FR2, and COs (Dhillon et al., 2010). It was also evident that winter cereals possess cold tolerance during the vegetative growth phase but that it is lost in the reproductive phase, although the precise mechanism remains elusive. Again, miR156 is particularly regulated under cold stress and delays flowering by inhibiting its target SQUAMOSA PROMOTER BINDING PROTEIN-LIKE3 (SPL3), which is a floral inducer at low temperature (Kim et al., 2012).
7 EFFECT OF SUGAR IN FLORAL INDUCTION UNDER STRESS CONDITION
Plants not only need sugar as their carbon source, but recent evidence suggests that this can help the plant withstand stress conditions and affect certain developmental processes, including floral induction (Yoon et al., 2021). A sharp increase in the sucrose level prior to floral induction has been reported in many plants, including Sinapis alba (Bodson and Outlaw, 1985), and Xanthium strumarium (Houssa et al., 1991). Being the main photosynthetic product, sucrose needs to maintain a balance between its components with the help of invertase and sucrose mobilizing enzymes. Under stress conditions, this well-established balance is hampered, which then affects the central circadian clock that consists of many important regulatory genes such as GI and, to a lesser extent, CIRCADIAN CLOCK ASSOCIATED 1 (CCA1) and TIMING OF CAB EXPRESSION (TOC1). These genes further affect the activity of the aforementioned enzymes (Bolouri Moghaddam and Van den Ende, 2013). Exogenous application of sucrose induces flowering in a non-inductive condition. In Brassica campestris (Friend et al., 1984) and Sinapis alba, flowering was induced under LD conditions upon sucrose treatment. In Arabidopsis, the late flowering phenotype caused by mutation in CO, GI, FVE, and FPA can be extricated by growing the plant in high-concentration sucrose-containing media (Roldán et al., 1999). Sucrose can be sensed by several identified sensors in many plants like patatin-1 in potato (Jefferson et al., 1990), SUCROSE TRANSPORTER 2 (SUT2) in Arabidopsis (Barker et al., 2000), Shrunken in maize etc. In Arabidopsis, one transcription factor, INDETERMINATE DOMAIN8 (IDD8), promotes flowering under normal conditions by upregulating certain sucrose synthase genes such as SUCROSE SYNTHASE4 (SUS4) (Seo et al., 2011). Studies in rice have shown that some abiotic stress conditions, such as heat and drought, result in the overexpression of sugar transporters, which ultimately rescues the plant from carbon starvation (Li et al., 2015). Sucrose promotes flowering in Brassica sp, Vitis sp., and Arabidopsis by acting downstream of CO and upstream of the FT gene when GI is present in its active form (Tognetti et al., 2013). A growing body of studies has shown that an abruptly high concentration of sucrose can delay flowering by extending the vegetative phase and inactivating the FT gene (Ohto et al., 2001). In Arabidopsis, KIN10 and KIN11 are protein kinase that makes the plant more tolerant to carbohydrate stress conditions by inducing catabolism and suppressing anabolism of sugar molecules and, along with that delays the floral induction (Baena-González et al., 2007). An expression analysis through qRT-PCR reveals that exogenous application of another disaccharide, namely Trehalose 6 phosphate, a close relative of sucrose, can induce the floral transition and also increase the level of FT gene expression (Wahl et al., 2013). Further increase in the sugar content after germination gradually decreases the expression level of miR156, which promotes adult traits (Yu et al., 2013, Yang et al., 2013). Exogenous application of sucrose promotes the expression of miR172, which causes early flowering by repressing the expression of the AP2-like transcription factor (Garg et al., 2021).
8 EFFECT OF SOME GASEOUS MOLECULES AND ANTIOXIDANTS ON THE REGULATION OF FLOWERING TIME
ROS is generated in response to stress conditions. Some antioxidants, such as glutathione (GSH) and ascorbic acid (vitamin C), affect floral induction under stress conditions. Along with that, nitric oxide (NO), nitrogen dioxide (NO2), carbon dioxide (CO2), and some heavy metals affect the transition. Exogenous application of glutathione inhibits the process of flowering under stress conditions, but simultaneous use of one GSH Inhibitor (L-buthionine sulfoxamine) promotes flowering (Ogawa et al., 2001). Ascorbic acid, a well-known co-factor for many antioxidants, delays flowering under LD but shows an opposite response under SD in Brassica sp. when exogenously applied (Daniela and De Tullio, 2007). Metal toxicity and accumulation beyond threshold value not only cause severe yield loss but also hampers normal growth and development. Higher amounts of copper accumulation in the soil delay floral induction (Brun et al., 2003). Some gaseous molecules alter the flowering process when present beyond its optimum level. High levels of CO2 in the atmosphere elevate the rate of photosynthesis and decrease photosynthetic capacity, thereby increasing the amount of sugar, which then affects the process of flowering (Albert et al., 2011). Studies on rice under elevated CO2 promote flowering (Jagadish et al., 2016) but delay it in Sorghum bicolor (Ellis et al., 1995) and Arabidopsis by activating flowering repressor genes FLC (Springer and Ward, 2007). CO2-mediated floral induction is also regulated by two miRNAs, miR156 and miR172, whose expression was downregulated under CO2-rich conditions (Figure 2) (May et al., 2013). NO inhibits the expression of flowering initiator genes and hinders floral induction (He et al., 2004), whereas exogenous application of NO2 promotes flowering in Arabidopsis thaliana (Takahashi and Morikawa, 2014).
9 ROLE OF MACRONUTRIENT ON FLORAL INDUCTION
Optimum nutrient level is another important factor that leads to better growth and helps maintain several physiological processes, including flowering. In the case of Landsberg erecta, it was proven that poor nutrition delays blooming (Tienderen et al., 1996), whereas another study reveals that Lansberg ercta blooms faster under low mineral nutrition availability (Kolár and Senková, 2008). Research on Pharbitis, an SD plant, has shown that nutrient scarcity induces flowering by upregulating the FT2 gene, an ortholog to the FLT gene in Arabidopsis, and hampers vegetative growth (Wada and Takeno, 2010). Nitrogen availability is also a determining factor in floral transition. Studies have shown that nitrogen-deprived conditions can both induce and delay the process of flowering in Arabidopsis and rice by affecting the expression of FT, CO, FLC and SOC1 genes (Lin and Tsay, 2017, Zhang et al., 2021). In Fagus crenata, higher amounts of nitrogen induce flowering (Miyazaki et al., 2014), whereas in Arabidopsis, low levels of nitrogen promote flowering (Castro Marín et al., 2011) Phosphorus is an essential macronutrient, and it has an antagonistic relationship with nitrogen (Kant et al., 2011b) Protein phosphorylation, which regulates floral induction, is greatly affected under nitrogen stress conditions. It was evident that the kinase activity of the SNF-related kinase (SnRK1) was reduced under low nitrogen conditions, which reduces the phosphorylation of its target BHLH4 gene. This, in turn, promotes the expression of FT and CO genes (Figure 2) (Sanagi et al., 2021). It was evident that phosphate (Pi) levels are upregulated under nitrogen-deprived conditions. Interestingly, these two minerals affect the floral induction in a similar pattern in Arabidopsis, where low nitrate levels induce flowering, but low phosphate hinders the process (Kant et al., 2011a). Similar results have been found in Trifolium sp. (ROSSITER, 1978), where low levels of phosphate delay flowering. Additionally, different amino acids, mainly glutamate and aspartic acid induce flowering in Lemna paucicostata (duckweed) even if they are present in minute concentrations in the nutrient solution (Khurana et al., 1988).
10 EFFECT OF VARIOUS BIOTIC STRESSES ON FLOWERING
10.1 Role of microbes in flowering
Nonpathogenic soil microbes, rhizosphere bacteria and plant growth-promoting bacteria play a pivotal role in promoting early flowering. Recent reports revealed that nonpathogenic soil microorganisms help plants reproduce by accelerating flowering under drought conditions (Jennifer A. Laua, 2012). Studies in Brassica repa indicated that plants grown in the presence of simple soil microbial communities reduce flowering while complex microbial associations enhance flowering (Lau and Lennon, 2011). A similar type of work has been done in a wild relative of Arabidopsis, Boechera stricta, elucidating the effect of soil microbiomes on phenotypic plasticity of floral timing (Wagner et al., 2014). (Panke-Buisse et al., 2015) reported that the whole ambit of floral induction was significantly regulated by soil microbiomes. It was shown that Arabidopsis and B. rapa inoculated with early flowering microbiomes (EF) such as cellulomonadaceae, clostridiaceae, and xanthomonadaceae promote flowering and late flowering microbiomes (LF) like Alcaligenaceae, Caldilineaceae, Corynebacteriaceae delays flowering with increase in reproductive biomass. Recently, in a report, it was found that the rhizospheric microorganism Arthrobacter promotes plant vegetative growth and delays flowering through the production of indole acetic acid (IAA) hormone from tryptophan (Li et al., 2018). Furthermore, the molecular pathway behind delayed flowering reveals that the microbiota produces (IAA) in rhizospheric soil, which changes the transcription of several floral integrator genes and significantly regulates the GA-mediated vernalization pathway (Tao Lu1†, 2018). Additionally, some rhizospheric bacteria such as Burkholderia, Mucilaginibacter, and Flavisolibactor overpopulate in the mutant plant (szl-1) of Arabidopsis lacking root secreted loliolide, accelerating flowering. In this context, wild type plants with root-secreted loliolide shows delayed flowering (Xin Chen, 2023). Another piece of evidence suggests that Arabidopsis plants treated with Burkholderia phytofirmans upregulate the synthesis of the GA3ox1 gene that plays a key role in producing bioactive GA during vegetative growth and thus promotes early flowering time (Figure 3).
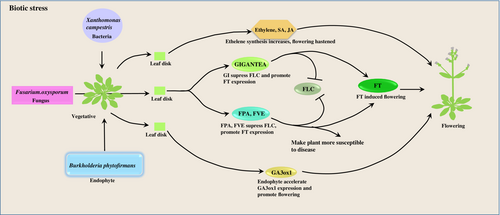
Fusarium oxysporium upregulates GI, FPA and FVE expression in Arabidopsis thaliana which further inhibit FLC expression and promotes FT induced flowering. FPA, FVE make plant more susceptible to disease. Xanthomonas campestris induced stress hormone like jasmonic acid, ethelene and salicylic acid accumulation and promote flowering. Endophyte Burkholderia phytofirmans promote flowering through the upregulation of GA3ox1 gene.
GIGANTEA- GI, FLOWERING LOCUS C- FLC, FLOWERING LOCUS T- FT.
10.2 Bacterial-induced flowering
Plants are sessile organisms that withstand several biotic stresses, which include bacterial infection. They can perceive the presence of pathogens in their environment and alter floral timing accordingly. This strategy either makes the plant susceptible to the infection, which promotes early flowering, or resistant to the infection, which delays the flowering time. Studies on Arabidopsis thaliana revealed that early flowering and reduced aerial branches on the inflorescence were observed upon infection with two bacterial species, namely Xanthomonas campestris, Pseudomonas syringae and an oomycetes Peronospora parasitica. Additionally, plants have accumulated several stress hormones such as salicylic acid, jasmonic acid and ethylene during pathogen infection, which triggers early flowering (Figure 3) (Korves and Bergelson, 2003).
10.3 Fungi-mediated early flowering
Fusarium oxysporum is a soil-borne fungal pathogen that causes severe wilt diseases in Arabidopsis thaliana. Previous literature has revealed that there is a correlation between fungal stress and flowering time. F. oxysporum infection promotes flowering by suppressing the expression of FLC and upregulating the FT gene (Lyons et al., 2015). Further, the gi mutant plant showed resistance upon infection and delayed flowering. On the other hand, upon infection in wild type plants, GI expression was enhanced, resulting in the plants being more susceptible to pathogen attack and accelerating flowering (Kundu and Sahu, 2021). Additionally, two genes of the autonomous pathway, namely FPA and FVE, make the plants susceptible to F. oxysporum infection and promote flowering through the suppression of the FLC gene and upregulation of the FT gene (Figure 3). In this context, it was also known that mutant fve and fpa plant becomes resistant to the fungal infection and results delayed flowering (Lyons et al., 2015). Trichoderma is a saprophytic fungus that colonizes the roots of plants (Prisa, 2012). Andrzejak et al. reported that an ornamental plant (Picotee Sunburst) was treated with Trichoderma spp. shows accelerated flowering (Andrzejak et al., 2021). Infection of Trichoderma results in early flowering in Gladiolus hybridus (Andrzejak and Janowska, 2022). Additionally, the inoculation of Trichoderma spp. This results in early flowering in Freesia refracta (Argentea) when the plant is underexposed to light. Moreover, it was observed that Trichoderma spp. enhances the potassium and calcium uptake, which are important for plants to mitigate many physiological activities like flowering (Janowska et al., 2020).
11 HERBIVORY
Different herbivores such as Pieris brassicae, Spodoptera and different aphids alter flowering in plants. Previous literature reports that Pieris infested plant promotes early flowering and increase the attractiveness of flowers to pollinators (Lucas-Barbosa et al., 2011), however, Spodoptera littoralis-infested plants accumulate a significant amount of glucobrassicanapin in their leaves and flowers which activate the plant indirect defence (parasitoid attraction) system and showed delayed flowering (Schiestl et al., 2014). Previous studies have also shown that Myzus persicae (green peach aphid) in Arabidopsis and Spodoptera littoralis (African cotton leaf worm) in Brasicca repa delays flowering (Züst et al., 2011). Plants can also prevent insect-mediated herbivory by delayed flowering, as reported in evening prime rose, which avoids the attack of Mampha brevivittella (Agrawal et al., 2013). Another study revealed that herbivory accelerates flowering and induces early seed production in Brassica nigra (Lucas-Barbosa et al., 2013). Further, it was evident that herbivore infestation in early developmental stages of a plant had a greater effect on inducing flowering phenology and reduced vegetative growth compared to the late developmental stage infested plant, as seen in Sinapis arvensis (Hoffmeister et al., 2016). Additionally, a recent report elucidates that late flowering plant Cardamine hirsute produces glucosinolates and other stress-related phytohormone to protect them against herbivores, which further alter the expression of main floral repressor gene FLC and delayed flowering (Rasmann et al., 2018). Moreover, Bustos-Segura et al. (2021) reported that herbivore (Leaf beetles) promotes early flowering and reduces the number of seeds in wild lima bean plants (Phaseolus lunatus).
12 COORDINATION BETWEEN FLOWERING AND POLLINATORS UNDER STRESS CONDITION
Pollinators are key factors that play an important role in plant reproduction. Flowering plants mainly depend on pollinators for their fertilization, and the majority of pollinators depend on plants for their food requirement, which portrays a mutual relationship between plants and pollinators. Additionally, pollinators help plants for their fertilization and genetic exchange; on the other hand, plants provide nectar and food for the pollinators' survival. In this way, plants and pollinators maintain a balance in the ecosystem. This mutual relationship is not maintained in many plant species due to environmental changes and flowering time alteration. It is an important reason which reduces the reproduction success and decreases seed set. Further, it is responsible for the food shortage of pollinators (Giannini et al., 2020, Richman et al., 2020, Whitney et al., 2008).
13 CONCLUSION
The rise in global population and fixed amount of cultivable land poses a challenge to the scientific community because of the increasing food demand that should be achieved within a short period (Kazan and Lyons, 2016). Flowering time is an important trait to fulfil the higher requirement of crop yield (Gao et al., 2014), as 60% of global food production is dependent on successful seed set and grain filling. Moreover, Majority of the mechanisms influencing the floral transition have been studied in Arabidopsis, and it can be concluded that the genetic mechanism underlying stress response in conjugation with flowering time is species-specific (Jung and Müller, 2009). Additionally, stress-induced flowering is not only species-specific but also depends on the timing of the stress. Mild stress delays flowering, whereas terminal stress accelerates it to ensure reproduction. In most cases, plants either avoid, tolerate or endure the abiotic or biotic stress condition to achieve reproductive success. An intricate interaction between the floral integrator genes and stress response pathway components at the transcriptional level regulates the whole ambit of the flowering process. Therefore, more research needs to be done in order to understand the link between crop yield and stress tolerance.
AUTHOR CONTRIBUTIONS
AM conceived the idea. DC and SP drafted the manuscript. All the authors read and agreed unanimously.
FUNDING INFORMATION
This work is supported by SERB-DST(SRG/2020/001618), University of Calcutta, for providing the basic infrastructure, DC acknowledges DST-INSPIRE Government of India for providing the fellowship (DST/INSPIRE Fellowship/2021/IF210466). SP acknowledges UGC government of India for providing fellowship (File no. 191620011821).
CONFLICTS OF INTEREST STATEMENT
The authors declare there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




