A precise balance of TETRASPANIN1/TORNADO2 activity is required for vascular proliferation and ground tissue patterning in Arabidopsis
Abstract
The molecular mechanisms guiding oriented cell divisions in the root vascular tissues of Arabidopsis thaliana are still poorly characterised. By overlapping bulk and single-cell transcriptomic datasets, we unveiled TETRASPANIN1 (TET1) as a putative regulator in this process. TET1 is expressed in root vascular cells, and loss-of-function mutants contain fewer vascular cell files. We further generated and characterised a CRISPR deletion mutant and showed, unlike previously described mutants, that the full knock out is additionally missing endodermal cells in a stochastic way. Finally, we show that HA-tagged versions of TET1 are functional in contrast to fluorescent TET1 translational fusions. Immunostaining using HA-TET1 lines complementing the mutant phenotype suggested a dual plasma membrane and intracellular localisation in the root vasculature and a polar membrane localisation in the young cortex, endodermal and initial cells. Taken together, we show that TET1 is involved in both vascular proliferation and ground tissue patterning. Our initial results pave the way for future work to decipher its precise mode of action.
1 INTRODUCTION
Oriented cell divisions are integral to the process of creating the three-dimensional shape of a plant (Metcalfe and Esau, 1966). This is particularly evident in the primary root apical meristem (RAM) of Arabidopsis thaliana (Arabidopsis), where cells are organised in a highly conserved pattern of concentric layers, achieved via strict spatiotemporal control of cell division orientation (Dolan et al., 1993). Anticlinal (perpendicular to the root surface and growth axis) division plane positioning adds cells to existing cell files and leads to longitudinal growth, whereas radial (perpendicular to the surface but parallel to the axis) and periclinal (parallel to both the surface and the growth axis) divisions increase the number of cell files (Smet and De Rybel, 2016). Under normal growth conditions, epidermal and ground tissues undergo periclinal divisions in the stem cell niche: the protoderm initials give rise to lateral root cap (LRC) and epidermis cell files, while cortex-endodermis initial (CEIs) daughter cells (CEIDs) produce cortex and endodermis. Upon exiting the stem cell region, LRC, epidermis, cortex and endodermis cells undergo anticlinal divisions while they retain the capacity to switch the division plane upon e.g. wounding (Marhavá et al., 2019) or ectopic expression of specific transcription factors (TFs) (De Rybel et al., 2013; Miyashima et al., 2019; Ohashi-Ito et al., 2014; Smet et al., 2019). The situation is different for vascular cells, as radial and periclinal divisions are needed during, e.g. the formation of the phloem lineage during primary growth (Miyashima et al., 2019; Rodriguez-Villalon et al., 2014).
Cell division orientation in the vasculature is controlled by a complex transcriptional network. One key regulator is the basic helix–loop–helix (bHLH) TF heterodimer formed by TARGET OF MONOPTEROS5 (TMO5) and LONESOME HIGHWAY (LHW). Upon misexpression, this complex is capable of triggering ectopic periclinal/radial divisions in any cell type (De Rybel et al., 2013; De Rybel et al., 2014; Katayama et al., 2015; Mor et al., 2022; Ohashi-Ito et al., 2014). Several factors acting both upstream and downstream of TMO5/LHW have been described (Vera-Sirera et al., 2015; Wybouw et al., 2023; Yang et al., 2021). Notably the DNA-BINDING WITH ONE ZINC FINGER2.1 (DOF2.1) TF, a direct TMO5/LHW target that is sufficient to trigger ectopic periclinal radial divisions and is related to the PEAR (PHLOEM EARLY DOF) TFs that control vascular cell division orientation independently of TMO5/LHW (Miyashima et al., 2019; Smet et al., 2019). Nonetheless, the post-transcriptional mechanisms involved in executing the switch in cell division plane positioning downstream of these transcriptional programs remain poorly understood.
Division plane formation is often viewed as a transient polarisation event and is, as such, expected to depend on mechanisms similar to those of other polarity establishment pathways (Glanc, 2022; Müller, 2019). Cell polarisation in plant and non-plant systems typically involves positive feedback loops that mechanistically rely on protein scaffolding (Chiou et al., 2017; Ramalho et al., 2022). Indeed, polarly localised scaffolding proteins, such as BASL, POLAR and SOSEKI proteins, have been shown to drive cell division processes in a polarised manner (Dong et al., 2009; Houbaert et al., 2018; Yoshida et al., 2019). In this work, we investigate the involvement of TETRASPANIN1/ TORNADO2 (TET1/TRN2), a transmembrane protein with a putative scaffolding function, in the regulation of cell division orientation during vascular development.
TET1 belongs to the TETRASPANIN (TET) protein family, which is conserved across multicellular eukaryotes and is represented by 17 members in Arabidopsis (Boavida et al., 2013; Wang et al., 2015; Wang et al., 2012). TETs are small membrane proteins with a conserved fold consisting of four membrane-spanning helices connected by three loops, a small extracellular, a small intracellular and a large extracellular loop (Reimann et al., 2017). TETs function in a variety of cellular and developmental processes in animal systems, ranging from endomembrane trafficking to signalling and cell adhesion (Charrin et al., 2014; Reimann et al., 2017). Scaffolding and partitioning of various multiprotein complexes into the so-called TETRASPANIN-Enriched Microdomains (TEMs) at the plasma membrane (PM) is a recurrent mode of TET action across their diverse functional roles (Reimann et al., 2017). Comparatively, less is characterised when it comes to the function and localisation of TETs in plants, although it has been shown that their PM localisation depends on the presence of “GCCK/RP” motifs and cysteine residues (Zhu et al., 2022). Most single mutants show no striking phenotypes, suggesting a high degree of functional redundancy in the family (Wang et al., 2015). One exception is the tet1/trn2 single mutant, which shows prominent pleiotropic defects ranging from dwarfed overall architecture to sterility and asymmetrical venation patterning (Cnops et al., 2000; Cnops et al., 2006; Olmos et al., 2003; Chiu et al., 2007). Additionally, the tet13 mutant shows a shortened meristem and reduced emergence of lateral roots, suggesting that TET13 plays a role in formation of the primary root (Wang et al., 2015). TET8 and TET9 contribute to plant immunity, as double mutants are susceptible to Botrytis cinerea. These double mutants also have a reduction of host sRNA in the excreted exosomes, required for silencing of fungal genes essential for infection, while the single tet8 mutant secretes less extracellular vesicles (Cai et al., 2018; He et al., 2021; Liu et al., 2020). TET5 and TET6 are reported to have a role in plant growth, as the tet5 tet6 double mutant has larger leaves and a longer primary root (Wang et al., 2015). Although it is thus clear that TETs are involved in a wide range of developmental aspects, a thorough understanding of their functionality is still lacking. In this study, we characterise TET1 in the context of vascular development, using cell biological and genetic approaches. We demonstrate that both the tet1 mutant phenotypes and the TET1 expression pattern argue for a role in the maintenance of root vascular tissues and ground tissue patterning. In addition, we show that an optimal balance of TET1 is required for proper growth and suggest that the mode-of-action might be linked to intracellular trafficking events.
2 MATERIALS AND METHODS
2.1 Bulk RNA sequencing and data analysis
The proRPS5A::DOF2.1-GR construct was cloned using the Multisite Gateway cloning system. 5-day-old seedlings were grown on half-strength MS medium plates and then transferred to plates supplemented with 10 μM Dexamethasone (DEX). The DEX treatment was performed for 1–2-4 h before harvesting the bottom 0.5 cm of ~300 roots, and three biological repeats per time point were used. Roots were harvested directly into liquid nitrogen, and RNA was extracted with the RNeasy kit (QIAGEN). The Arabidopsis thaliana Col-0 TAIR10 Genome data was used for mapping and annotation. The analysis was performed with R software package edgeR (R version 3.5.1). Genes with expression values higher than 0.35 cpm (5 read counts) on at least 3 samples were retained for the analysis. TMM normalisation was applied using the calcNormFactors function. Variability in the dataset was assessed with an MDSplot. The treatment and genotype factors were collapsed into one factor. Trended negative binomial dispersion parameters were estimated based on an additive no intercept model with the collapsed factor and a batch effect using the estimateDisp function and down-weighting outlying genes. A quasi-likelihood negative binomial regression model was then used to model the over-dispersed counts for each gene separately, as implemented in the function glmQLFit. The interest was in the simple tests of effect: testing for a treatment effect in the genotype and testing for a genotype effect under treatment conditions. Contrasts were estimated using empirical Bayes quasi-likelihood F-tests. P-values were corrected using the FDR method described by Benjamini and Hochberg (1995) for each contrast separately. All edgeR functions were applied with default values. A gene was called differentially expressed when the FDR value was <0.05.
2.2 Data overlap analysis
From the proRPS5A::DOF2.1-GR dataset, genes with fold change >1.5 across all time points and P-values <0.001 across all time points were retained. From the published scRNA-seq dataset (Wendrich et al., 2020), procambium and early phloem cell clusters were selected, and those differentially expressed genes showing high expression in these clusters (pct.1 > 0.99) were retained. Overlap analysis of these gene lists was performed using an online Venn diagram tool (https://bioinformatics.psb.ugent.be/webtools/Venn/).
2.3 Plant material and growth conditions
Arabidopsis thaliana Columbia-0 (Col-0) background was used for the generation of mutant and transgenic lines. Seeds were sterilised using 75% EthOH and 25% bleach for 5 min, after which they were rinsed twice with 70% EtOH and once with 96% EtOH. Seedlings were grown at 22°C under continuous light on ½ Murashige & Skoog (MS) with 1% agar without sucrose after a stratification period of 48 h. 7 DAG seedlings were placed on Jiffy peat pods for phenotypic analysis at the rosette stage and propagation. The trn2-1 (Cnops et al., 2000) and trn2-7 (GK-254G01.02) (Wang et al., 2015) mutants were described previously. The tet1-cko1 mutant and all transgenic lines described in this study were generated by transforming the respective constructs into Col-0, trn2-7+/− or tet1-cko1+/− using the floral dip method (Clough and Bent, 1998). The AGI identifiers for the genes used in this study were as follows: DOF2.1: AT2G28510; TET1: AT5G46700; UBC: AT5G25760; EEF1α4: AT5G60390.
2.4 Molecular cloning
All primers and vectors used in this study can be found in Table S3 and Table S4, respectively. Promoters and coding sequences were commercially synthesised (Eurofins) or PCR-amplified using the Q5 high fidelity polymerase (NEB) according to the manufacturer's instructions. Constructs were generated by the Multisite Gateway (Karimi et al., 2007) or the Golden Gateway (Karimi and Jacobs, 2021) methods. All entry clones were verified by Sanger sequencing. All sequence data were handled using CLC Main Workbench Version 21.0.4.
2.5 CRISPR/CAS9 mutant generation
To generate a full knockout of TET1, the CRISPR-CAS9 technique was used, as described previously (Decaestecker et al., 2019). Briefly, gRNA sequences targeting the TET1 5′ and 3’ UTRs were designed using the Cas-Designer tool (Park et al., 2015) and cloned into the pFASTRK-AtCas9-AG destination vector. The TET1-cKO construct was transformed into Col-0, and candidate genome-edited T1/M1 plants were identified by PCR using primers flanking the gRNA target sites. The excision of TET1 was confirmed in Cas9-free T2/M2 plants by PCR and Sanger sequencing (Figure 3).
2.6 DNA extraction and genotyping
Genomic DNA used for PCR-based genotyping was isolated using the CTAB extraction buffer (0.1 M Tris pH 7.5, 0.7 M NaCl, 0.01 M EDTA and 0.03 M CTAB) as described previously (Wybouw et al., 2023). Primers used for genotyping can be found in Table S3.
2.7 Microscopy and image analysis
GUS staining and imaging were performed as described previously (Wybouw et al., 2023). Fluorescent reporter lines were imaged on Leica SP8 (Leica Microsystems), Zeiss LSM 710 or LSM880 (Zeiss) confocal microscopes equipped with 40x NA 1.1 W (Leica), 20x NA 0.8 or 40x NA 1.2 W (Zeiss) objectives. sYFP and mCitrine were excited at 514 nm and detected at 520–580 nm. mPS-PI staining was done as described previously (Arents et al., 2022). Briefly, 7 DAG seedlings were fixed in 50% methanol/10% acetic acid at 4 degrees overnight, incubated in 1% periodic acid and stained with freshly prepared propidium iodide in Schiff reagent solution for 2 hours or until visibly stained. Seedlings were then mounted in chloral hydrate and imaged in XZ mode on Leica SP8 with a 40x NA 1.1 water immersion objective, excited at 560 nm and detected at 600–700 nm. Immunofluorescence was performed as described previously (Sauer et al., 2006) using the InSitu PRO VSI robot (Intavis) and the following antibodies: mouse αHA (Santa Cruz Biotech) 1:500, rabbit αPIN1 (Baster et al., 2013) 1:1000, AlexaFluor Plus 488-conjugated goat αMouse (Invitrogen) 1:500, AlexaFluor Plus 647-conjugated goat αRabbit (Invitrogen) 1:500. BFA (Sigma) treatment was applied in liquid ½MS medium as indicated in the figure legend from a 1000x stock solution in DMSO. Images were processed and analysed using FIJI (Schindelin et al., 2012), and figures were assembled in Inkscape version 1.1.2.
2.8 RNA isolation and qRT-PCR
Samples from 8-week-old Arabidopsis rosettes were snap-frozen and ground in liquid nitrogen. RNA was extracted using Trizol and the ReliaPrep RNA Miniprep System (Promega). Trizol was added to each sample for 5 min, followed by chloroform. After centrifugation, the top aqueous phase was mixed with isopropanol, incubated for 10 min at −20°C and then this solution was processed further using the Reliaprep Kit as per the manufacturer's instructions. cDNA was synthesised from 1 μg of total RNA with the qScriptTM cDNA Supermix kit (Quanta BioSciences). qPCR was performed on a Roche Lightcycler 480 with SYBR Green I (Roche). Analysis was done with qbase+ Version: 3.3 (https://cellcarta.com/genomic-data-analysis/). Primers used are listed in Table S3.
2.9 Quantification and Statistical Analysis
Quantification, statistical analysis and plotting were performed using Microsoft Excel or R version 4.1.0 using RStudio version 1.4.1106 and the ggplot2 package. All data and tests used for statistical analyses are summarised in Table S5.
3 RESULTS
3.1 Identification of TET1 as regulator of division orientation in vascular cells
To identify novel regulators of cell division orientation during vascular development, we harnessed a published single-cell RNA sequencing (scRNA-seq)-based transcriptomic atlas of the Arabidopsis root (Wendrich et al., 2020). Specifically, we focused on transcripts enriched in young protophloem and/or procambium cells, which undergo a switch from anticlinal to periclinal or radial cell division orientation, most often outside of the stem cell niche (Miyashima et al., 2019). We further reasoned that key factors involved in vascular division plane positioning would likely act downstream of the TMO5/LHW-DOF2.1 pathway (De Rybel et al., 2013; Smet et al., 2019). With this rationale, we transcriptionally profiled the pRPS5A::DOF2.1-GR inducible misexpression line (Smet et al., 2019) in a time-course induction using bulk RNA-seq (Table S1) and overlapped the transcripts upregulated upon DOF2.1 induction with the phloem/procambium-enriched candidates obtained from the scRNA-seq dataset (Wendrich et al., 2020) (Table S1, Figure 1A). To further narrow down the search space, we focused on genes linked to entities involved in the execution of cell division, such as the cytoskeleton, plasma membrane or the cell wall (Glanc, 2022; Livanos and Müller, 2019). Among the shortlist of candidate genes identified by this approach, TETRASPANIN1/ TORNADO2 (TET1/TRN2) drew our attention for several reasons. Firstly, besides its enrichment in young protophloem, procambium and the stem cell niche, lower amounts of the TET1 transcript were detected in most other cell types as well, which would be expected for a regulator of core cellular processes such as cell division (Figure S1). Secondly, TET1 was consistently and significantly upregulated upon DOF2.1-GR induction from 1 h after DEX treatment onwards (Table S1) and tet1/trn2 mutants have strong pleiotropic growth and patterning defects, which include lateral root cap (LRC)/epidermis cell type misspecification, presumably caused by irregular protoderm initial cell divisions (Cnops et al., 2000; Cnops et al., 2006; Lieber et al., 2011; Olmos et al., 2003; Yamaguchi et al., 2017). Thirdly and most importantly, TET proteins are conserved across multicellular eukaryotes and function as molecular scaffolds in a plethora of multiprotein complexes at the plasma membrane in animal cells (Reimann et al., 2017; Termini and Gillette, 2017). As protein scaffolding is a common feature of numerous cellular polarity establishment pathways (Ramalho et al., 2022), this provides a mechanistically plausible role for TET1 in plant cell division orientation, which is thought to be tightly linked to cell polarity (Glanc, 2022; Müller, 2019). In summary, based on the overlap analysis of two different transcriptomic datasets, we selected TET1 as a putative regulator of cell division orientation during vascular development.
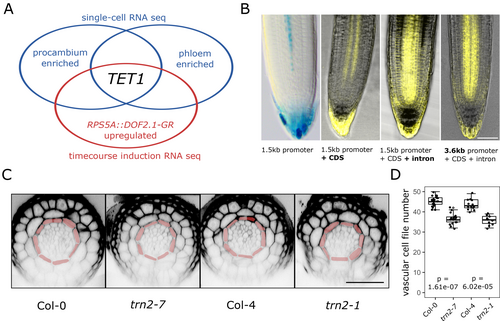
Although the scRNA-seq data (Wendrich et al., 2020) suggested the presence of TET1 mRNA in most cell types of the root meristem with an enrichment in young protophloem and procambium (Figure S1), this is contradicted by the expression pattern of the previously described pTET1::nlsGUS-GFP transcriptional reporter line (Wang et al., 2015). This line reporting on TET1 promoter activity revealed weak expression in a small subset of vascular and root cap cells and only in lines with multiple T-DNA insertions (Wang et al., 2015). We thus hypothesised that endogenous TET1 expression depended on additional regulatory elements in a longer promoter region and/or the protein-coding region than was used previously. To test this hypothesis, we generated a series of native promoter-driven translational reporter constructs varying in the length of the promoter fragment and the presence/absence of the single 595 bp intron. The expression pattern of the cDNA-derived pTET11500::TET1-sYFP was very similar to the previously described pTET1::nlsGUS-GFP, while the gDNA-derived, intron-including pTET11500::TET1g-sYFP showed a comparably stronger and broader expression, corresponding to the predicted scRNA-seq data (Figure 1B). To test whether TET1 expression in the root meristem is regulated by additional elements further upstream in the promoter region, similar to the situation in developing flowers (Yamaguchi et al., 2017), we generated a pTET13600::TET1g-mCitrine construct using a 3600 bp promoter fragment spanning the whole region between the start codon of TET1 and the stop codon of the 5′ neighbouring gene, bHLH071. In the root meristem, the expression patterns of pTET11500::TETg1-sYFP and pTET13600::TET1g-mCitrine were indistinguishable (Figure 1B), indicating that while the longer promoter fragment is important for correct TET1 expression in flowers and perhaps other tissues, expression in the root meristem depends primarily on regulatory elements present in the intron.
To further validate the role of TET1 in the regulation of vascular development, we probed the known tet1/trn2 mutants for defects in the anatomy of the root apical meristem vascular bundle. Specifically, we analysed the number of vascular cell files, which is a reliable readout of the relative frequency of periclinal/radial cell divisions (Arents et al., 2022; De Rybel et al., 2013; Smet et al., 2019). At least 7 different tet1 alleles, including T-DNA insertion lines and mutants obtained via EMS mutagenesis, have been described to date; all causing the characteristic tornado phenotype which includes dwarfism, sterility and severe twisting of roots, leaves and other organs (Cnops et al., 2000; Cnops et al., 2006; Lieber et al., 2011; Olmos et al., 2003; Yamaguchi et al., 2017). The root vascular phenotype of tet1/trn2 mutants has, however, not been examined so far. We thus quantified the number of cell files within the vascular bundle in the primary root meristem of two different tet1 alleles: trn2-1 (in the Col-4 background) and trn2-7 (in the Col-0 background). In both cases, we observed a significant reduction in vascular cell file numbers compared to the respective wildtype control (Figure 1C,D, and Table S5). This result indicated a lower frequency of a division plane switch from anticlinal to periclinal/radial during the development of primary root vasculature in tet1 mutants. Collectively, the TET1 expression pattern and loss-of-function mutant phenotype strongly support a role for TET1 during root vascular proliferation, presumably in the control of cell division orientation.
3.2 Generation of a tet1 deletion mutant reveals both vascular and ground tissue defects
To get an insight into a possible molecular mechanism for TET1 function, we next aimed to determine its subcellular localisation. To avoid artefacts caused by non-functional reporters, we first tested the functionality of our fluorescent protein fusions (Figure 1B) by a genetic complementation assay. To this end, we transformed the constructs into trn2-7 heterozygote plants, as all known homozygous tet1 mutants are sterile (Cnops et al., 2000; Cnops et al., 2006). After screening T1 and T2 populations of 13 different TET1 translational reporter constructs, consisting of different combinations of endogenous and constitutive promoters, gDNA and cDNA fragments and different N- and C-terminal tags (Table S2), we consistently observed ~25% of plants with the characteristic tornado phenotype (Figure S2), and never found a tet1/trn2-7 homozygote with a wildtype-like phenotype. This indicated that none of the 13 tested constructs rescued the tet1/trn2 mutant. Such lack of rescue could, in theory, be caused by an incorrect expression pattern of the transgenes or non-functional fusion proteins. Second-site mutations being causal to the tornado phenotype can be effectively ruled out given the number of phenotypically identical independent tet1 mutants (Cnops et al., 2006). All previously described tet1 alleles harbour mutations that disrupt the TET1 open reading frame in or after the large extracellular loop between transmembrane domains 3 and 4 (Cnops et al., 2006). Therefore, the existing tet1/trn2 alleles might produce partial TET1 transcripts, and the resulting truncated TET1 proteins could cause the tornado phenotype in a weakly semidominant-negative manner, which could have been easily misinterpreted as recessive loss-of-function. Supporting this theory, we see that the previously described trn2-7 allele does, in fact, produce N-terminal transcripts of TET1 (Figure S3). To test this hypothesis, we cloned fragments of TET1 mimicking the trn2-1 and trn2-7 alleles and drove them from the strong constitutive 35S promoter in the Col-0 background. Both p35S::TRN2-1 and p35S::TRN2-7 constructs indeed yielded plants with the tornado phenotype (Figure 2), albeit only partially penetrant. Surprisingly, though, we observed the tornado phenotype also among plants harbouring the full-length p35S::TET1g construct, as well as p35S::TET1-GFP and pTET13600:TET1g-TurboID-HA (Figure 2 and S2). We further confirmed that the p35S::TET1g construct indeed resulted in TET1 overexpression by qRT-PCR. Despite we also observed lower levels of endogenous TET1 expression, there was no correlation between the amount of endogenous or transgenic TET1 transcripts and the penetrance of the tornado phenotype in a population of independent p35S::TET1g T1 transformants (Figure S2). This result argued against the knock-down of endogenous TET1 due to co-suppression (Depicker and Van Montagu, 1997) causing the tornado phenotype in p35S::TET1g plants. Collectively, these results suggested that ectopic expression of full-length, truncated or tagged versions of TET1 cause the tornado phenotype.

To decisively uncouple the effects of loss of TET1 function from potential dominant-negative effects of residual truncated TET1 proteins, we utilised the CRISPR-Cas9 technology to produce a bona fide tet1 full knockout or deletion mutant. By co-expressing Cas9 with gRNAs targeting the TET1 5′ and 3’ UTRs, we were indeed able to isolate a mutant with the TET1 coding region cleanly excised and named it tet1-cko1 (Crispr KnockOut 1) (Figure 3A-E). The overall seedling and rosette stage phenotype of the tet1-cko1 mutant was indistinguishable from the previously described tet1/trn2 alleles (Figure 3B,D and S4A). We next analysed vascular cell file numbers of tet1-cko1 roots and observed a similar reduction compared to wildtype as in the trn2-1 and trn2-7 alleles (Figure 3F,G). Among other defects, previously described tet1/trn2 mutants often lack one or more epidermal cell files, which are replaced by ectopic lateral root cap cells, presumably due to defects in cell division orientation of the protoderm initials (Cnops et al., 2000; Fendrych et al., 2014). Notably, in ~20% tet1-cko1 roots, we observed analogical defects in the ground tissue, specifically missing endodermal cell files, resulting in cortex cells directly neighbouring with pericycle cells (Figure 3F).
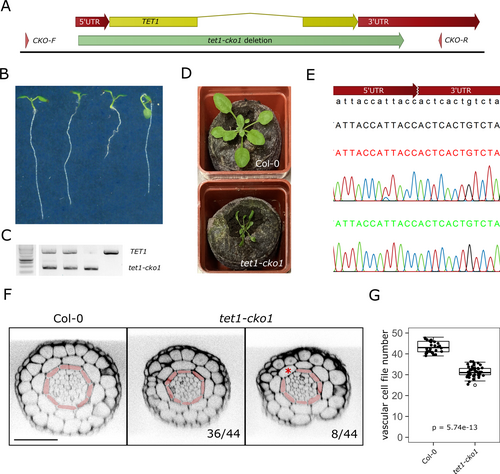
Collectively, these results confirmed that the tornado phenotype of the previously described tet1/trn2 alleles is indeed recessively caused by the loss of TET1. Nonetheless, they also show that gain-of-function of full-length, truncated or tagged versions TET1 can phenocopy tet1 loss-of-function in a dominant-negative manner. We thus conclude that the tet1 deletion allele tet1-cko1, which phenocopies other tet1/trn2 alleles and displays an additional defect in ground tissue patterning, is a superior genetic background for further functional characterisation of TET1.
3.3 Subcellular localisation supports a function for TET1 in ground tissue patterning
We next re-assessed the functionality of TET1 fluorescent fusions by complementing the tet1-cko1 deletion mutant. As we have had issues complementing the trn2-7 allele with tagged constructs, we initially decided to use the non-tagged pTET1::TET1g construct to look for complementation in the tet1-cko1 mutant background. We observed full rescue of the tet1-cko1 mutant by the non-tagged pTET1::TET1g construct, but similarly to the trn2-7 allele, no rescue by pTET1::TET1g-mCitrine, pTET1::TET1g-mScarlet or pTET1::mCitrine-TET1g (Figure S4B-D). As these constructs consisted of identical promoter and coding regions and differed only in the presence/absence of the fluorescent tags, we conclude that the lack of rescue was indeed caused by the non-functionality of the fusion proteins, rather than discrepancies between the endogenous and transgenic expression pattern and/or levels. Difficulties in obtaining functional translational reporters of transmembrane proteins caused by lack of functionality of N- or C-terminal fluorescent fusions have been reported before (Abas et al., 2006; Wisniewska et al., 2006; Xu and Scheres, 2005). In the case of the PIN-formed (PIN) auxin efflux carriers, this obstacle was successfully circumvented by inserting the fluorescent protein into the central hydrophilic loop or by terminal fusion to the hemagglutinin epitope (HA) tag, which is much smaller than the relatively large fluorescent proteins (Abas et al., 2006; Ntoukakis et al., 2011; Wisniewska et al., 2006; Xu and Scheres, 2005). Similarly, we inserted the mCitrine tag into the single short intracellular loop within the predicted TET1 structure. Nonetheless, this in-loop fusion protein did not complement the trn2-7 mutant, displayed cytoplasmic localisation in T1 generation and got silenced by T2, and caused the tornado phenotype in a dominant-negative manner similar to the p35S::TRN2-7 and p35S::TRN2-1 constructs, indicating it was not functional either (Figure S5). Next, we tagged TET1 N- and C-terminally with three repeats of the HA tag. The pTET1::3xHA-TET1g (HA-TET1) construct yielded tet1-cko1−/− T1 plants with wildtype-like phenotype, which produced viable T2 seeds, and thus fully rescued the tet1 loss-of-function mutant, as further confirmed in T2 and T3 generations (Figure S4E). These results indicated that the HA-TET1 fusion protein is functional and finally allowed us to assess the subcellular localisation of TET1 reliably. The expression pattern of pTET1:: 3xHA-TET1g was consistent with the scRNA-seq data and the pTET1::TET1g-sYFP reporters (the comparably weaker LRC signal was likely caused by enhanced fluorescent protein signal and/or lower immunostaining efficiency in these cells). This once again confirms that the lack of rescue by the fluorescent reporters was due to the non-functionality of the fusion proteins rather than incorrect expression of the transgenes (Figure 4A). On the subcellular level, the functional HA-TET1 fusion protein displayed dual localisation, at the cell periphery presumably representing the PM and in the endomembrane system, with the presumable PM signal being more pronounced in younger cells and in the xylem (Figure 4A,B). Upon treatment with the ARF-GEF inhibitor Brefeldin-A (BFA), the HA-TET1-positive endomembrane compartments aggregated partially into the so-called BFA bodies together with PIN1 (Lam et al., 2009) (Figure S6A). These results indicate that endomembrane trafficking of HA-TET1 occurs in part along BFA-sensitive secretory and/or recycling pathways.
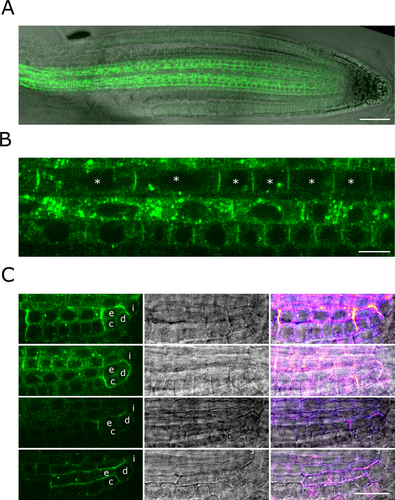
The presumable PM pool of HA-TET1 was distributed uniformly across the individual PM domains of the meristematic and transition zone cells (Figure 4B). This contrasted with the subcellular localisation of TET1-sYFP, which was often enriched at the centres of individual apical/basal PM domains of cortex and epidermis cells when expressed from the endogenous pTET1 or strong meristematic pRPS5A promoters (Figure S6B), reminiscent of the subcellular localisation of the receptor-like kinase IRK in Cortex-Endodermis Initials (CEIs), (Campos et al., 2019) the choline transporter CHER1 in the phloem sieve tubes (Dettmer et al., 2014), or the “super-polar” distribution of PINs (Kleine-Vehn et al., 2011) (Figure S6). Nonetheless, as HA-TET1, but not TET1-sYFP, rescued the tet1 mutant, we favour the hypothesis that the “super-polar” distribution of TET1-sYFP was an artefact caused by incorrect folding, ectopic interactions of the fusion protein and/or simply polar secretion visualised by the non-functional fusion. The uniform presumable PM distribution of HA-TET1 was a better proxy of the endogenous TET1 localisation. Notably, in the CEIs, CEIDs and young cortex and endodermis cells, HA-TET1 localised almost exclusively at the cell periphery, presumably at the PM, and its distribution was often asymmetric, with signal maxima at the apical, basal and/or lateral PM domains (Figure 4C).
Together with the missing endodermis cell file phenotype of the tet1-cko1 mutant (Figure 3F), this result suggests that TET1 might also be involved in the control of ground tissue patterning.
Taken together, the TET1 expression pattern, the mutant phenotype of the newly generated tet1-cko1 deletion allele, and the subcellular localisation of the functional HA-TET1 fusion protein all support a role for TET1 during vascular proliferation and ground tissue patterning.
4 DISCUSSION
A major unanswered question at the interface of plant cell and developmental biology is how tissue and cell polarity cues are integrated into precisely positioning the cell division plane (Glanc, 2022). In order to find novel regulators of this process in the context of developing vascular cells of the Arabidopsis root, we took a transcriptomics approach to pinpoint those factors being expressed at the correct spatiotemporal location. Based on overlap analysis of transcripts enriched in young phloem and procambium with those upregulated upon ectopic activation of the TMO5/LHW-DOF2.1 pathway (De Rybel et al., 2013; Smet et al., 2019), we identified TET1/TRN2, a member of the evolutionary conserved tetraspanin protein family (Reimann et al., 2017) as a promising candidate of vascular cell division orientation. By analysing the promoter region of TET1, we found that the expression domain depends more on regulatory elements in the intron compared to the length of the promoter and, most importantly, for its function, that TET1 is expressed in vascular cells as was predicted by single-cell transcriptomics (Wendrich et al., 2020). Moreover, trn2-7 and trn2-1 mutants showed a significant reduction in the number of vascular cell files in the root meristem, supporting a possible role of TET1 in the controlled switch from anticlinal to periclinal/ radial division plane positioning during vascular development. To prevent potential side effects of truncated TET1 proteins in existing mutants, we generated a full knock-out TET1 mutant, tet1-cko1. Besides reiterating the reduction of vascular cell file numbers in the root meristem as the previously described alleles, an additional novel phenotype in the ground tissue was found, with a proportion of the mutants missing an endodermal cell file. To further characterise TET1, we generated and analysed protein localisation in the tet1-cko1 mutant complemented with the functional HA-TET1 protein. This revealed TET1 localisation at the cell periphery, presumably representing the plasma membrane and endomembrane system of the root vasculature and asymmetrical polar distribution at the CEI and young cortex and endodermal cells. Our study has thus established TET1 as a regulator of vascular proliferation and also revealed the involvement of TET1 in ground tissue patterning, providing the necessary foundation for future work on understanding the molecular mechanisms behind these phenomena. It is tempting to speculate that TET1 might exert its developmental roles by contributing to the control of cell division orientation. Nonetheless, additional in-depth analysis will be needed to properly test this hypothesis.
Besides these biological insights, our work again demonstrates the importance of mutant complementation and careful analysis of generated plant material. Cross-checking expression profiles with publicly available single-cell datasets allows validation of reporter line expression profiles, as is the case with TET1, where meristem expression depends on the presence of the intron. Initially, we aimed to complement trn2-7 and trn2-1 mutants with fluorescent protein fusions. After screening a multitude of constructs, we did not find a single one that could rescue the tet1/trn2 phenotype (Cnops et al. 2006). As all 7 of the previously characterised TET1 mutant alleles have mutations around the large extracellular loop (Cnops et al. 2006), we investigated whether our failure to complement these mutants is because of a dominant-negative effect due to possible expression of truncated protein. We were surprised to find the tet1 phenotype present not only in Col-0 lines carrying truncated versions of TET1, but also in overexpression lines harbouring full-length TET1 and ruling out potential silencing issues. These results demonstrate that the tet1 phenotype can be caused by both gain-of-function and loss-of-function and argue that a precise balance of TET1 activity is necessary for plant development. We finally managed to complement a true full-deletion mutant, tet1-cko1, with the pTET1::TET1g and pTET1::HA-TET1g constructs but failed to do so by pTET1::TET1g-mCitrine, pTET1::mCitrine-TET1g or pTET1::TET1g-HA, which differed only in the type and position of the tags. This result confirmed that fluorescent C-terminal, as well as N-terminal, TET1 fusions are not functional. This necessitates a careful re-evaluation of any data involving such reporters, as well as well as fluorescent fusions of other TET homologues whose functionality had not been tested (Yamaguchi et al., 2017; Zhu et al., 2022). Overall, we hope that our work demonstrates the importance of generating full knockout mutant lines and checking for full complementation before continuing any sort of in-depth analysis of generated material, as well as providing a solid foundation for further work on TET proteins in root development.
AUTHOR CONTRIBUTIONS
M.G. and B.D.R. conceived the project; B.D.R., M.G., E.M. and N.K. designed experiments and analysed data; E.V., J.N., K.V., E.M., N.K. and M.G. performed experiments; F.W. provided unpublished constructs and transgenic lines; D.V.D. contributed to data interpretation and revising the manuscript; B.D.R. and M.G. supervised the project; M.G., N.K. and B.D.R. wrote the paper with input of all authors.
ACKNOWLEDGEMENTS
The authors thank Veronique Storme for assistance in data analysis, Thomas Eekhout for help with data submission, Mieke van Lijsebettens for sharing unpublished constructs and lines and critical reading of the manuscript, the VIB Bioimaging core and the IEB ASCR Imaging facility for imaging assistance, and all members of the Vascular Development team for valuable discussions. M.G. is grateful to Matyáš Fendrych for hosting him in his laboratory during the final stages of the project and to all members of the Fendrych lab, as well as Roman Pleskot and Nemanja Vukašinović, for their feedback and support.
FUNDING INFORMATION
This work was funded by The Research Foundation - Flanders (FWO; Odysseus II G0D0515N and project G0G2621N); the European Research Council (ERC Starting Grant TORPEDO; 714055); the European Union's Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 885979 “DIVISION BELL” and an EMBO long term fellowship (ALTF 1005–2019).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All quantitative data supporting the findings of this study and respective statistics are available as Table S5. Materials and resources of this study are available from the corresponding author upon reasonable request. Raw data for the bulk RNA-seq analysis can be accessed at NCBI with GEO number: GSE244902.




