Transcriptomics and metabolomics revealed the synthesis and potential role of flavonoids in pea roots under continuous cropping obstacles
Abstract
With the continuation of intensive and monoculture production in modern agriculture, the harm of continuous cropping obstacles is becoming more prominent. Pea has important nutritional and economic value, but it is easy to have continuous cropping obstacles in production. However, there is limited knowledge of the regulatory mechanisms of pea to cope with continuous cropping obstacles. In this study, we found that the number of differential expressed genes (DEGs) and differential metabolites (DAMs) increased in the pea roots with increasing continuous cropping times, and the number of DEGs and DAMs in roots of sensitive pea was more than that of continuous cropping tolerant pea. Comprehensive analysis of the omics data revealed that the flavonoid and isoflavonoid biosynthesis pathways play key roles in the response of pea roots to the continuous cropping obstacles. Most of the DEGs involved in these two pathways were up-regulated. Meanwhile, most of the flavonoid compounds and total flavonoid content increased. With increasing continuous cropping times, the isoflavones category in DAMs increased, and the isoflavones category in the roots of continuous cropping tolerant pea were higher than in sensitive pea. Additionally, the isoflavonoid (biochanin A, calycosin, genistein) in the roots of continuous cropping tolerant pea have the ability to inhibit the growth of fungi in pea soil and possess antioxidant activity. These findings revealed the important role of flavonoids in pea continuous cropping obstacles and laid a foundation for effectively alleviating pea continuous cropping obstacles in the future.
1 INTRODUCTION
Pea (Pisum sativum L.) is a legume crop used for grain, vegetable and fodder production. It is rich in various nutrients such as fiber, protein, lipids, minerals and vitamins (Fahmi et al., 2019; Kumari & Deka 2021). In addition, polyphenols such as flavonoids and tannins are also important bioactive components in peas (Devi et al., 2019; Han et al., 2023). Therefore, peas have important nutritional and economic value and are widely cultivated worldwide (Kour et al., 2020). In recent years, a better understanding of the nutritional value of peas has led to an increased demand in the market (Raghunathan et al., 2017). Although China is one of the largest pea producers in the world, due to limited land resources and continuous cropping, it is easy to cause continuous cropping obstacles and reduce pea production (Nayyar et al., 2009), resulting in the need to import to meet market demand (Hao et al., 2022). Therefore, to increase pea production under limited arable land is crucial for the sustainability of the industry.
Due to the limitation of arable land, continuous cropping has become a common cropping pattern in modern intensive and large-scale agricultural production (Tan et al., 2021). According to statistics, by the end of 2013, about 95% of China's 1.3 million hectares of arable land was under continuous cropping (Tan et al., 2021). After years of continuous cropping, obstacles may occur, even with good field management, and may lead to slow growth and development of crops, as well as lower yield and quality (Tan et al., 2021). This is related to the degradation of the soil environment after continuous cropping, including soil nutrient depletion, accumulation of autotoxins, and changes in the structure of the soil microbial community (Ma et al., 2023). Studies have confirmed that pea plants in continuous cropping systems have stunted growth and reduced biomass (Ma et al., 2026; Wang et al., 2021; Yang et al., 2020; Zhang et al., 2021). This phenomenon is closely related to the alteration of the soil microbiota. When exposed to adverse stress, the plant mitigates the damage caused by adversity by regulating the synthesis and accumulation of metabolites (Ma et al., 2020). The mechanisms by which pea plants regulate their metabolic activities to adapt to the continuous cropping system require further investigation.
Plant-microbe interactions dynamically influence plant growth, development, and health. The mechanisms of their association are largely mediated by specific host-derived secondary metabolites (Wang et al., 2022). Flavonoids are a very abundant and widely studied group of secondary metabolites in plants (Shen et al., 2022). Flavonoid biosynthesis occurs at the intersection of the shikimate and the acetic acid pathways, and its initial product, phenylalanine, undergoes a series of enzyme-catalyzed reactions, such as phenylalanine ammonia-lyase (PAL) (EC:4.3.1.24), chalcone synthase (CHS) (EC:2.3.1.74), chalcone isomerase (CHI) (EC:5.5.1.6), resulting in the formation of various forms of flavonoid compounds (Shen et al., 2022). After synthesis, these compounds are mainly transported to vacuoles for storage, some can be transported to the cell compartment or extracellular, and some are secreted into the rhizosphere through the root system (Wang et al., 2022). These substances play an important role in the response of plants to biotic stresses. Through transcriptomics and metabolomics, Long et al. (2019) found that the accumulation of flavonoids increased the resistance of cotton (Gossypium spp.) mutants to Verticillium dahlia. Bai et al. (2020) transgenically increased proanthocyanidins, anthocyanins, and flavonols in poplar and improved resistance of Populus to Botrytis cinerea and Dothiorella gregaria. Other studies have shown that down-regulation of genes in the flavonoid synthesis pathway and a decrease in their content increased the incidence of pathogen infection in barley (Hordeum vulgare L.) (Karre et al., 2019). This suggests that flavonoids may enhance plant resistance to biotic stresses by inhibiting pathogens. In addition, flavonoids may reduce plant damage in adversity by inhibiting the accumulation of reactive oxygen species (ROS) (Li et al., 2017).
Currently, there is limited research on the stress response and regulatory mechanisms of pea plants in continuous cropping environments. The response of pea root flavonoids to continuous cropping stress remains to be investigated. To this end, this study analyzed the transcriptomics and metabolomics of pea roots from two genotypes under three treatments to reveal possible molecular mechanisms of flavonoids in pea roots in response to continuous cropping. In addition, through the antimicrobial test and antioxidant capacity measurement, the potential mechanism of the action of flavonoid compounds in pea roots was clarified to provide theoretical references for the future alleviation of pea continuous cropping obstacles.
2 MATERIALS AND METHODS
2.1 Plant materials and treatment
The soil for the experiment was collected from Yuhe Village, Fengxiang Town, Anding District, Dingxi City, Gansu Province, in China, and the cultivation history of the plot is shown in Figure 1. The soil collection consisted of soil not planted (RT) with two genotypes of pea (continuous cropping sensitive: Ding wan 10 (D10); continuous cropping tolerant: Yun wan 8 (Y8)), continuous cropping once treatment (CC1) and continuous cropping twice treatment (CC2). Soil from each plot was mixed after soil collection and dried naturally. Soil from different treatments was packed in equal amounts in polyethylene pots of 31 cm height and 27.5 cm diameter, with 4 kg soil per pot, and infiltrated by pouring 500 mL of water and left for use. Pea seeds of both genotypes were sterilized with 75% alcohol and 5% sodium hypochlorite and placed in petri dishes lined with two layers of filter paper for germination. Pea seeds with uniform germination were selected and sown in different soil treatments, 15 pea seeds per pot at 3–4 cm depth, with 9 replicates per treatment (Figure 1). Watering was done every 3 days during the growth period.
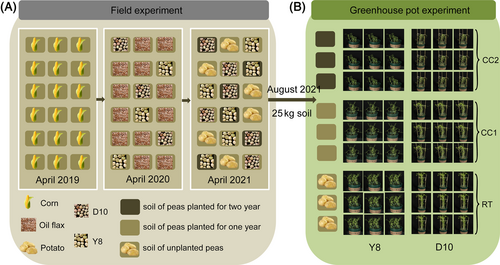
Plant samples were collected when pea plants were 16 days (d). Three biological samples were randomly collected from each treatment, snap-frozen in liquid nitrogen and stored at −80°C for transcriptomic and metabolomic analysis.
2.2 Metabolomics analysis
Three biological replicate samples for each RT, CC1, and CC2 treatment were analyzed by LC–MS/MS for metabolomics. Samples were lyophilized, ground, and centrifuged prior to LC–MS/MS analysis. Data acquisition systems included ultra-performance liquid chromatography (UPLC) and tandem mass spectrometry (MS/MS). UPLC analysis conditions: The chromatographic column was Agilent SB-C18 (1.8 μm, 2.1 mm ⤫ 100 mm). Mobile phase: phase A is ultrapure water (with 0.1% formic acid added), phase B is acetonitrile (with 0.1% formic acid added); elution gradient: 0.00 min B-phase ratio was 5%, the B-phase ratio increased linearly to 95% within 9.00 min and maintained at 95% for 1 min, from 10.00–11.10 min, the B-phase ratio was decreased to 5% and equilibrated at 5% for 14 min; flow rate was 0.35 mL·min−1; column temperature was 40°C; injection volume was 4 μL.
The repeatability between samples was evaluated by PCoA and Pearson correlation analysis. The significance (p-value) of the difference between the compounds was calculated using a t-test. Differential accumulation metabolites (Demmig-Adams et al., 2018) were identified using a combination of fold change (FC), p-value and VIP value of the OPLS-DA model. Screening criteria were FC >1, p < 0.05, VIP >1. Metabolite analysis was performed using BMK Cloud (http://www.biocloud.net/).
2.3 Transcriptome analysis
RNA was extracted from RT, CC1, and CC2-treated samples using an RNA extraction kit (Tiangen Biotechnology Co., Ltd.). RNA concentration, purity and integrity were determined using Nanodrop 2000 (ThermoFisher Scientific.) and Agient 2100 (Agilent Technologies). After mRNA purification, cDNA was synthesized, purified and ligated, and the sequencing library was constructed by PCR enrichment. The quality of the library was evaluated using Agilent Bioanalyzer 2100 (Agilent Technologies) (effective concentration of the library >2 nM). Libraries were sequenced on the Illumina platform. Low-quality sequencing data from the raw data were filtered to obtain clean data and compared to the pea reference genome (https://urgi.versailles.inra.fr/Species/Pisum). Gene expression levels were estimated using the fragments per kilobase of transcript per million mapped reads (FPKM) metric. Differential expression gene (DEG) screening was based on the criteria of |FC| > 1.5 and false discovery rate (FDR) < 0.05. Statistical enrichment of DEGs in the KEGG pathway was performed using KOBAS (Kanehisa & Goto 2000; Mao et al., 2005).
2.4 Determination of total flavonoids in pea roots
The pea root samples under different treatments were placed in an oven at constant temperature until the weight was constant. After pulverization, about 0.02 g of sample was weighed, 2 mL of 60% ethanol was added, and the sample was extracted by shaking at 60°C for 2 h. Then, the sample was centrifuged for 10 min (10000 g, 25°C), and the supernatant was extracted, detected by a flavonoid kit (Suzhou Keming Biotechnology Co.), and the content of flavonoids was calculated.
2.5 Antimicrobial effects of key isoflavonoid metabolites
The key isoflavonoid metabolites biochanin A, calycosin, and genistein (Yuanye Biotechnology technology company) screened by transcriptomics and metabolomics was dissolved in dimethyl sulfoxide and configured into 0.313, 0.625, 1.25, 2.5, 5, and 10 mg mL−1 solutions, respectively. The number of culturable bacteria and fungi in potting soil under RT and CC2 treatments was determined by the dilution plate plating method (Chen et al., 2023). Beef paste peptone medium was used for bacteria, and potato dextrose agar medium was used for fungi (Wang et al., 2015). The experimental method was as shown in Figure S1, 10 g of fresh soil under different treatments was weighed, and soil extract was prepared with sterile water and diluted to different concentrations. The 10−4 extract was applied to the bacterial solid medium, and the 10−3 extract was applied to the fungal medium, with a total of nine replicates for each treatment. The medium was placed in a constant temperature incubator for inverted incubation (bacteria: 37°C, fungi: 28°C) for 3 d, and after 5 d, the number of bacteria and fungi were counted, respectively (Mo et al., 2022).
Bacteria and fungi cultured under CC2 treatment were inoculated into the corresponding liquid medium respectively, placed in a constant temperature shaker and cultured until the bacterial and fungi concentration reached 106 CUF mL−1 (Shao et al., 2023), and 0.1 mL of different bacterial solutions were aspirated and applied to the solid medium of the corresponding bacteria and fungi. Sterile drug-sensitized tablets were immersed in different concentrations of biochanin A, calycosin, genistein solution for 2 h and placed at regular intervals on plates coated with microorganisms and placed in a thermostat for incubation. The bacteria were incubated at 37°C for 24 h, and the fungi were incubated at 28°C for 48 h (Chen et al., 2023). It was observed whether the ring of inhibition was produced or not.
2.6 Determination of DPPH (1,1-Diphenyl-2-picrylhydrazyl) free radical scavenging rate
DPPH (Yuanye Biotechnology Technology Company) was determined by slightly modifying the method of Meng et al. (2023) and Chen et al. (2023). DPPH was configured with dimethyl sulfoxide into a solution of 0.5 mmol L−1. Biochanin A, calycosin, genistein were configured with dimethyl sulfoxide into solutions with concentrations of 0.0095, 0.0195, 0.039, 0.078, 0.156, 0.313, 0.625, 1.25, 2.5, 5, 10 mg mL−1 respectively. Different concentrations of biochanin A, calycosin, genistein solution 100 uL and 0.5 mmol L−1 DPPH master batch 100 uL were added into 96 well enzyme labelling plate, and the absorbance values at 517 nm were measured after storing at 25°C for 30 min away from light. The control was Vc standard solution at the same concentration.
2.7 Data analysis
Duncan's statistical analysis was performed using SPSS 22.0 software (IBM Corporation). Data were statistically significant at the p < 0.05 level, and results were expressed as mean ± SD. Tables and bar graphs were generated using Microsoft Excel 2010 (Microsoft Corporation) and Origin 9 (OriginLab Corporation), and heat maps were generated using TBtools.
3 RESULTS
3.1 Characterization of phenotypic changes in pea plants under continuous cropping conditions
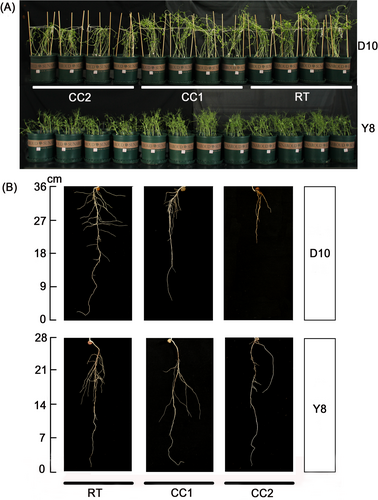
The effect of continuous cropping on pea phenotype varied with continuous cropping times and pea genotypes. As can be seen from the aboveground, the growth of two genotypes of peas was inhibited by CC2 treatment, especially in D10, where the aboveground part of the pea plants wilted significantly (Figure 2A). In contrast, Y8 growth was inhibited but without significant wilting (Figure 2A). By comparing the height and biomass of pea plants under different treatments, it was found that continuous cropping inhibited their growth, and the degree of inhibition was exacerbated under CC2 treatment (Table S1). By observing the roots, it was found that the root length of D10 was inhibited with the increase of continuous cropping times and the roots of the pea were significantly aged under CC2 treatment (Figure 2B). By analyzing the root length and root biomass, it was also found that continuous cropping inhibited root elongation and biomass formation, especially under the CC2 treatment (Table S1). In addition, compared with RT, the effect of continuous cropping on Y8 roots was less than that of D10.
3.2 Metabolomic analysis of pea roots under continuous cropping conditions
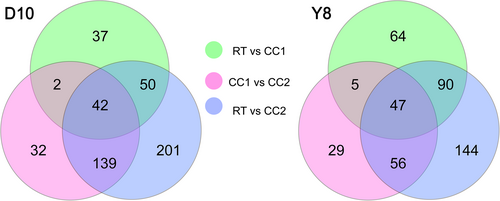
Through non-targeted metabolomics analysis, 1001 and 1000 metabolites were identified in the roots of D10 and Y8, respectively. PCoA analysis showed that the first principal components of D10 and Y8 could explain 72.23 and 63.78% of the total variance, respectively, and there was a significant separation between the samples of different continuous cropping treatments (Figure S2A, C). The correlation coefficients between the samples from the continuous cropping treatments and the RT samples were all lower than those of the three biological replicates of RT, and the correlation coefficients between the samples from the continuous cropping treatments and the RT samples decreased as the continuous cropping times increased (Figure S2B, D). Compared with RT, the number of DAMs in the roots of the two genotypes of peas under CC2 was higher than that under CC1 (Figure 3).
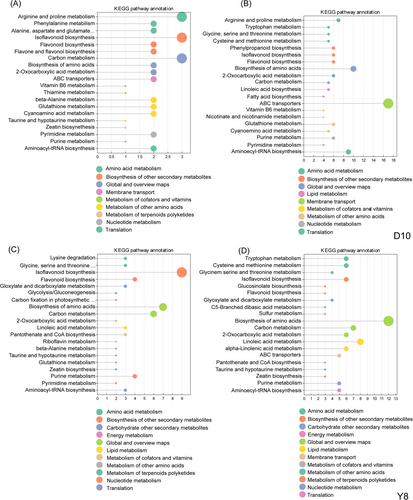
KEGG classification analysis of DAMs showed that in the biosynthesis of other secondary metabolites, CC1 treatment of D10 involved isoflavonoid biosynthesis (Ko 00943), flavonoid biosynthesis (Ko 00941), flavone and flavonol biosynthesis (Ko 00944) (Figure 4A). In D10, CC2 treatment involved phenylpropanoid biosynthesis (Ko 00940)、isoflavonoid biosynthesis (Ko 00943)、flavonoid biosynthesis (Ko 00941) (Figure 4B). The CC1 treatment of Y8 involved isoflavonoid biosynthesis (Ko 00943) and flavonoid biosynthesis (Ko 00941) (Figure 4C). In Y8, CC2 treatment involved isoflavonoid biosynthesis (Ko 00943), flavonoid biosynthesis (Ko 00941), and glucosinolate biosynthesis (Figure 4D). Metabolic pathways related to the synthesis of flavonoids were present in different genotypes of peas under different continuous cropping treatments, suggesting that the pea roots regulate the synthesis of flavonoids as an important strategy in response to continuous cropping.
3.3 Differential analysis of flavonoid accumulation in pea roots under continuous cropping treatments
We further analyzed the flavonoids that were differentially accumulated between treatments. A total of 5 differentially accumulated flavonoids were identified in the CC1 treatment of D10. Two of them were down-regulated, and three were significantly accumulated (Table S2). A total of 11 differentially accumulated flavonoids were identified in the CC2 treatment; 8 were up-regulated, and three were down-regulated (Table S2). A total of 13 differentially accumulated flavonoids were identified in the CC1 treatment of Y8, of which 12 were up-regulated, and one was down-regulated (Table S3). 7 flavonoids were identified in the CC2 treatment, and all were up-regulated (Table S3). This indicated that continuous cropping caused a large amount of flavonoid synthesis in pea roots. In addition, we performed a secondary classification of the different flavonoids and found that the percentage of isoflavones in the D10 roots was 20% under CC1 treatment, whereas 46.15% of isoflavones were found in Y8. Under CC2, the percentage of isoflavones in D10 roots was 45.45%, whereas 57.16% of isoflavones were present in Y8 (Table S2, S3). It was shown that the isoflavonoid species increased with the increase in the number of successive crops. Meanwhile, under different treatments, there were more isoflavone species in Y8 than in D10.
3.4 Response of total flavonoids from pea roots to continuous cropping obstacle
We further determined the total flavonoid content of pea roots under different continuous cropping treatments. It was found that the total flavonoid content of the two genotypes of pea roots increased significantly with the increase of continuous cropping times (Figure 5A, B). In D10, the total flavonoid content increased by 46.99% and 195.86% under CC1 and CC2 treatment, respectively, compared to RT (Figure 5A). In Y8, the total flavonoid content under CC1 and CC2 treatment were 37.52% and 64.85% higher than RT, respectively (Figure 5B). The results indicated that continuous cropping treatment significantly promoted the accumulation of total flavonoid content in pea roots. At the same time, the number of up-regulated flavonoid compounds in metabolomics was more than down-regulated after continuous cropping, which was basically in line with the trend of total flavonoids.
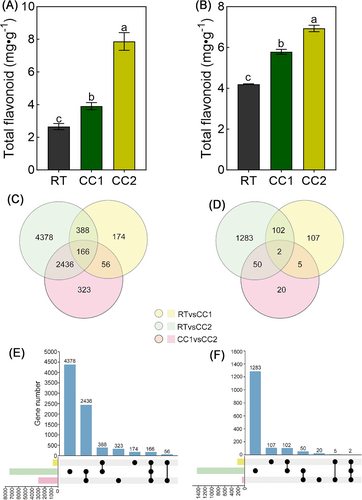
3.5 Transcriptomic sequencing analysis of pea roots under continuous cropping conditions
Differentially expressed genes were identified from the samples under different continuous cropping conditions based on transcriptomics profiling. CC2 treatment had the highest number of differentially expressed genes compared to RT samples, while CC1 treatment had the lowest number of differentially expressed genes compared to RT samples (Figure 5C-F). The alterations in the number of DEGs highlight adjustments in the transcript levels within the pea roots. These adjustments imply that continuous cropping is a primary factor driving these changes.
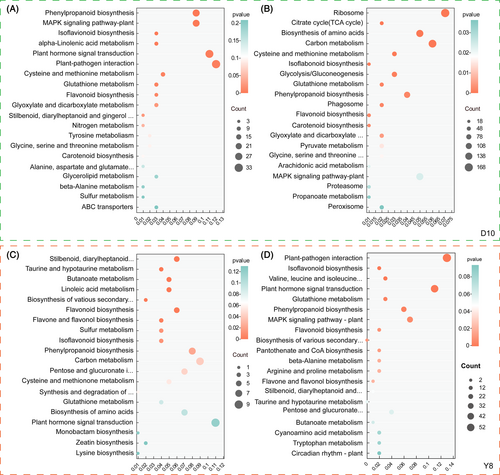
In order to clarify the effect of continuous cropping on the important metabolic pathways at the transcriptional level of pea roots, we performed KEGG enrichment analysis on DEGs. There are metabolic pathways that respond specifically to different continuous cropping treatments, but there are also many pathways that are identical. These identical metabolic pathways may be the main drivers of pea root response to continuous cropping. Among them, phenylpropanoid biosynthesis (Ko 00940), isoflavonoid biosynthesis (Ko 00943), and flavonoid biosynthesis (Ko 00941) were significantly enriched under different continuous cropping treatments of D10 (Figure 6A, B). These metabolic pathways were also significantly enriched in the CC1 treatment of Y8, with the addition of the flavone and flavonol biosynthesis pathway (Ko 00944) in the CC2 treatment (Figure 6C, D). The results show that the changes at the transcriptome level are related to flavonoid synthesis metabolic pathways and associated with the observed changes in flavonoid levels.
3.6 Analysis of DEGs in the flavonoid biosynthesis pathway
To further understand the molecular mechanism of flavonoid biosynthesis in the roots of continuous cropping peas, we examined the specific expression patterns of genes involved in the isoflavonoid biosynthesis (Ko 00943) and flavonoid biosynthesis (Ko 00941) pathways. In D10, a total of 9 DEGs between CC1 and RT treatment were identified. 7 of them were up-regulated, and 2 were down-regulated. 62 DEGs were identified between CC2 and RT treatment, 55 of them were up-regulated, and 7 were down-regulated (Table S4). In Y8, a total of 8 DEGs were identified between CC1 and RT treatment, of which 7 were up-regulated and 1 was down-regulated. A total of 21 DEGs were identified between CC2 and RT treatment, of which 18 were up-regulated, and 3 were down-regulated (Table S5). It indicated that with the increase of continuous cropping times, the number of DEGs related to the isoflavonoid biosynthesis pathway (Ko 00943) and flavonoid biosynthesis pathway (Ko 00941) in the roots of different genotypes of peas increased, and a large number of DEGs were up-regulated. These up-regulated DEGs may actively promote the synthesis and accumulation of flavonoids. In addition, we found 1 new annotated DEGs in CC1 treatment of D10 and 14 new annotated DEGs in CC2 treatment. 2 new annotated DEGs were found in both CC1 and CC2 treatment of Y8. These newly annotated DEGs may be useful for studying isoflavonoid biosynthesis and flavonoid biosynthesis in pea roots in continuous cropping.
3.7 Combined analysis of transcriptomics and metabolomics in pea roots under continuous cropping conditions
To clarify the relationship between DAMs and DEGs, we used correlation analysis to perform a joint analysis of transcriptomics and metabolomics data (R2 > 0.8, or < −0.8 and p < 0.05). The results showed that the flavonoid biosynthesis pathway (Ko 00941) and the isoflavone biosynthesis pathway (Ko 00943) linked the transcriptome and metabolome when comparing CC1 and RT treatment, CC2 and RT treatment of the two genotype peas (Table S6, S7). The continuous cropping obstacles mainly caused changes in flavonoid-related genes and metabolites in the pea roots, further illustrating the important role of flavonoids in the pea roots in response to continuous cropping. Therefore, the above metabolic pathways are further analyzed below.
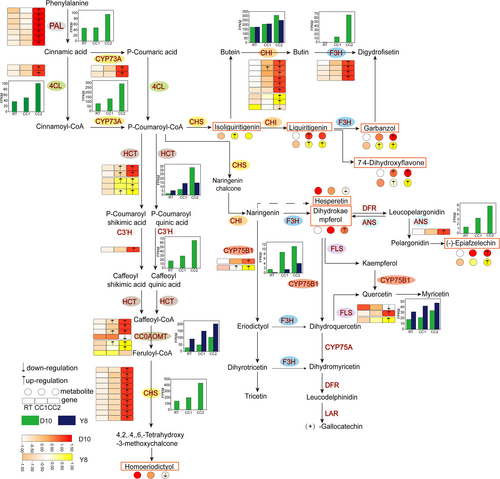
In the flavonoid biosynthesis pathway (Figure 7), D10 was significantly up-regulated in the expression of HCT, CHI, and CCoAOMT under CC1 treatment, and the expression of the remaining genes involved in the flavonoid biosynthesis pathway were not significantly different. The types and numbers of genes involved in flavonoid synthesis were increased under CC2 treatment, and the differences were significant compared with RT treatment. Continuous cropping increased the levels of liquiritigenin, garbanzol, dihydrokaempferol, epiafzelechin, and 7,4′-dihydroxyflavone in D10 and differed significantly from RT under CC2 treatment (except for liquiritigenin). The genes PAL, 4CL, CYP73A, CHS, CHI, ANS and F3H involved in the synthesis of these substances were up-regulated under CC2 treatment. In addition, continuous cropping inhibited the levels of hesperetin and homoeriodictyol in D10, and the degree of inhibition increased with the increase of continuous cropping times. However, the genes related to their synthesis, PAL, 4CL, CYP73A, HCT, C3'H, and CHS, were up-regulated, while among the genes encoding CCOAOMT, three were upregulated and one was down-regulated. The expression of HCT, CCOAOMT, CYP75B1, CHI, and FLS in the flavonoid synthesis pathway increased with the continuous cropping times in Y8. Among them, HCT and CYP75B1 were significantly up-regulated under CC1 treatment. The levels of the metabolites isoliquiritigenin, liquiritigenin, garbanzol, and 7,4′-dihydroxyflavone were significantly increased in CC1. HCT, CYP75B1, and FLS were significantly up-regulated for expression under CC2 treatment. Two of the three genes encoding CHI and CCOAOMT were significantly up-regulated expression, and the levels of metabolites liquiritigenin, 7,4′-dihydroxyflavone, epiafzelechin and garbanzol were significantly increased.
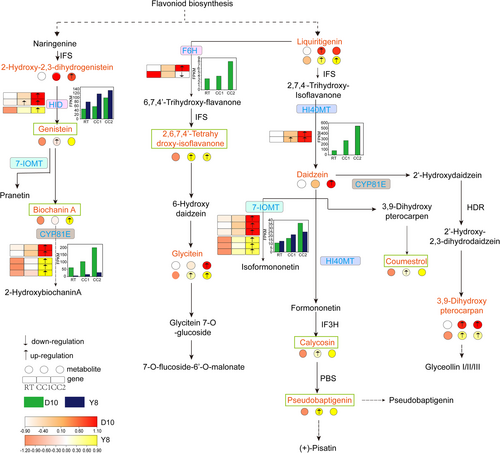
In the isoflavone biosynthesis pathway (Figure 8), the expression levels of HID, 7-IOMT, HI40MT and CYP81E increased under different continuous cropping treatments of D10, especially under CC2 treatment, which was significantly different from RT. At the same time, the metabolic levels of daidzein, 3,9-dihydroxypterocarpan, glycitein and 2-hydroxy-2,3-dihydrogenistein increased and were significantly different from RT under CC2 treatment. In Y8, the expression of HID, 7-IOMT, and CYP81E was enhanced with the increase in the number of continuous cropping and differed significantly from RT under CC2 treatment. At the same time, the metabolic levels of genistein, biochanin A, 3,9-dihydroxypterocarpan, 2,6,7,4′-tetrahydroxy-isoflavanone and glycitein were higher than those of RT under continuous cropping treatment, and the differences were significant under CC2 treatment (except for genistein). In addition, we found that genistein, biochanin A, 2,6,7,4′-tetrahydroxy-isoflacanone, calycosin, pseudobaptigenin, and coumestrol were DAMs only in Y8 and were not differentially expressed in D10. It is suggested that these compounds may be directly related to the ability of pea to tolerate continuous cropping.
In summary, the flavonoid biosynthesis pathway and the isoflavone biosynthesis pathway are the key metabolic pathways in the pea roots in response to continuous cropping. Among them, the metabolites involved in the isoflavone biosynthesis pathway in the roots of the two genotypes had different responses to continuous cropping.
3.8 Validation of the antimicrobial effects of isoflavones
To investigate whether the DAMs of the isoflavonoids screened by transcriptomics and metabolomics, which were only present in Y8, had an antimicrobial effect, we selected genistein, biochanin A and calycosin for antimicrobial assays. We measured the number of bacteria and fungi in two genotypes of peas planting soil under RT and CC2 treatment by plate counting. Among them, the CC2 treatment in D10 had higher bacterial counts in the soil than the RT treatment, but there was no significant difference (Figure S3A). Continuous cropping did not affect the soil bacterial populations in Y8 (Figure S3A). While the number of fungi in the soil of two pea genotypes was significantly higher under CC2 than RT treatment, the number of Y8 fungi was lower than that of D10 (Figure S3A). This indicated that the increase in the number of bacteria and fungi under CC2 treatment may be related to the growth inhibition of pea plants.
To this end, we selected bacteria and fungi cultured under CC2 treatment of D10 to determine the antibacterial effect of biochanin A, calycosin, and genistein. We found that biochanin A showed no inhibitory effect on bacteria at low concentrations (0.313, 0.625, 1.25 mg mL−1) and showed an inhibitory effect when the concentration was increased to 2.5, 5 mg mL−1 (Figure S3B). Different concentrations of biochanin A in fungi showed an obvious antibacterial effect, and the antibacterial effect was the strongest at 0.625 mg mL−1 (Figure S3B). Calycosin concentrations of 0.625,1.25 mg mL−1 showed inhibitory effects on bacteria but were not obvious. When the concentration increased, it did not show an obvious antibacterial effect. When the concentration of calycosin was 2.5, 5, 10 mg mL−1, there was an obvious inhibitory effect on fungi, and with the increase of concentration, the antibacterial effect was enhanced. However, it did not show the effect of inhibiting fungi at low concentrations (Figure S3B). Genistein at a concentration of 1.25, 2.5 mg mL−1, has the effect of inhibiting bacteria. At low concentrations (0.313,0.625,1.25 mg mL−1) have the effect of inhibiting fungi (Figure S3B). This indicated that the antibacterial inhibitory effect of these isoflavones on bacteria was lower than that on fungi, and the inhibitory effect varied with concentration and metabolite type.
3.9 Antioxidant capacity of isoflavones in vitro
DPPH has unpaired electrons that can accept electrons from other substances, thereby reducing free radical production, and is often used to evaluate antioxidant capacity. With the increase in concentration, the scavenging rate of Vc and three isoflavones on DPPH free radical showed an increasing trend (Figure S3C). The concentrations of the three isoflavones between 0.0047 and 0.095 mg mL−1 showed significantly higher scavenging rates of DPPH free radicals than Vc (Figure S3C). When the concentration was greater than or equal to 0.039 mg mL−1, the scavenging rate of Vc on DPPH free radical was significantly higher than that of three isoflavones (Figure S3C). In addition, when the concentration was between 0.0047 and 0.0195 mg mL−1, there was no significant difference in the DPPH free radical scavenging rate of the three isoflavones (Figure S3C). When the concentration was higher than 0.0195 mg mL−1, the DPPH free radical scavenging rate of calycosin was significantly higher than that of biochanin A and genistein (Figure S3C). When the concentration was between 0.039 and 1.25 mg mL−1, the scavenging rate of biochanin A on DPPH free radicals was higher than that of genistein (Figure S3C). When the concentration was higher than 1.25 mg mL−1, the scavenging rate of genistein on DPPH free radical was significantly higher than that of biochanin A (Figure S3C). This indicated that these three isoflavonoids have some ability to scavenge free radicals, and the scavenging ability varies depending on the isoflavonoid category.
4 DISCUSSION
Continuous cropping obstacles are one of the most important factors limiting the sustainable development of the modern agricultural industry (Chen et al., 2022). Currently, the related research on continuous cropping obstacles mainly focuses on the exploration of its causes, including the accumulation of autotoxic substances in soil and the changes in soil microbial community structure and diversity (Chen et al., 2020; Gao et al., 2019; Liu et al., 2021; Cheng et al., 2020). Understanding how plants adapt their physiological and molecular mechanisms under continuous cropping conditions is crucial for revealing key factors of plant adaptability and stress resistance, which are essential for promoting the sustainable development of modern agriculture.
Poor plant growth and development after continuous cropping is the main cause of yield reduction. This study found that in the continuous cropping system, the aboveground parts of D10 grown for 16 d were inhibited to different degrees under CC1 and CC2 treatments, especially under CC2 treatment (Figure 1A). However, the growth of Y8 was inhibited only under CC2 treatment (Figure 1A). By observing the roots of the two genotypes of peas, it was found that the root growth of D10 was significantly inhibited under CC2 treatment, while the degree of inhibition of Y8 was significantly lower than that of D10 (Figure 1B, Table S1). This indicated that the deterioration of the soil environment in the continuous cropping system resulted in stunted root growth of peas. The number of lateral roots in the ageing roots was also significantly reduced, which further limited the uptake of nutrients and water from the soil, as well as transport to the aboveground, resulting in poor aboveground growth of peas.
Some specific metabolites in plants are important for plants to resist adversity stress (Zahedi et al., 2021). In this study, the changes of metabolites in pea roots under different continuous cropping times were analyzed by metabolomics. It was found that continuous cropping times increased the number of DAMs of pea roots, and the number of DAMs in the sensitive to continuous cropping pea was higher than that of pea tolerant to continuous cropping (Figure 3). These results indicated that the pea roots respond to continuous cropping stress by altering the metabolite composition under continuous cropping treatments. In particular, peas sensitive to continuous cropping show more extensive metabolic adjustments in response to the increased stress caused by these conditions. Through KEGG classification analysis of DAMs, we found that in other secondary metabolic biosynthesis pathways, the DAMs of the roots of the two genotypes under different continuous cropping treatments were mainly involved in flavonoid and isoflavone biosynthesis pathways (Figure 4). These results indicated that flavonoids are the major DAMs in response to continuous cropping. Legumes are rich in flavonoid compounds (Thilakarathna & Rupasinghe 2013), which play a key role in their resistance to stress (García-Calderón et al., 2020). Studies have confirmed that stress can lead to the synthesis and accumulation of flavonoids in plants (Ahmed et al., 2021; Guo et al., 2021). Similar results were also found in this study, the number of up-regulated flavonoids was more than down-regulated under different continuous cropping treatments of D10, and the types of up-regulated flavonoids increased under CC2 treatment (Table S2). It indicates that pea may respond to the stress caused by continuous cropping by enhancing their antioxidant and anti-stress metabolic pathways, which is a defence mechanism. In Y8, the number of up-regulated flavonoids was greater under the CC1 treatment than in D10, and all were up-regulated under the CC2 treatment (Table S3). This may mean that continuous cropping tolerant pea have a more efficient stress response mechanism and can quickly adjust their metabolism to cope with the mild challenges caused by continuous cropping. Under CC2 treatment, the flavonoid DAMs involved in the continuous cropping tolerant pea roots were up-regulated, indicating that it has a stronger ability to adapt to severe continuous cropping stress. Differences in flavonoid synthesis in the roots of these two pea genotypes may be related to the ability of pea plants to withstand continuous cropping. In addition, we further determined the total flavonoid content of pea roots under different treatments and found that continuous cropping increased the total flavonoid content of pea roots, especially under CC2 treatment (Figure 5A, B). This further suggests that flavonoids in pea root systems respond positively to continuous cropping. Flavonoids are involved in many processes of plant growth, such as signal transduction, growth hormone polarity transport and pigmentation (Bag et al., 2022; Bhakta et al., 2022; Chen et al., 2022). In addition, flavonoids are important antioxidants that scavenge reactive oxygen species produced under biotic and abiotic stresses (Baskar et al., 2018; Marone et al., 2022). Therefore, the accumulation of flavonoid compounds induced by continuous cropping has an important regulatory role in the growth of pea plants and may play an important role in scavenging excessive ROS caused by continuous cropping.
In this study, we further analyzed the transcriptomics of pea roots under different continuous cropping treatments. The results showed that the gene expression of pea root samples had a strong response to continuous cropping treatment. With the increase of continuous cropping times, the number of DEGs in the roots of the two pea genotypes increased, and the number of DEGs in D10 was more than that in Y8 (Figure 5C-F). This may be due to the fact that pea, which are sensitive to continuous cropping, needs to activate more stress-responsive genes to cope with the continuous cropping environment. In addition, differences in genetic and physiological traits between sensitive and tolerant peas may lead to different responses to continuous cropping stress. Continuous cropping tolerant peas have more effective mechanisms to mitigate the negative effects of continuous cropping and, therefore, do not show as drastic changes in gene expression as sensitive peas. This indicated that the pea roots adopted a specific molecular mechanism for the continuous cropping treatment and was affected by the number of continuous cropping and the pea genotypes, which changed the transcriptional activity of the pea roots to different degrees. We further performed KEGG enrichment analysis of DEGs, and found that flavonoid biosynthesis and isoflavone biosynthesis pathways were significantly enriched in pea roots under different continuous cropping treatments and different genotypes (Figure 6). Sugar beet was also significantly enriched in genes related to flavonoid compound synthesis in its roots in the continuous cropping system, and the number of genes related to flavonoid synthesis in its roots increased with the increase in the number of years of continuous cropping, which was similar to the results of the present study (Huang et al., 2021). Under different continuous cropping treatments, the number of DEGs involved in flavonoid and isoflavone biosynthesis pathways was significantly higher up-regulated than that of down-regulated DEGs (Table S4, S5), which may be the reason for the accumulation of flavonoid compounds under different treatments.
The comprehensive analysis of different omics data is helpful for us to explore and further understand the response mechanism of plants to adversity stress (Huang et al., 2023; Asakura et al., 2021; Lu et al., 2022). In this study, comprehensive transcriptomic and metabolomic analyses showed that the flavonoid biosynthetic pathway and the isoflavonoid biosynthetic pathway were co-annotated in both CC1vsRT and CC2vsRT of different genotypes of pea (Table S6, S7). Previous studies have shown that the expression of key genes PAL, 4CL, CHS, F3H, C3'H and ANS involved in flavonoid synthesis was up-regulated after plants were subjected to stress (Zhuang et al., 2023). In this study, these genes were significantly up-regulated under CC2 treatment of D10, but there was no differential expression under different treatments of Y8 (Figure 7). We speculate that these genes may be specific expression genes for the sensitive genotype in response to severe continuous cropping. The continuous cropping degree set in this study cannot induce the expression of these genes in the roots of continuous cropping tolerant peas, which may also be the key to continuous cropping tolerance of Y8. CHI plays a key role in flavonoid biosynthesis, and overexpression of CHI can increase the levels of flavanones and flavonoids in citrus (Zhao et al., 2021). In addition, adversity stress can lead to an increase in the expression of CHI in plants (Li et al., 2021; Zhao et al., 2021). In this study, we found that the expression of CHI in the roots of the two pea genotypes was up-regulated as a whole (Figure 7). This may be a result of differences in the ability of the two genotypes of peas to tolerate continuous cropping. The flavonoid compounds isoliguirtigenin, liquiritigenin, dihydrokase mpferal, epiafzelechin, 7′4-dihydroxyflavone, which are involved in the regulation of these genes, were increased in their levels to different degrees under different continuous cropping treatments (Figure 7). It indicated that the up-regulated expression of these genes in the flavonoid biosynthesis pathway is a determinant of their metabolite accumulation. Previous studies have shown that overexpression of HID promotes isoflavone accumulation (Shimamura et al., 2007). In addition, CYP81E encodes isoflavone 2′-hydroxylase and isoflavone 3′-hydroxylase (Akashi et al., 1998; Liu et al., 2003). In this study, the expression of HID and CYP81E in the roots of two pea genotypes was activated under different continuous cropping treatments (Figure 8). At the same time, a variety of metabolites in the isoflavone biosynthesis pathway also increased with the increase of continuous cropping times. This indicated that HID and CYP81E positively regulate the biosynthesis of different isoflavones. However, this positive regulatory relationship needs to be verified by sufficient experiments in the future. Interestingly, there were more DAMs involving flavonoids than DAMs involving isoflavonoids in D10 under continuous cropping, whereas there were more DAMs involving isoflavonoids than DAMs involving flavonoids in Y8. and there were more DAMs involving isoflavonoids than D10 in Y8 under the CC1 treatment (Table S2, S3). Isoflavonoids can act as signalling molecules in plant-microorganism interactions, as well as induced antimicrobial or anti-insect compounds (Xia et al., 2023). Therefore, we speculate that the roots of continuous cropping peas resist continuous cropping by synthesizing more isoflavones under continuous cropping stress, which may be the key to the different tolerance of the two genotypes to continuous cropping. This requires a large number of experiments to verify the possibility of the results further in the future.
In this study, biochanin A, calycosin, and genistein were found to be DAMs only in the roots of Y8, suggesting that these isoflavonoids may be associated with pea sensitivity to continuous cropping and deserve to be looked at. Studies have confirmed that the change of soil microbial community structure is the key to the occurrence of continuous cropping obstacles (Tan et al., 2021), and crops are subjected to oxidative stress in continuous cropping systems (Wang et al., 2021; Yang et al., 2020). To this end, we further validated the antimicrobial and antioxidant effects of the isoflavonoids biochanin A, calycosin, and genistein in the Y8 roots. It was found that the number of culturable fungi in the soil planted with two genotypes of peas increased significantly under CC2 treatment, and the number of fungi in D10 was more than that in Y8 (Figure S3A). This showed that the occurrence of pea continuous cropping obstacles may be induced by microorganisms in soil. Bacteriostatic test of biochanin A, calycosin, and genistein was carried out by mixing and culturing separately culturable bacteria and fungi in soil under CC2 treatment of D10, and it was found that the three isoflavones were found to have little inhibitory ability against bacteria. And the ability to inhibit fungi varied depending on the type and concentration of isoflavones, and all three substances had the ability to inhibit fungi. This may be one of the main reasons for the lower fungal populations in Y8 soils than in D10. In addition, in vitro antioxidant assays have shown that biochanin A, calycosin and genistein have the ability to scavenge free radicals, which may indicate that Y8 has a stronger antioxidant system than D10 to minimize oxidative damage. The potential role of these isoflavones may be an important reason for the resistance of Y8 to continuous cropping.
5 CONCLUSIONS
In this study, transcriptomics and metabolomics revealed the response characteristics of flavonoids in pea roots to continuous cropping. With increasing continuous cropping times, the growth of peas was inhibited, and the two genotypes of pea responded differently to continuous cropping. Flavonoid and isoflavonoid biosynthesis pathways were altered at the transcriptional and metabolic levels in pea roots of both genotypes. There were differences in the categories of isoflavones in the roots of the two genotypes of pea, with a higher category of isoflavones in the roots of the continuous cropping tolerant pea. Antibacterial and in vitro antioxidant tests confirmed that biochanin A, calycosin, and genistein in the roots of continuous cropping tolerant pea have the ability to inhibit fungi and scavenge DPPH free radicals. The results of this study not only help to understand the molecular regulatory mechanism of different genotypes of pea roots on flavonoid biosynthesis under continuous cropping treatment, but also provide important information for alleviating the obstacles of pea continuous cropping by molecular breeding and exogenous application in the future.
AUTHOR CONTRIBUTIONS
Experimental design: Lei Ma and Sheng Li. Field experiment and soil sample collection: Lei Ma, Xiaojie Feng. Pot test and index determination: Lei Ma, Ruonan Wei, Congcong Zhang, Ling Xu. Data analysis and integration: Lei Ma, Shaoying Ma, Guiping Chen, Xu Lu. Shaoying Ma provided the test site. Lei Ma finished the manuscript. Sheng Li, Xucheng Zhang and Qiang Chai revised the manuscript. All authors read and approved the manuscript.
ACKNOWLEDGMENTS
This work was supported by the Major Science and Technology Project of Gansu Province (20ZD7NA007), Modern Agro-Industry Technology Research System of China (CARS-22-G-12), National Natural Science Foundation of China (31460382), and the Natural Science Foundation of Gansu Province (21JR7RA822).
Open Research
DATA AVAILABILITY STATEMENT
The RNA-Seq data in this study have been uploaded to National Center for Biotechnology Information (NCBI) with bioproject accession number PRJNA889933.




