The cytochrome P450 enzyme WsCYP71B35 from Withania somnifera has a role in withanolides biosynthesis and defence against bacteria
Abstract
The medicinal properties of Ashwagandha (Withania somnifera L. Dunal) are attributed to withanolides, which belong to the triterpenoid steroidal lactones class of compounds. Though it is proposed that intermediates of the universal phytosterol pathway are utilized by cytochrome P450 (CYP450) enzymes to form withanolides, studies on the functional characterization of these enzymes have been sparse. This study reports the functional characterization of a CYP450 candidate from W. somnifera, WsCYP71B35 that exhibited induced expression in response to methyl jasmonate treatment and showed higher expression in tissues that accumulate withanolides. Biochemical assay with yeast microsomal fraction expressing recombinant WsCYP71B35 indicated no activity when phytosterols and their intermediate 24-methylene cholesterol were used as substrates. However, WsCYP71B35 catalyzed product formation with withaferin A, withanolide A, withanolide B, and withanoside IV among the tested substrates. Moreover, virus-induced gene silencing (VIGS) and transient overexpression of WsCYP71B35 in W. somnifera leaves modulated the levels of withaferin A, withanolide A, and withanolide B, indicating the role of WsCYP71B35 in withanolides pathway. Furthermore, VIGS of WsCYP71B35 in W. somnifera reduced its tolerance to Pseudomonas syringae (DC3000) infection, whereas overexpression enhanced the tolerance to the bacterium in W. somnifera and transgenic tobacco. Overall, these results provide insights into the role of W. somnifera WsCYP71B35 in withanolides biosynthesis and bacterial defence.
1 INTRODUCTION
Terpenoids represent the largest and most diverse group of plant specialized metabolites and are often specific or unique to individual plant species or groups of species. They provide overall fitness to the plant in terms of their ecological roles and defence against stresses. Among the terpenoids, triterpenoids/steroids are the most numerous groups of plant metabolites, and their structural diversity lies in the modification of side chains (Thimmappa et al., 2014). Besides their beneficial role in plants, these triterpenoids have immense value to humans due to their medicinal properties (Banerjee et al., 2019). Withanolides are a group of secondary metabolites belonging to the class of tetracyclic triterpenoid steroidal lactones that contain more than 600 structurally distinct compounds, found mostly in plants of the Solanaceae family (Knoch et al., 2018). The best-known plant to produce withanolides is Withania somnifera, also known as Indian ginseng or Ashwagandha. It is a medicinal plant that has been used for over 3,000 years in traditional Indian medicine or Ayurveda and is also relevant in modern medicine (Bharti, Malik and Gupta, 2021). Traditional healers have been using roots and leaves of this plant for over 6000 years to treat a wide variety of ailments, including asthma, fever, inflammation, abdominal pain, constipation, helminths, tuberculosis, and cardiac disorders and to improve overall fitness (Joshi and Joshi, 2021). These healing properties are credited to the presence of the active ingredients, withanolides. So far, over 40 withanolides have been reported from this plant, which include withaferin A, withanolide A, withanolide B, withanone, withanolide D, 12-Deoxywithastramonolide, various hydroxyl- and deoxy- withanolides, and several withanosides. The concentration of these compounds varies based on the type of tissue and stage of the plant, ranging from 0.001 to 0.5% of the dry weights (Tetali et al., 2021). Modern research has shown that a number of withanolides have promising pharmacological properties for the treatment of inflammation-associated chronic diseases, including cancer, arthritis, autoimmune and neurodegenerative diseases (White et al., 2016; Wang, 2020). Though several withanolides are found in this plant, only withaferin A and withanolide D have been extensively studied for their medicinal properties (Dhar et al., 2015). Both Withaferin A and Withanolide D have been reported to inhibit angiogenesis, Notch-1 and NFjB in cancer cells and induce apoptosis in breast cancer cells (Kaileh et al., 2007; Koduru et al., 2010; Hahm et al., 2011). Plant extracts of W. somnifera, as well as purified withanolides, have demonstrated diverse pharmacological activities such as anti-inflammatory, anti-tumor, cardioprotective, neuroprotective and anti-bacterial properties (Mirjalili et al., 2009; Sehgal et al., 2012).
Despite their immense pharmacological and therapeutic potential, commercial exploitation of withanolides has been limited owing to low in planta accumulation (in the range of 0.001 to 0.5% of DW), resulting in limited availability in purified forms (Agarwal et al., 2018). Also, the accumulation of withanolides is influenced by various factors such as growth rate, tissue type, geographical and environmental conditions, and chemotype (Dhar et al., 2013). Moreover, successful metabolic engineering to improve withanolide production requires a complete understanding of the genes involved in the biosynthetic pathway. Structurally, withanolides are C28 steroids with ergostane skeleton in which C26 and C22, or C26 and C23, are oxidized in order to form a δ- or γ-lactone (Chen, He and Qiu, 2011). Withanolides have been reported to be derived through the universal phytosterol pathway (Figure 1). It is proposed that the intermediates of the phytosterol pathway derived via cycloartenol undergo various biochemical transformations such as hydroxylation and glycosylation postulated to be carried out by cytochrome P450 enzymes (CYP450s) and glycosyltransferases (GTs) leading to the biosynthesis of withanolides and withanosides, respectively (Lockley, Rees and Goodwin, 1976; Sangwan et al., 2008; Singh et al., 2015). CYP450 enzymes are a large class of heme-thiolate proteins that are ubiquitous in their presence across all genera of organisms and catalyze diverse reactions in pivotal molecular pathways. They have been studied and classified by Nelson, 2009 and are available at https://drnelson.uthsc.edu/CytochromeP450.html , and an estimated 80 CYP450s have been assigned biochemical functions related to the plant triterpene metabolism (Ghosh, 2017).
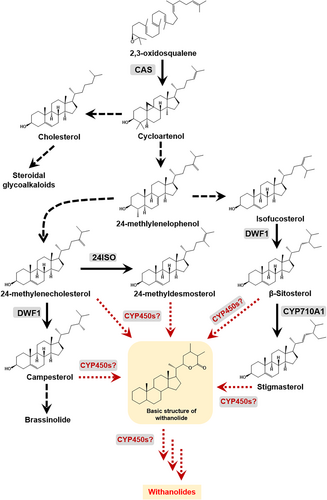
Efforts have been made by different research groups to identify and characterize CYP450s in Withania somnifera. Elicitor-responsive WsCYP98A and WsCYP76A have been found to express abundantly in stalk and root, respectively, correlating in a positive manner to withanolides accumulation (Rana et al., 2014). In another study, it was shown that WSCYP93Id protein catalyzed the conversion of withaferin A to an unidentified hydroxylated product (Srivastava et al., 2015). Functional characterization of WsCYP85A69 through miRNA and transient overexpression resulted in the modulation of castasterone, stigmasterol and withanolides. Further, it was shown that WsCYP85A69 catalyzed the in vitro conversion of 6-deoxocastasterone into castasterone (Sharma et al., 2019). Despite these studies, the knowledge of the biochemical and in planta role of CYP450s in withanolides biosynthesis is poorly understood. Recently, we have performed molecular and in planta characterization of three CYP450 genes (WsCYP749B1, WsCYP76 and WsCYP71B10) that showed their involvement in withanolides formation and defence in W. somnifera (Shilpashree et al., 2022). Here, we present the functional characterization of another CYP450 gene (WsCYP71B35) from W. somnifera and show its participation in withanolide biosynthesis and defence using biochemical, in planta silencing, and overexpression studies.
2 MATERIALS AND METHODS
2.1 Plant material and methyl jasmonate treatment
W. somnifera cv. Poshita (National Gene Bank, CSIR-CIMAP, India) seeds were sown using the casting method in a seed bed tray. The planting mixture was composed of sterile soilrite, vermicompost and soil in the ratio of 1:1:1. Two-leaf staged seedlings were transplanted into pots and were maintained in a growth chamber at 25°C with 16/8 h light/ dark cycle and 70% humidity. Plants of the appropriate leaf stage (described in respective methods sections) were used to carry out VIGS and overexpression experiments. Methyl jasmonate (MeJA) treatment was performed using 10-week-old plants as described previously (Singh et al., 2017). Samples were harvested at different time intervals (h) and stored at 80°C until further use. To analyze the gene expression in different tissues, berries, flowers, leaves, roots and stems were collected from W. somnifera plant and stored at −80°C for further use.
2.2 Phylogenetic analysis
Amino acid sequences of cytochrome P450s belonging to the CYP71 family that has been shown to be involved in secondary metabolism, as well as three CYPs from W. somnifera characterized previously (Shilpashree et al. 2022), were used to construct a phylogenetic tree. Neighbourhood joining method was used for tree construction using MEGAX with default setting and bootstrap value changed to 1000 (Tamura et al., 2013). GenBank accession numbers for all CYP450 sequences used in the phylogenetic tree are given in Table S1.
2.3 RNA isolation, cDNA synthesis and qRT-PCR analysis
Total RNA from different tissues was extracted from 100 mg tissue using the TRIzol reagent or SpectrumTM Plant Total RNA kit (Sigma-Aldrich) following the manufacturer's instructions. On-column DNase digestion was performed with DNase I (Sigma-Aldrich) to remove contaminating genomic DNA. cDNA was synthesized with 2 μg total RNA and random hexamer primers using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems) as per the manufacturer's instructions. A linear range of cDNA was determined with 18S rRNA (endogenous control) and used for qRT-PCR analysis. qRT-PCR analysis was carried out with gene-specific primers that were designed using Primer Express software outside the gene region used for cloning into the pTRV2 vector (Table S2). The reaction was performed using 2X SYBR green mix (Thermo Scientific) and run in StepOne Real-Time PCR System (Applied Biosystems). The qRT-PCR conditions used were as follows: 94°C for 10 min, followed by 40 cycles of 94°C for 15 s and 60°C for 45 s. Fold-change differences in gene expression were analyzed using the comparative cycle threshold (Ct) method.
2.4 Generation of VIGS and overexpression constructs
For the generation of the VIGS construct, a 500 bp fragment corresponding to WsCYP71B35 was amplified from leaf cDNA using gene-specific primers. The amplicon was cloned into a pGEMT-easy vector, sequence confirmed by nucleotide sequencing, and subsequently sub-cloned into XbaI and XhoI sites of pTRV2 plasmid that was procured from TAIR (www.arabidopsis.org), resulting in pTRV2::WsCYP71B35 construct (Figure S1, Table S2). To generate the plant overexpression construct, first, the open reading frame (ORF) of WsCYP71B35 was PCR amplified using leaf cDNA, and the resulting amplicon was cloned into pJET1.2/vector and confirmed by nucleotide sequencing. The WsCYP71B35 fragment was restriction digested from pJET1.2 construct using XbaI and SacI and subcloned into the respective sites of pBI121 vector under 35S promoter to form pBI121::WsCYP71B35 construct (Figure S1, Table S2). The plasmids (pTRV1, pTRV2, and pBI121) and constructs (pTRV2::WsCYP71B35 and pBI121::WsCYP71B35) were individually transformed into A. tumefaciens by freeze–thaw method.
2.5 VIGS and transient overexpression of WsCYP71B35
VIGS and transient overexpression of WsCYP71B35 were performed as described earlier (Singh et al., 2017). For VIGS, the transformed A. tumefaciens (strain GV3101) cultures were grown in 100 mL YEP medium at 28°C. The overnight grown cultures were harvested by centrifugation and resuspended in infiltration buffer (10 mM MgCl2, 10 mM MES and 100 μM acetosyringone, pH 5.6) to a final OD600 of 1.6 and incubated at 28°C for additional 4–5 h. Infiltration was performed using 1:1 ratio of Agrobacteria cultures harboring TRV1 and TRV2 or its derivatives using 1-mL needleless syringe on the abaxial side of leaves in 4-leaf-staged plants. Leaves exhibiting viral infection phenotype were harvested 30 days post infiltration and used for transcript and metabolite analyses. Transient overexpression was performed by co-infiltration of Agrobacteria harbouring pBI121::WsCYP71B35 and p19, and pBI121 and p19 vectors (control). Briefly, overnight grown Agrobacteria cultures were pelleted and resuspended in infiltration buffer (50 mM MES, 2 mM Na3PO4, 0.5% glucose, and 100 μM acetosyringone with pH adjusted to 5.6) to a final OD600 of 0.2 and incubated at 28°C for 3–4 h. Leaf infiltration of agro-suspension was performed as described above in the first pair of leaves of 6–8 leaf-staged plants and placed in dark condition for 48 h. The infiltrated leaves were harvested and stored at −80°C for metabolite and transcript analyses (Singh et al., 2015).
2.6 Overexpression of WsCYP71B35 in yeast and CYP450 assay
The open reading frame of WsCYP71B35 was amplified and cloned into BamHI and EcoRI sites of yeast expression vector pYEDP60u, resulting in pYEDP60u::WsCYP71B35 (Figure S1, Table S2). The empty vector and the overexpression constructs were transformed into Saccharomyces cerevisiae WAT11 strain (with yeast reductase replaced by the ATR1 reductase from Arabidopsis thaliana, under GAL10-CYC1 promoter andCPR1 terminator) (Urban et al., 1997). The confirmed colonies were cultured using a high-density procedure as described in Pompon et al., 1996. Briefly, an individual colony was streaked onto an SG(A)I plate. A loopful of culture from the plate was inoculated into 30 mL of SG(A)I and grown to stationary phase (overnight). A 1:50 dilution was made into 500 mL YPGE medium (yeast extract-10 g, Bacto-Peptone- 10 g, glycerol- 20 mL and ethanol- 10 mL per liter of H2O) and cells were grown at 30°C in a shaking incubator until cell density (OD600) reached 8 x 107 cells per ml (~24–36 h). Induction was initiated by the addition of 10% (v/v) of sterile aqueous solution of galactose (20 mg mL−1). The induction was continued for 8–15 h until the cell density reached 2–5 x 108 cells per mL. The cultures were pelleted, cells were mechanically broken and centrifuged for microsomal isolation. Protein content in isolated microsomes was determined by Bradford's method using Bovine Serum Albumin (BSA) as a standard. CYP450 assay was performed using potassium phosphate buffer (50 mM, pH 7.4) containing 1 mM NADPH and 100 μM of potential substrate (phytosterol intermediates and different commercially available withanolides/withanosides). The reaction was initiated by addition of 0.2–0.5 mg of a microsomal protein fraction of S. cerevisiae harbouring either pYEDP60u (control) or pYEDP60u::WsCYP71B35, and the mixture was incubated at 30°C for 1 h in a water bath. The reaction was terminated by chilling the mixture on ice and adding 3 N HCl. The reaction products were extracted by the addition of chloroform: methanol (7:3), and the extract was used for detection using HPLC analysis as described below.
2.7 Extraction and analysis of withanolides
Withanolides extraction and analysis from W. somnifera was performed following Singh et al. (2015). Leaf tissues were oven-dried at 55°C, and 20 mg of dried tissue was ground using 1 mL absolute methanol, sonicated, and the supernatant was collected. The ground tissue was re-extracted twice, and the methanolic extracts that were obtained were pooled in a scintillation vial and allowed to evaporate. The remaining residue was resuspended in 3 mL of 70% methanol. This was extracted thrice with 3 mL chloroform. The lower layer of a chloroform extract (9 mL) was decolorized using charcoal and dried. To the dried samples, 200 μL of 7:3 chloroform:methanol was added and used for HPLC analysis. Whereas the CYP450 enzyme assay products were directly used for HPLC analysis. Withanolides were analyzed using HPLC (Model: Nexera, Shimadzu) fitted with a C-18 column (250 x 4.60 mm, 5 μm) as per the program described previously. Briefly, Potassium dihydrogen phosphate buffer (1.02 mM) and acetonitrile were used as aqueous and organic solvents. A gradient program was used to separate withanolides. Initially, solvent A concentration was set at 95%, then changed to 55–20% at 18 and 25 min. 20% of solvent A was maintained for the next 10 min, later, it was increased to 55% at the 35th min and 95% at the 40th min. The flow rate was set to 1.5 mL min−1 with UV detection at 227 nm. All standards of withanolides were from Natural Remedies Pvt. Ltd. (Bangalore, India), and the standard stock concentration was 1 mg mL−1. To 36 μL of withanolides extract, 4 μL of 1 mg mL−1 catharanthine (Sigma-Aldrich) was added as internal standard, and 20 μL of this mixture was injected into the HPLC system. The area of individual withanolides was determined after normalizing with the peak area of catharanthine (Singh et al., 2015, 2017).
2.8 Bacterial growth curve assay
A bacterial growth assay was performed using the model plant pathogen P. syringae pv. tomato DC3000. The bacterium was cultured in 5 mL nutrient broth and grown in an incubator shaker at 28°C. The overnight grown culture was centrifuged, and the pellet was resuspended in 10 mM MgCl2 to achieve an OD600 = 0.002. Bacterial infiltration and cfu determination were performed as described previously (Singh et al., 2015, 2017). Leaf discs from infected and buffer-infiltrated control plants were collected 3 days post-infiltration and used to assess the bacterial infection rate. Leaf discs were homogenized using 10 mM MgCl2, and the cfu cm−2 was determined by plating serial dilutions of leaf extracts on Pseudomonas-specific agar plates.
2.9 Generation of transgenic tobacco
Leaf disc co-cultivation with A. tumefaciens harbouring pBI121 and pBI121::WsCYP71B35 was carried out to transform tobacco as described in Singh et al., 2017. Transformed shoots regenerated on MS (Murashige and Skoog, 1962) medium supplemented with kanamycin (100 mg L−1) selection media were transferred to ½ strength MS agar for rooting. Plantlets with well-developed roots were removed from the bottle and transferred to pots containing sterile soilrite mix for hardening. Further, the plants were PCR screened and positive lines were transferred to the growth chamber. Seeds were collected from T0 lines and used to generate T1 plants. PCR-screened positive lines of T1 plants were used for transcript and bacterial growth analyses (Singh et al., 2017).
2.10 Statistical analysis
Means, standard deviations and standard errors were calculated using the GraphPad QUICKCALC online tool. The statistical significance between control and treated samples was calculated using an unpaired Students t-test. Asterisks indicate significant difference between samples (*P < 0.05; **P < 0.01; and ***P < 0.001).
3 RESULTS
3.1 Identification and sequence analysis WsCYP71B35
In our previous work, WsCYP450 genes were mined from an in-house generated transcriptome along with the “Withanomics” database curated by Senthil et al. (2015) (www.im-crop.snu.ac.kr, the site is no longer active). Initially, 159 sequences were annotated to the CYP450 family of enzymes. After filtering for specific FPKM values, duplicity, and partial sequences, 18 sequences were shortlisted. Of these, three genes having induced expression upon MeJA treatment were identified and characterized at in planta level previously (Shilpashree et al., 2022). Here, a fourth CYP450 candidate belonging to the CYP71 family (WsCYP71B35) has been functionally characterized. WsCYP71B35 contains an open reading frame of 1515 bp that encodes a protein of 504 amino acids with a calculated molecular mass of 57190.50 Da (GenBank accession number: MW298520.1). Analysis of the deduced amino acid sequences of the encoded protein showed the presence of [FW]-[SGNH]-x-[GD]-{F}-[RKHPT]-{P}-C-[LIVMFAP]-[GAD] consensus pattern for cysteine heme-iron ligand signature, where C is the heme iron ligand (Figure 2). Phylogenetic analysis with candidates of the CYP71 family from other plant species indicated that WsCYP71B35 has 58.57% identity with Hyoscyamus muticus CYP71 followed by 46.8% identity with MgCYP71D95 from Mentha x gracilis (Figure 2A). WsCYP71B35 possessed the most conserved motif among CYP450 enzymes, which is the heme-binding region FxxGxRxCxG (also known as the CxG motif), containing the axial Cys ligand that binds to the heme (Figure 2B). Also, WsCYP71B35 contained ExxR and PER motifs that form the E-R-R triad, which is important for locking the structure of the heme pocket in place and ensuring the stabilization of the core structure (Córdova et al., 2017). Prediction of subcellular localization using different tools indicated that CYP71B35 is membrane-targeted, which is a characteristic of CYP450 enzymes (Table S3).
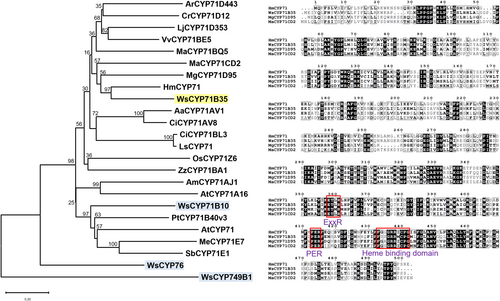
Sequence analysis of WsCYP71B35 with other related plant CYP450 enzymes. (A) Phylogenetic tree showing the relationship of WsCYP71B35 with other plant CYP450s of the CYP71 family as well as CYPs from W. somnifera characterized previously (blue). The tree was constructed using MEGAX software (Kumar et al., 2016), and statistical reliability of individual nodes of the tree was assessed by bootstrap analyses with 1000 replicates. The GenBank accession numbers of sequences used for constructing the phylogenetic tree are shown in Table S1. (B) Multiple sequence alignment of WsCYP71B35 with related CYP71 family enzymes from Catharanthus roseus (ACM92061), Hyoscyamus muticus (ABS00393), and Manihot esculenta, (Q6XQ14). The characteristic heme-biding domain of CYP450s is shown in a red box.
3.2 Expression of WsCYP71B35 in response to MeJA and in different tissues
MeJA is a known inducer of pathway genes and transcription factors involved in secondary metabolism, including genes related to withanolide biosynthesis. To determine whether expression of WsCYP71B35 is induced in response to MeJA, qRT-PCR was performed using leaf tissues collected at different time intervals after MeJA treatment. It was found that WsCYP71B35 exhibited a drastic induced expression of almost 80-fold at 6 h upon exposure to MeJA (Figure 3A). However, WsCYP71B35 expression reverted back to 0 h level at 12 h, and remained the same at 24 and 48 h after MeJA treatment. The observed MeJA-induced expression of WsCYP71B35 indicated its possible involvement in secondary metabolism and possibly in withanolides biosynthesis (Figure 1). Further, expression analysis using different tissues revealed that WsCYP71B35 possessed the highest expression in tissues that predominantly accumulate withanolides, i.e., leaf (~70-fold), followed by fruit (~50-fold), flower and root (~30-fold each) with respect to the stem where the least expression was seen (Figure 3B).
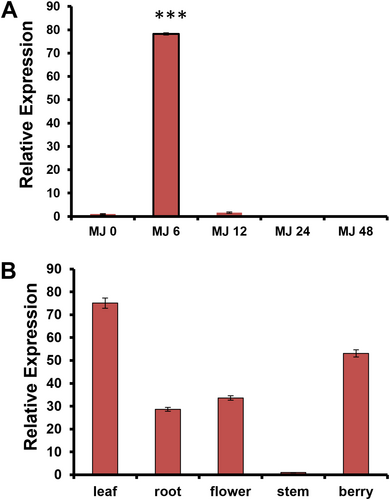
Gene expression analysis of WsCYP71B35. (A) Effect of methyl jasmonate treatment on WsCYP71B35 mRNA levels at time intervals of 0, 6, 12, 24, and 48 hours. The expression is represented relative to the control leaf tissue. (B) Relative transcript abundance of WsCYP71B35 in different tissues of W. somnifera. In each graph, the tissue having the least Ct was set to one-fold to determine the relative abundance of transcripts in other tissue. 18S rRNA was used as an internal reference for normalization. The data shown are from three independent experiments. Student's t-test: ***, p < 0.001. Error bars indicate mean ± SE.
3.3 Biochemical characterization of WsCYP71B35
To understand the biochemical function of the WsCYP71B35 enzyme, the ORF was cloned into a yeast overexpression vector, and the recombinant protein was expressed in yeast. Since withanolides are reported to be derived from the universal sterol pathway in W. somnifera, phytosterols such as campesterol, cholesterol, stigmasterol, beta-sitosterol and one of their intermediates 24-methylenecholesterol (presumed to be the precursor of withanolides) were used as substrates. Enzyme assay using yeast microsomal fraction expressing recombinant WsCYP71B35, the phytosterol substrates, and NADPH as a cofactor, followed by HPLC analysis of the reaction products indicated no activity with any of the tested phytosterols. Further, an independent CYP450 assay with seven different withanolides (withanolide A, withanolide B, withanone, 12- deoxywithastramonolide, withanodise IV, withaferin A and withanoside V) as substrates and NADPH cofactor, showed that four substrates among the tested seven exhibited product formation. It was found that WsCYP71B35 catalyzed the product formation in the presence of withaferin A, withanolide A, withanolide B, and withnoside IV as substrates (Figure 4). A comparison of UV spectra from the PDA detector of the HPLC instrument revealed that the product peak formed in each assay had different absorption maxima, indicating that the products formed were different from each other (Figure S2).
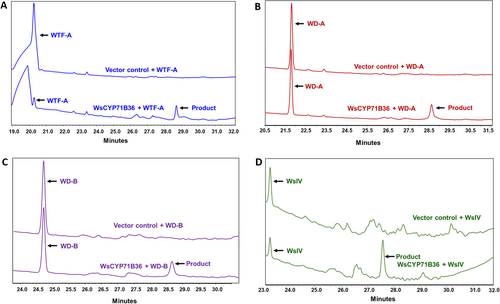
HPLC analysis of reaction products of CYP450 assay. The yeast microsomal fraction expressing the recombinant WsCYP71B35 along with different withanolides, A) Withaferin A, B) Withanolide A, C) Withanolide B and D) Withanoside IV as substrates were used in the assay. Assay with substrates that showed product formation are only shown here. The chromatograms at the top and bottom of each sub-figure represent reactions using microsomal fraction of yeast transformed with empty vector (control) and pYeDP60u::WsCYP71B35 construct, respectively.
3.4 Effect of silencing and overexpression of WsCYP71B35 on withanolide biosynthesis
To determine the in-planta role of WsCYP71B35 in formation of different withanolides (Figure 5A), virus-induced gene silencing (VIGS) was performed. As VIGS is prone to off-target silencing (Dinesh-Kumar et al., 2003), the sequence region unique to WsCYP71B35 was chosen to generate the silencing constructs. Thirty days post-infiltration, leaves of similar developmental stages exhibiting typical viral infection symptoms were collected along with empty vector control for transcript and metabolite analyses. qRT-PCR analysis showed 80% reduction in the expression of WsCYP71B35 compared to EV controls (Figure 5B). The degree of silencing of WsCYP71B35 in this study was comparable to the VIGS of other related genes of W. somnifera reported in earlier studies as well as our own previous study (Singh et al., 2015, 2017; Agarwal et al., 2018; Shilpashree et al., 2022). Subsequent analysis of metabolites through HPLC indicated that VIGS of WsCYP71B35 did not significantly alter the levels of withanolides in the plants except for withanolide A. In the silenced plants, it was observed that withanolide A levels were reduced by 75% compared to EV (Figure 5C). In addition, withaferin A showed an increased level that was not statistically significant in WsCYP71B35-vigs plants. In continuing to characterize the role of WsCYP71B35 in planta, we proceeded to overexpress this gene by agroinfiltration in W. somnifera leaves. Two days post infiltration, leaves were collected for metabolite and transcript analyses. qRT-PCR analysis showed that WsCYP71B35 was overexpressed almost 8-fold in the infiltrated tissue (Figure 5D). HPLC analysis of the metabolites revealed an increased level of withanolide A by around 160% and, surprisingly, a decreased level of withanolide B by about 90% as compared to vector control (Figure 5E).
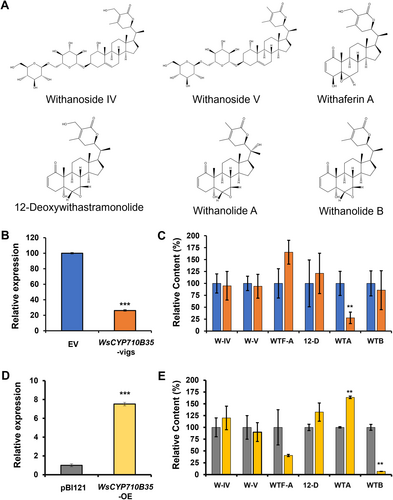
Effect of silencing and overexpression of WsCYP71B35 on withanolide content. (A) Chemical structures of W-IV, withanoside IV; W-V, withanoside V; WTA, withanolide A; WTB, withanolide B; WTF-A, withaferin A and 12-D, 12-deoxywithastramonolide that were analysed in the WsCYP71B35 silenced and overexpressed leaves of W. somnifera. (B) Quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis of WsCYP71B35 expression in silenced leaves. Expression level of WsCYP71B35 was normalized to 18S rRNA and set to 1 in an empty vector (EV) control to determine the relative reduction in WsCYP71B35 -silenced leaves. (C) High performance liquid chromatography (HPLC) quantification of different withanolides in control and WsCYP71B35 -silenced leaves. (D) Quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis of WsCYP71B35 expression in overexpressed (OE) leaves. (E) withanolides levels in control and WsCYP71B35 overexpressing leaves quantified through HPLC. Levels of different withanolides are represented relative to EV. The peak area of individual withanolides was determined after normalizing with peak area of internal standard (catharanthine). The results shown are from (a) three, (b) five and (d) 10 independent experiments. Student's t-test: **, p < 0.01; ***, p < 0.001. Error bars indicate mean SE.
3.5 Role of WsCYP71B35 in bacterial defense
In our previous studies in W. somnifera, it was observed that VIGS and overexpression of genes related to phytosterol and withanolides pathway modulate the expression of defence-related genes and affect pathogen defence (Singh et al., 2015, 2017). Moreover, withanolides have been shown to possess antibacterial and antifungal properties (Choudhary et al., 1995). As silencing and transient overexpression of WsCYP71B35 resulted in modulation of withanolides content, we proceeded to study their effect on PR genes. We focused on salicylic acid (SA)- dependent PR1 and jasmonate-dependent PR3 genes. It was observed that PR1 and PR3 expression did not show statistically significant changes in expression in the silencing (VIGS) background. In stark contrast, overexpression of WsCYP71B35 led to significant upregulation of both PR1 and PR3 by 6.2- and 16.9-fold, respectively (Figure 6). Following this, a bacterial growth assay was performed to determine WsCYP71B35s' role in defence. Fully expanded leaves from WsCYP71B35-vigs and EV control plants were infiltrated with P. syringae DC3000. Leaves from WsCYP71B35-vigs background developed severe disease symptoms and sustained more tissue damage than EV leaves. Further, bacterial growth assay using extract isolated from infiltrated leaves 3 dpi showed that WsCYP71B35-vigs samples exhibited a drastic increase in the growth of P. syringae DC3000 than that of EV control. The log cfu cm−2 for WsCYP71B35-vigs was found to be 7.1, whereas it was ~6 cfu cm−2 for EV control (Figure 7A). Leaves overexpressing WsCYP71B35 remained healthy and exhibited no necrotic phenotype compared to control at 4 dpi of P. syringae DC3000. Subsequent bacterial growth assay revealed a significant reduction in the growth of P. syringae. The log cfu cm−2 in WsCYP71B35 overexpressing tissue was 5.3, while it was 7.2 in vector control (Figure 7B). The observed phenotype and bacterial growth in P. syringae infiltrated samples correlated with elevated and reduced expression of WsCYP71B35 (Figure 5B,D).
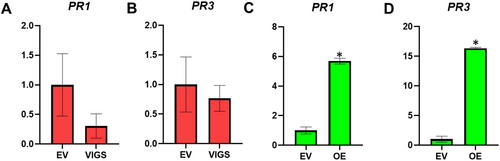
Relative expression of PR genes in WsCYP71B35 modulated plants. A) Expression of PR1 and B) PR3 in VIGS background. C) Expression of PR1 and D) PR3 in overexpression background. Relative gene expression was analysed using qRT-PCR and calculated using the 2−ΔΔCt method. Graphs were plotted using mean, error bars show ± SE from three independent experiments. Statistical significance represented as *p < 0.01.
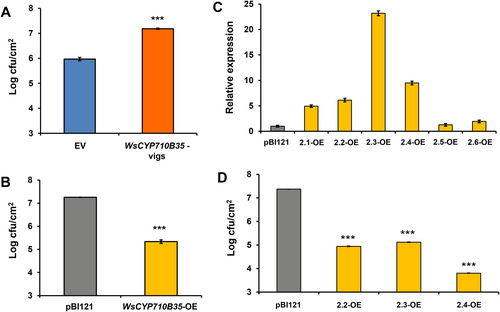
WsCYP71B35 confers tolerance to bacterial growth in W. somnifera and tobacco. Pseudomonas syringae growth assay in WsCYP450s silenced (A) and overexpressing (B) W. somnifera leaves. (C) Expression level of WsCYP71B35 in different transgenic tobacco lines, normalized to internal reference NtEF1α and represented as expression relative to pBI121 control that was set to 1. (D) Bacterial growth assay of transgenic tobacco lines with P. syrinage (DC3000) strains. The bacterial growth (CFU) at 3 dpi was obtained by plating serial dilutions of leaf disc extracts. Error bars show mean ± SE of three independent experiments. Statistical significance represented as ***p < 0.001.
3.6 Overexpression of WsCYP71B35 in transgenic tobacco confers tolerance to bacteria
Heterologous overexpression of plant CYP450s has been shown to confer tolerance to various stresses, including tolerance against bacteria (Pandian et al., 2020). To investigate the defensive role of WsCYP71B35 in a heterologous system, independent transgenic tobacco lines overexpressing WsCYP71B35 were generated. The transcript abundance of the WsCYP71B35 gene in 6 independent lines was determined by RT-qPCR and was found to be between 1- to 24-fold (Figure 7C). The three highest expressing lines and control plants were chosen for bacterial growth assays. Analysis of bacterial growth using extracts isolated from leaf discs of P. syringae infiltrated leaves at 3 dpi significantly repressed the multiplication of P. syringae as compared to that of empty vector infiltrated plants (Figure 7D). Similar to thisese results, overexpression of Panax ginseng PgCYP76C9 conferred enhanced resistance to P. syringae in transgenic Arabidopsis (Balusamy et al., 2017).
4 DISCUSSION
Withanolides are bioactive constituents of W. somnifera belonging to the triterpenoid steroidal lactone class of plant specialized metabolites. The terminal phytosterol pathway genes responsible for the biosynthesis of these immensely important molecules are yet to be identified. It is speculated that precursors derived from the universal sterol pathway undergo hydroxylation and other biochemical modifications postulated to be carried out by WsCYP450 enzymes, leading to the formation of diverse withanolides (Srivastava et al., 2015). Therefore, understanding the specific WsCYP450s involved is essential to elucidate the biosynthesis of these valuable compounds. In this study, we set out to identify and characterize WsCYP450s involved in withanolides biosynthesis through biochemical, molecular and in planta studies. The newly identified WsCYP71B35 possessed the characteristic cysteine heme-iron ligand domain structure and belonged to the CYP71 clan, which is the largest set of P450s in plants (Nelson et al., 2004). The families and subfamilies within the CYP71 clan have diverged remarkably during plant evolution, and are known to have diverse roles in the biosynthesis of terpenoids, phytoalexin, as well as indolic derivatives, cyanogenic glucosides, flavonoids, aldoximes and nitriles (Nelson et al., 2004; Nelson and Werck-Reichhart, 2011; Hamberger and Bak, 2013; XU, WANG and GUO, 2015). Phylogenetic analysis showed that the closest related enzyme to WsCYP71B35 was premna-spirodiene oxygenase from Hyoscyamus muticus (HmCYP71), which shared 58.57% sequence identity. HmCYP71 was characterized to carry out the regio-specific (C-2) hydroxylation of several eremophilane-type (decalin ring system) sesquiterpenes, including 5-epi-aristolochene (Takahashi et al., 2007). The second closest related enzyme in the tree was (−)-(4S)-limonene-3-hydroxylase from Mentha x gracilis (MgCYP71D95), which also carries out regio-specific hydroxylation of limonene at C-3 to (−)-trans-isopiperitenol (Bertea et al., 2003). Further, WsCYP71B35 also showed homology to the CYP71 enzymes involved in triterpenoid metabolism (Malhotra and Franke, 2022). They included Arabidopsis CYP71A16, that converts marneral/marnerol to 23-hydroxymarneral/23-hydroxymarnerol (Kranz-Finger et al., 2018), Melia azedarach CYP71BQ5 and CYP71CD2 which utilize dihydroniloticin and tirucalla-7,24-dien-3β-ol, respectively, forming corresponding products, melianol and dihydroniloticin (Hodgson et al., 2019), Lotus japonicas CYP71D353 that catalyzes the conversion of dihydrolupeol/20-hydroxylupeol to 20-hydroxylupeol/20-hydroxybetulinic acid (Krokida et al., 2013), and Ajuga reptans CYP71D443 that is involved in the conversion of 3β-hydroxy-5β-cholestan-6-one to 3β,22R-dihydroxy-5β-cholestan-6-one (Tsukagoshi et al., 2016). Given these similarities of WsCYP71B35 with other CYP71 enzymes involved in specialized terpenoid metabolism, it may be assumed that WsCYP71B35 may also possess regio-specific hydroxylation activity of withanolides. Interestingly, WsCYP71B35 did not share significant similarity with the three previously characterized CYP450s in our lab, indicating that this enzyme arose through a different lineage (Shilpashree et al., 2022).
The expression of pathway genes involved in plant secondary metabolism and the corresponding secondary metabolites are often induced in response to phytohormone elicitor MeJA. W. somnifera is no exception to this phenomenon wherein withanolides accumulation and expression of related genes have been shown to be induced in response to biotic and abiotic stresses (Dhar et al., 2015), and also in response to MeJA (Bhat et al., 2012; Dhar et al., 2014; Singh et al., 2015, 2017). Similarly, genes of phytosterol biosynthesis and a WRKY transcription factor were highly induced in response to MeJA (Singh et al., 2015, 2017). Also, genes encoding CYP450s that have been implicated in the formation of withanolides are shown to be induced in response to MeJA treatment (Dhar et al., 2015). Similar to the above reports, the expression of WsCYP71B35 was induced at the 6th hour of MeJA treatment and was higher in all tissues that are known to accumulate withanolides, indicating its possible role in withanolides biosynthesis (Figure 3). The highest expression of WsCYP71B35 was found to be in the leaf, which is known to accumulate phytosterols such as cholesterol, crinosterol and stigmasterol, and withanolides such as withaferin A, withanolide A, withanolide B, and withanone (Tetali et al., 2021). It has been reported that withanolides are formed via the universal phytosterol pathway, and 24-methylene cholesterol is suggested to be the starting precursor for all downstream withanolides (Lockley, Rees and Goodwin, 1976). Since CYP450 enzymes are known to utilize a wide range of substrates, a biochemical assay using yeast microsomal fraction expressing WsCYP71B35 with different phytosterols was performed. This revealed that there was no activity with any of the phytosterol substrates. The fact that this enzyme did not form any product when incubated with phytosterols indicated that they are likely to be involved in the formation of downstream and eventual withanolide products using the intermediate substrates made by other CYP450 enzymes of the metabolic pathway. However, when withanolides were employed as substrates, WsCYP71B35 exhibited the ability to catalyze the conversion of withaferin A, withanolide A, withanolide B, and withanoside IV, resulting in the formation of a distinct peak corresponding to an unidentified product, possibly resulting from hydroxylation (Figure 4). Elucidating the chemical structures of these products could shed light on the role of WsCYP71B35 in the withanolide biosynthetic pathway. However, as the withanolide biosynthesis is a complex pathway, deciphering the correct substrate for each CYP is difficult. For instance, a CYP450 from W. somnifera WSCYP93Id was found to convert withaferin A to a hydroxylated product, putatively thought to be 17-hydroxy withaferin A based on the correspondence of peak retention time of the product (Srivastava et al., 2015). In our attempts to identify the enzyme products of the biochemical assay using LC-ESI-MS/MS, we found it difficult to identify the hydroxylated products of withanolides due to the similarity of masses and fragmentation pattern with other withanolides. Nevertheless, the determination of the exact chemical structure of these products could shed some light on the role of WsCYP71B35 in the withanolide biosynthetic pathway.
Since biochemical assay products could not be confirmed, performing in planta studies would provide a better understanding of the gene function. To this end, VIGS and transient overexpression have been successfully adapted to study the in planta function of genes involved in the biosynthesis and regulation of withanloides in W. somnifera. Hence, WsCYP71B35 was specifically silenced to determine its role in the formation of withanolides. It was found that the degree of WsCYP71B35 silencing was comparable to the VIGS of other related genes of W. somnifera reported in earlier studies by other groups as well as our own previous studies (Singh et al., 2015, 2017; Agarwal et al., 2018; Shilpashree et al., 2022). Silencing of WsCYP71B35 resulted in a significant reduction of withanolide A, while its overexpression resulted in a drastic increase in withanolide A along with a reduction in withanolide B content (Figure 5). This suggests that WsCYP71B35 may play a direct role in withanolide A biosynthesis and have some indirect role in the regulation of other withanolide biosynthesis. Curiously, the hydroxylation of the 20th carbon of withanolide B forms withanolide A. However, this is unlikely to be the activity of WsCYP71B35 as enzyme assay with withanolide B clearly showed products at a much different retention time (Figure 4). Also, the fact that this enzyme did not form any detectable product when incubated with phytosterols indicated unknown steps and precursor/s in the pathway between phytosterols and withanolide A. Besides, though not statistically significant, withaferin A also showed some modulation as WsCYP71B35 silenced plants showed increased levels and overexpressing plants showed reduced levels. Withaferin A shares a common skeletal structure with withanolide B but lacks the C-20 hydroxylation found in withanolide A. Based on metabolite analysis, it can be construed that withanolide A, withanolide B and even withaferin A may have a common precursor and that their levels may be modulated by the expression of WsCYP71B35. The higher expression of WsCYP71B35 leads to higher levels of withanolide A, which depletes the precursor and leads to lower levels of withanolide B and withaferin A. Therefore, silencing of WsCYP71B35 should lead to the depletion of withanolide A, accumulation of the unknown precursor and, in turn, accumulation of withanolide B and withaferin A. While we do see a marginal increase in withaferin A, we do not see a significant increase in withanolide B levels, likely due to some unknown regulation or accumulation of a precursor not analysed in this study. Similarly, when we analyse the in-planta data of the three WsCYP450s from our previous study (Shilpashree et al., 2022), we hypothesize that WsCYP71B10 is diverting the carbon flux away from withanolide A and towards withanolide B, WsCYP76 diverts the carbon flux from 12-deoxywithastramonolide to withanolide A, and WsCYP749B1 diverts the flux from 12-deoxywithastramonolide to both withanolide A and B (Figure 8). Nevertheless, more investigations involving multiomics-driven research would be necessary to fully establish the role of the characterized CYPs and yet-to-be-discovered pathway enzymes in withanolide biosynthesis. In recent times, the multi-omics approach has led to the elucidation of several pathways involved in the biosynthesis of pharmaceutically important compounds, including the recent discovery of key steps of leonurine biosynthesis (Gupta, Sharma and Nagegowda, 2024; Li et al., 2024).
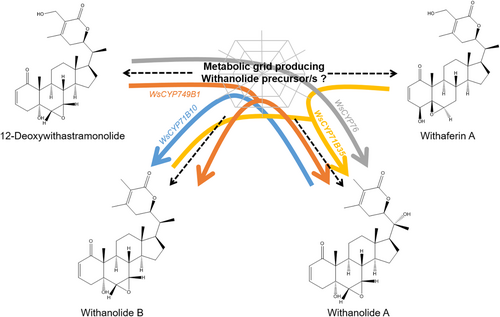
Proposed role of characterized WsCYP450s in withanolides biosynthesis. Schematic model depicting the role of four WsCYP450s based on the metabolite data obtained from the in planta silencing and overexpression of WsCYP71B35 (this study) as well from the data from our previous work (Shilpashree et al., 2022). The results indicate that WsCYP71B35 plays a role in the formation of withanolide A from a yet-to-be elucidated metabolic grid that forms precursor(s) common between withanolide B, 12-deoxywithastramonolide and withaferin A. The yellow arrow indicates the carbon flux being diverted from withanolide B and withaferin A towards withanolide A by WsCYP71B35. Similarly, the blue arrow shows the diversion of carbon flux from withanolide A to withanolide B by WsCYP71B10, grey arrow shows the diversion from 12-deoxywithastramonolide to withanolide A by WsCYP76, and finally the orange arrow shows the diversion from 12-deoxywithastramonolide to both withanolide A and B by WsCYP749B1.
CYP450s play important roles in plant defence through their involvement in phytoalexin biosynthesis, hormone metabolism and the biosynthesis of some other secondary metabolites (XU, WANG and GUO, 2015). When we assessed the role of WsCYP71B35 in plant defence, it was found that modulation of expression of WsCYP71B35 not only modulated withanolide levels in the plant but also modulated the expression of defence-related PR genes, PR1 and PR3. Further, it was observed that VIGS of WsCYP71B35 led to significantly reduced tolerance of plants to P. syringae growth, and in contrast, the overexpression of WsCYP71B35 resulted in increased tolerance to the same bacterium (Figure 7). It is interesting to note that these results are similar to our previous characterization of three CYPs from W. sominifera, indicating that cytochrome P450 enzymes play a crucial role in defence in this plant (Shilpashree et al., 2022). Moreover, AtCYP76C2 and AtCYP71A12 from Arabidopsis were found to be associated with a defence mechanism against Pseudomonas syringae infection (Godiard et al., 1998; Kempthorne et al., 2021). Hence, it is clear that WsCYP71B35 is involved in bacterial defence, however, the exact mechanism of signalling needs further investigation. It is possible that the products (one or more withanolides) formed by this enzyme could be involved in providing tolerance through their antibacterial activity or in signalling. Similar to our results, it has been previously reported that overexpression of Panax ginseng PgCYP76C9 conferred enhanced resistance to P. syringae in transgenic Arabidopsis (Balusamy et al., 2017). When we overexpressed WsCYP71B35 in a transgenic N. tabacum, we observed an increased tolerance to P. syringae infections. This shows that WsCYP71B35 has an independent role in plant defence. In conclusion, this study identified and characterized a novel CYP450 enzyme from W. somnifera and gave some insight into the biosynthetic pathway of withanolides. This will aid future work in the field of synthetic biology for the recombinant production of withanolides. This study also gives insight into biotechnological approaches for improving withanolide content by gene editing. We also show the role of WsCYP71B35 in plant defence.
In summary, this study has presented biochemical evidence for the utilization of certain withanolides as substrates by WsCYP71B35. Moreover, it has demonstrated the in planta role of WsCYP71B35 in both withanolides biosynthesis and defence against a model bacterial pathogen. Further elucidation of the enzymatic products through Nuclear Magnetic Resonance (NMR) analysis would offer valuable insights into the precise biochemical nature of WsCYP71B35. The characterized CYP450 could also be utilized in the metabolic engineering of W. somnifera cell cultures or the whole plant for the targeted enhancement of specific withanolides, and for the development of plants tolerant to bacterial pathogens.
AUTHOR CONTRIBUTIONS
H.B.S, A.K.N., S.R.K., and V.B. performed the experiments. H.B.S, A.K.N and D.A.N analysed the data. D.A.N conceived and coordinated the research. H.B.S, A.K.N., and D.A.N wrote the manuscript.
ACKNOWLEDGEMENTS
H.B.S and A.K.N are recipients of research fellowships from Indian Council of Medical Research (ICMR). The authors are thankful to the Director, CSIR-CIMAP, for the support throughout the study. This manuscript bears the institutional communication number CIMAP/PUB/2023/147. Authors declare no conflict of interest.
FUNDING INFORMATION
This work was supported by the GAP-376 project (SERB funded project EMR/2016/002746) of CSIR-Central Institute of Medicinal and Aromatic Plants and Phyto-pharmaceutical Mission HCP010.
Open Research
DATA AVAILABILITY STATEMENT
All the data generated or analysed during the study are available from the corresponding author upon request.




