High throughput phenotyping of functional traits and key indices for selection of salt tolerant Mustard [Brassica juncea (L.) Czern & Coss] genotypes
Abstract
The current scanty knowledge about the salt tolerance mechanism underlying the ability of plants to tolerate salt stress hinders the potential production of numerous crops, including Indian mustard. To explore the traits and mechanism for salt tolerance, high throughput phenotyping of 250 stabilized F7:8 recombinant inbred lines (RILs) mapping population of Indian mustard were conducted under control and salinity (ECiw 12 dS m−1) for 54 morpho-physio-seed-quality traits. Most of the traits were reduced with variable percentages under salt stress. The stress tolerance index (STI) of YPP showed a significant negative association with Na+ concentration of root (RNa), indicating that RILs with low Na+ concentration have high seed yield and a positive significant association with STI of yield-related traits, photosynthesis rate (Pn), intrinsic water use efficiency (inWUE), fresh weight of upper leaf (USFW), fresh weight of branches (BrFW), fresh weight of basal leaf (BLFW), and fresh weight of middle leaf (MLFW) revealed that by improving these traits seed yield per plant (YPP) was improved. Based on principal component analysis (PCA) of 54 STI and new index composite selection index (CSI), RILs viz., R114, R150, R164, R170, and R206 were identified as stable performers which can be exploited for quantitative trait loci (QTLs)/gene discovery and serve as potential donors to combat salt stress. Our research will serve to determine the relative importance of different functional traits of salt tolerance mechanisms that can be used to screen colossal germplasm.
1 INTRODUCTION
In Asian regions, agriculture is the main source of employment, food security, and income for those living in rural regions (Singh & Sharma 2016). A cascade effect of climate change has been seen over the past few decades, leading to a fast change in natural ecosystems, agricultural production, and cultivation techniques (Kumar et al., 2022; Sharma et al., 2022; Gupta et al., 2023). In general, the Indian mustard crop may grow in a variety of worldwide climates. However, due to groundwater depletion, deterioration of the land, and salt stress, mustard farming systems have begun to see a small drop in seed production (Singh et al., 2022). Globally, one of the biggest problems faced by mustard farmers is a gradually increasing saline condition because it has a negative impact on oil quality and seed yield (Singh et al., 2014). Worldwide, salinity and sodicity affect an area of 932.2 mha, of which 6.73 mha is in India and is expected to increase up to 16.25 mha by 2050 (Singh et al., 2014). Groundwater that is utilized for irrigation contains salty or brackish water between 32–84%. Salinity brought on by irrigation results in an annual loss of 10 mha of land (Kumar 2014). Mustard plant growth, seed germination rates, early seedling development, photosynthetic rate, plant height, and seed yield are all negatively impacted by salinity (Singh & Sharma 2016). By improving crop tolerance to salt or by draining salt from the soil, this low yield in saline areas can be combated.
Indian mustard [Brassica juncea (L.) Czern & Coss: AABB, 2n = 36, Genome size: 1068 Mb] is an amphidiploid species developed from interspecific hybridization between the two diploid progenitors viz. B nigra (2n = 16; BB) and B. campestris (2n = 20; AA) (Nagaharu & Nagaharu 1935; Kang et al., 2021). Indian mustard is a significant oilseed crop with global adaptability. It originated in Central Asia and was recently introduced in Australia and Canada (Kang et al., 2021). Globally, India ranked second in rapeseed-mustard cultivation after China and third in production behind Canada and China (Kumari et al., 2019). Worldwide production, yield, and acreage of rapeseed-mustard were 72.42 mt, 1974 kg/ha, and 36.68 mha, respectively (Singh et al., 2021). It ranks India's third-largest edible oilseed crop after peanut and soybean, which accounts for 24.36% of the country's oilseed market among nine edible oilseed crops.
One of the main initiatives in Brassica development is cultivar improvement for saline tolerance. The amphidiploid species appear to be superior to the diploid species in terms of saline tolerance, as per previous research (Zhang et al., 2020). The introduction of salt mitigation or salt tolerance mechanisms into target crop plants proceeds slowly due to a lack of research or inadequate information in these areas (Zhang et al., 2020; Prakash et al., 2022). Therefore, there is a need to define a standard protocol for Indian mustard salinity characteristics. The development of salinity-tolerant Indian mustard cultivars with higher yield under the salt-affected semi-arid tropics will be more effective and efficient by following standard protocol with the integration of morphological, physiology, growth, and seed quality studies through high through-put phenotyping.
To improve mustard genotypes genetically, physiological traits such as photosynthetic rate, stomatal conductance, transpiration rate and water use efficiency may be used as a secondary selection technique since they are less influenced by environmental conditions and have higher genetic stability than factors that affect seed production. This could only be accomplished by creating genotypes with high yields, early maturation, prolonged seed-filling length, climatic smart, and abiotic stress tolerance (Kumawat et al., 2023). By the incorporation of agro-physiological and seed quality characteristics, breeding programs are supported, and their performance is increased by the application of multidimensional multivariate analytic techniques to obtain precision in verification and selection (Kumawat et al., 2023). This might lead to deeper comprehension and an integrated method that incorporates several factors of salt-adaptive traits. To accurately examine phenotypic traits produced by highly computational modelling of multidimensional data and to offer a more thorough knowledge of the complex mechanisms under salt stress, a combination of analyses must be used (Singh & Sastry 2011; Sanwal et al., 2022). To preserve as much information as possible, PCA, for example, lowers the number of variables in a data collection and uses an orthogonal transformation to turn a set of observations of potentially correlated variables into a set of values of uncorrelated variables (Kumawat et al., 2021; Sabouri et al., 2022).
Several selection indices, including SSI, RSI, TOL, MP, YSI, HM, GMP, STI, and YI, have been suggested based on the association between seed yield under control and stress conditions. However, selecting the steady and tolerant genotypes based on a mix of the top indices using different statistical methods might be more suitable for identifying them in both conditions (Sabouri et al., 2022). A combination of indicators could offer a more suitable selection criterion for breeding programs. Therefore, it is essential to establish a generic index that is efficient and easy to calculate. In this research, a new index called composite selection index (CSI), which is significantly associated with seed yield under stress-free and stressed circumstances is used. The effectiveness of multivariate analysis and selection by CSI was examined as well as the outcomes of both analyses. Different stress indices, correlation analysis, and multivariate analysis of variance are examples of screening techniques with their advanced forms that may be used as a combined method of screening tests and for identifying sources of variation (Kumawat et al., 2021; Sabouri et al., 2022). The main objectives of this research were: (1) to create a screening procedure to know the effect of salt stress on morpho-physio and seed quality traits, (2) to create a procedure for selection of best indices to pinout the salt tolerant lines based on association study, (3) to assess 250 RILs for multivariate analysis based on 54 STI, (4) to exploit a new index: CSI and compare its results with PCA for final screening of RILs mapping population to select salt tolerate RILs.
2 MATERIALS AND METHODS
2.1 Research material and experimental site
The experimental materials consisted of 250 stabilized F7:8 recombinant inbred lines (RILs) mapping population of Indian mustard developed by the crossing of two contrasting parents; CS 614–1–1-100-13, a salt-sensitive mutant line developed with λ-ray irradiation treatment and stabilized for M6 generation and CS 56, a national released high yielding salt tolerant variety by ICAR-CSSRI, Karnal (Sharma et al., 2008). These 250 RILs along with parents grown during two consecutive Rabi seasons 2020–21 and 2021–22 under control and irrigation water salinity ECiw 12 dS m−1 in the pots with three replications at Indian Council of Agricultural Research-Central Soil Salinity Research Institute (ICAR-CSSRI), Karnal (29°43'N, 76°58′E; 245 m above the average sea level) (Singh et al., 2020). The average weekly meteorological data during the experimentation period is presented in Figure S1.
2.2 Experimental procedure details
The RIL mapping population was cultivated in pots of 20 kg capacity in sand culture in a factorial experiment using a randomized complete block design (RCBD). For the salinity stress, irrigated with saline water of ECiw 12 dS m−1 throughout the experiment. The chloride and sulphate salts of Na+, Ca2+, and Mg2+ to keep the SAR (Sodium absorption ratio) within the permissible limits used for the preparation of ECiw 12 dS m−1 saline irrigation water. Seeds were surface sterilized for 5 min in a 1% sodium hypochlorite solution before sowing, and then washed with distilled water (Singh et al., 2019). Twenty seeds of each RIL were planted in a plastic pot filled with properly washed river sand at a depth of one centimeter. Each pot's bottom was dug out to allow any extra water to drain. The pots were watered with Hoagland's solution, a nutrient solution, and kept at maximum field capacity. Throughout the experiment, salinity levels were kept constant by draining the salt out of the pots every day (Singh et al., 2019).
2.3 Data collection
The following observations were recorded at different stages (seed germination, seedling, flowering, harvesting, and final seed quality) of the Indian mustard growing cycle to standardize an ideal protocol for the characterization of components/functional traits and elite genotypes.
2.3.1 Morphological traits measured from germination to harvesting stage
The observation and measurements of thirteen morphological traits were taken as follows: germination percentage (G), days to 50% flowering (DF; number), days to maturity (DM; number), plant height (PH; cm), number of primary branches per plant (PBR; number), number of secondary branches per plant (SBR; number), number of siliquae per plant (NSPP; number), main shoot length (MSL; cm), number of siliquae on main shoot (MSS; number), siliqua length (SL; cm), seeds per siliqua (SPS; number), test weight (TW; g), and seed yield per plant (YPP; g).
2.3.2 Measurement of photosynthetic traits at seedling stage
Photosynthetic traits viz., photosynthesis rate (Pn; μmol CO2 m−2 s−1), transpiration rate (E; μmol CO2 m−2 s−1), stomatal conductance (gSW; mmol CO2 m−2 s−1), intracellular CO2 assimilation (Ci/Ca), instantaneous water use efficiency (iWUE), and intrinsic water use efficiency (inWUE) were measured at seedling stage. Randomly selected three plants from each RIL and parents under control and ECiw 12 dS m−1 salinity regime were used for photosynthetic data and measured on the fully expanded leaf using a portable photosynthetic system: IRGA: Infrared gas analyzer LI-6800XT (Li-COR) (Singh et al., 2019). All the photosynthetic traits were measured between 10:00 and 12:00 AM in sunlight under these weather conditions; PAR ~700 μmol m−2 s−1, temperature ~ 25 ± 1 relative humidity ~70%, and air CO2 355 μmol mol−1 (Figure S2).
2.3.3 Growth and ionic traits measurement at the flowering stage
Growth (LAI, NDVI, fresh weight, and dry weight), and ionic (Na+ and K+) traits were measured at the flowering stage. The leaf area index (LAI) was recorded by CI-110 Plant Canopy Imager (CID Bio-Science, Inc.) from both control and saline conditions to compare the growth and development in both conditions. The plant vigour or health of mustard plants was measured with a green seeker (Green Seeker-Handhold crop sensor, Trimble Inc.), which gave Normalized Difference Vegetation Index (NDVI) that depicts the distinction between vegetation's visible and near-infrared absorption (Figure S2). For fresh weight measurement, randomly ten plants (50 days old) from each RIL under control and salinity ECiw 12 dS m−1 conditions were uprooted and washed with distilled water to record the fresh weight (g) of different plant parts viz., root (RFW), middle stem (MSFW), upper stem (USFW), branches (BrFW), basal leaf (BLFW), middle leaf (MLFW), and upper leaf (ULFW). The oven-dried plant samples at 55–65 for 5–6 days were used for reading of dry weight (g) of different plant parts viz., root (RDW), middle stem (MSDW), upper stem (USDW), branches (BrDW), basal leaf (BLDW), middle leaf (MLDW), and upper leaf (ULDW).
At the flowering, stage mega ion partitioning is done. The Na+ concentration of different plant parts viz., root (RNa), middle stem (MSNa), upper stem (USNa), branches (BrNa), basal leaf (BLNa), middle leaf (MLNa), and upper leaf (ULNa) and K+ concentration of different plant parts viz., root (RK), middle stem (MSK), upper stem (USK), branches (BrK), basal leaf (BLK), middle leaf (MLK), and upper leaf (ULK) measured with Inductively coupled plasma-optical emission spectrometry (ICPE-9800; Shimadzu) after checking the standards (Piper 2019) (Figure S2).
2.3.4 Seed quality parameters
The quality parameters of the mustard seed were measured with Fourier Transform Near-Infrared reflectance spectroscopy (FT-NIR spectrometer; Perkin Elmer). The intact seed samples were deposited in glass containers and set in a sample holder to capture the spectrum of the FT-NIR measurement (Figure S2). With 32 scans, NIR spectra were recorded in reflectance mode for the wavelength range of 1000–2500 nm (10000–4000 meters). The software Spectrum10 from Perkin Elmer was used for instrument management and spectral acquisition, and the program spectral Quant+ from Perkin Elmer was used for calibrations (Singh & Sharma 2016). Oil content, erucic acid content, crude fiber content, and protein content were all evaluated as indicators of seed quality.
2.4 Statistical analysis
We used Origin Pro 2023 (Origin Lab Corporation) for the diagrammatic presentation of data i.e., frequency distribution, Pearson correlation chord diagram, rain cloud plot, correlation matrix, and 3D plot for principle component analysis. Analysis of variance (ANOVA) was calculated using R studio 4.2.1. Different salinity tolerant and susceptibility indices including reduction percentage were performed for each RIL. The ten indices used in this research, including the new Composite Tolerance Index (CSI) with their calculation formula are described in Table 1.
| S. No. | Index | Formula | Description | References |
|---|---|---|---|---|
| 1. | Stress Susceptibility Index | SSI = |
Evaluates the difference in yield between adverse and favourable environments. A lower number implies smaller yield differences, indicating more tolerance for salt. | (Fischer & Maurer 1978) |
| 2. | Relative Stress Index | RSI = | The genotypes will be more tolerable if their RSI value is high. | (Fischer & Wood 1979) |
| 3. | Tolerance | TOL = Yp – Ys | Greater TOL scores suggest a genotype's vulnerability. | (Rosielle & Hamblin 1981) |
| 4. | Yield Stability Index | YSI = | A higher YSI score denotes a genotype that is more stable under stressful and non-stressful circumstances. | (Bouslama & Schapaugh Jr 1984) |
| 5. | Yield Index | YI = | The genotypes with high YI values are appropriate under conditions of salt stress. | (Gavuzzi et al., 1997) |
| 6. | Mean Productivity | MP = | Greater MP values correspond to better productivity rates. | (Rosielle & Hamblin 1981) |
| 7. | Geometric Mean Productivity | GMP = | High-yielding genotypes have GMP values that are higher under both stressful and non-stressful conditions. | (Fernandez 1992) |
| 8. | Harmonic Mean | HM = | The genotypes will be more tolerable if their HM value is high. The genotypes will be more tolerable if their HM value is high. | (Bidinger et al., 1987) |
| 9. | Stress Tolerance Index | STI = | A higher STI value denotes a high yield both under stressful and non-stressful settings. | (Fernandez 1992) |
| 10. | Composite Tolerance Index | CSIi = | This CSI is a linear combination of significant indices. This index is described as the average of the combination of the association values of seed yield under control and stress conditions in significant indices. If the correlation coefficient between the index and seed yield was judged significant in both cases, the index was deemed significant. | (Sabouri et al., 2022) |
- Note: Yp and Ys: yield per plant under control saline conditions, respectively. and : mean of yield per plant overall RIL mapping population under control and saline conditions, respectively.
3 RESULTS
3.1 Pooled ANOVA for studied traits
The pooled ANOVA revealed that the environment showed a significant mean sum of square (p < 0.05) for all the studied traits (photosynthetic, growth, ionic traits, seed quality, and morphological traits), indicating significant differences between the environments. The significant mean sum of squares (p < 0.05) due to genotypes (RILs and both parents) for all the studied traits showed a significant difference among the 250 RILs and both parents. Genotypes × environment interaction (G × E) was significant (p < 0.05) for all the recorded traits demonstrating how different genotypes respond to the manifestation of characteristics about salinity (Table S1). The pooled mean performance of all the studied traits for the 250 RILs with parents (control and salinity 12 dS m−1 environments) were given in Table S2. Figure 1 showed continuous normal or near-normal distribution of seed yield per plant under control and salinity ECiw 12 dS m−1 of both seasons, indicating a polygenic inheritance of them and an ideal model for the study of salt tolerance.

3.2 Effect of irrigation water salinity on the performance of parents and RILs
The salt-tolerant parent CS 56 recorded high value for most of the studied traits over the environments as compared to salt-sensitive parent CS 614–1–1-100-13 except DF, iWUE, inWUE, erucic acid content, and Na+ concentration that was higher in CS 614–1–1-100-13 in comparison to CS 56. Both the parents were statistically differing (p ≤ 0.05) for all measured traits except DF over the environment (Table S2 and Figure 2). Salinity stress conditions reduced (S-21 and S-22) almost all measured traits in RILs, P1 and P2 in contrast to control conditions (C-21 and C-22) with variable extent except DF, iWUE, inWUE, erucic acid content, and Na+ concentration of different plant parts (Table S3). The effect of irrigation water salinity on morphological, photosynthetic, growth, and ionic traits is discussed in detail under the following heads:
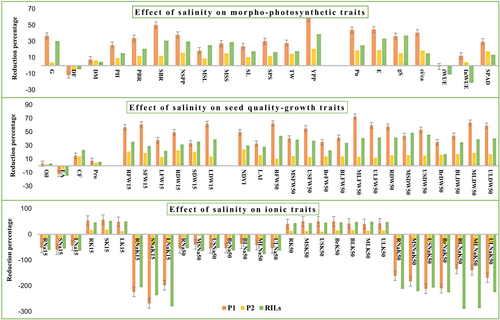
3.2.1 Effect on morphological traits
Salt stress induces a remarkable reduction in morphological traits from seed germination to the harvesting stage under saline stress in both parents and RILs compared to control conditions except for days to flowering (Table S2 and Figure 1, 2). The highest reduction recorded among the morphological traits in YPP (38.47%) was followed by the germination percentage (30.22%) and SBR (29.66%). YPP is the most important trait, which was reduced severely under saline conditions, with the highest reduction observed in RIL 154 (65.19%), whereas the lowest reduction was noted in RIL 144 (12.33%) as compared to the control (Table S4).
3.2.2 Effect on photosynthetic traits
NaCl-induced salt stress has a serious impact on photosynthetic traits. Salinity reduced Pn, E, gSW, and Ci/Ca in all RILs and parents (Table S2, S4, and Figure 2), whereas iWUE and inWUE increased at ECiw 12 dS m−1 as compared to the control environment. The highest reduction recorded among the photosynthetic traits in gSW (37.03%) was followed by the E (33.32%) and Pn (25.10%) (Table). The iWUE and inWUE are also significantly affected by salinity stress. The iWUE increased by 10.91% and inWUE enhanced by 21.14% under saline conditions over the environment.
3.2.3 Effect on growth attributing traits
All growth-attributing traits were reduced severely under saline conditions. NDVI showed a reduction of 29.84%, among RILs, the highest reduction was noted in RIL 109 (58.17%), whereas, the lowest was in RIL 17 (10.17%) as compared to the control (Table S4). NaCl salt reduced LAI by 28.06%, furthermore, RIL 149 (52.90%) showed the highest reduction, whereas CS 56 (15.55%) and RIL 130 (20.14%) showed the lowest reduction as compared to the control. Salt of Na+ reduced the fresh weight of different plant parts; RFW (41.58%) reduced severely, followed by ULFW (39.37%), and MLFW (38.73%), furthermore, among the dry weight of different plant parts, MSDW showed the highest reduction (45.59%) followed by USDW (44.08%), and RDW (39.19%) as compared to control (Table S2, S4; Figure 2).
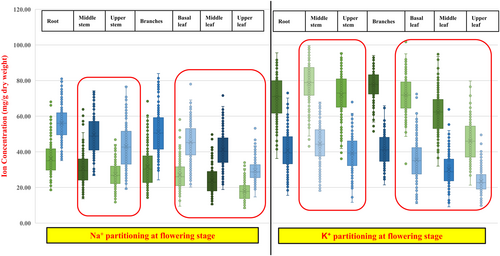
3.2.4 Effect on ionic concentration in different parts of the plant
The effect of salinity on the concentration of ions (Na+ and K+) is shown in Figures 2 and 3, indicating a clear-cut increment in Na+ concentration and a reduction in K+ concentration in different parts (from root to leaf). The ionic concentration of Na+ increased drastically under saline conditions, among Na+ concentrations of different plant parts; MLNa (−92.14%) followed by BLNa (−77.28%), and BrNa (−77.25%) at the flowering stage increased to the greatest extent under salt stress as compared to the control condition. Salt stress reduced K+ concentration in different plant parts, MLK (48.17%) reduced highest followed by BLK (47.84), and ULK (46.58%) (Table S2 and S4).
3.2.5 Effect on seed quality traits
Salinity also has a detrimental effect on seed quality traits like crude fiber content that was reduced severely (23.19%) under saline conditions. Seed quality traits such as oil (3.20%), protein (5.83%), and crude fiber content (23.19%) of mustard reduced under a saline environment whereas erucic acid content (−15.40%) was increased (Table S2; Figure 2).
3.3 Identification of the best indices for salt tolerance based on their relationship to seed yield per plant
Ten stress indices were measured and their association with YPP was analyzed to characterize RILs for salt tolerance (Table S5). Although several significant positive correlation coefficients were measured i.e. STI, YSI, YI, MP, GMP, and HM, and negative correlation with SSI and TOL, we considered highly significant (p ≤ 0.001), and positive correlation with YPP under control and salt stress condition (Table S6). Meanwhile, among all stress indices, STI showed a highly significant and positive association with YPP under control and salt stress conditions (0.512***, and 0.900***). Selection based on STI will improve seed yield per plant due to the highly significant and positive association of STI with Yp and Ys.
3.4 Salt tolerance index of measured traits
For all studied variables, the STI revealed extremely substantial genotype-to-genotype differences as sources of variance resulting from discrepancies between optimum and salt treatment. Intriguingly, for the majority of measured indices, there was a substantial fluctuation between big and small values, with a few exceptions (Oil content, DM, protein, and crude fiber content) (Table S7; Figure 4), which showed little variation between higher and lower value. The seven tested indices (STI of RNa, USNa, ULNa, MSNa, BrNa, BLNa, and MLNa) had big values that were double or more than the tiny ones, indicating the effectiveness of these indices in demonstrating genetic diversity among the RILs utilized. However, in all salt tolerance indices (in some RILs), except one index (NDVI) were greater than one. STI demonstrated significant variation between various indices within the same RIL and between the same indices among other RILs. As a result, it is challenging to determine each genotype's level of salt tolerance using the STI of a single index.
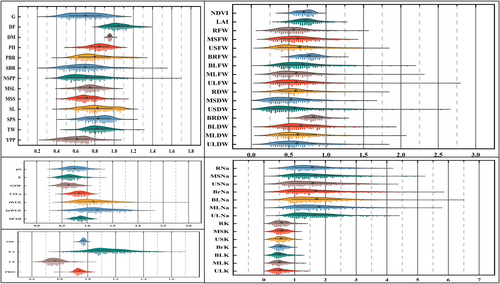
Rain Cloud Plot showing significant variation of 54 Salt Tolerance indices.
* p<=0.05 ** p<=0.01.
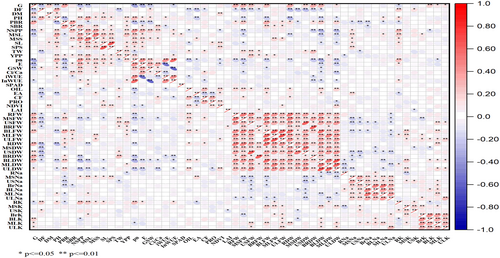
3.5 Association study of STI
A correlation matrix was performed among 52 STIs to understand the association among measured indices (Figure 5). STI of yield per plant showed a significant and positive correlation (p ≤ 0.1, and ≤0.01) with STI of MSL (0.265**), USFW (0.244**), Pn (0.182**), NSPP (0.178**), TW (0.171**), PH (0.168**), SPS (0.152*), SL (0.147*), PBR (0.135*), inWUE (0.134*), BrFW (0.134*), BLFW (0.129*), SBr (0.126*), and MLFW (0.124*) whereas significant negatively associated with STI of RNa (−0.154*).
3.6 Principal Component Analysis
The estimated variable (54 indices) was categorized by the PCA into 18 principal exhaustive indices based on eigenvector value (eigenvalue >1), which have cumulative contribution rates of 68.41% [Table S8 and Figure 6 (a)]. PCA showed that the PC1 explained 11.76% of the variations and exhibited significant association among BLDW, BLFW, ULDW, RDW, MSFW, RFW, USFW, USDW, MLFW, ULFW, and G, hence, the PC1 can be designated as a component of growth [Table S9 and Figure 6 (b)]. The PC2 explained 7.02% of the variations and exhibited significant association among NSPP, Pn, MLFW, MSL, MLDW, MSS, RK, SL, ULFW, SPS, E, and YPP, hence, the PC2 can be designated as a component of productivity. The PC3 explained 5.62% of the variations and exhibited significant association with BrK, MLK, BLK, BLNa, BrNa, ULNa, MLNa, ULK, USNa, and MSNa hence, PC 3 can be designated as an ionic component.
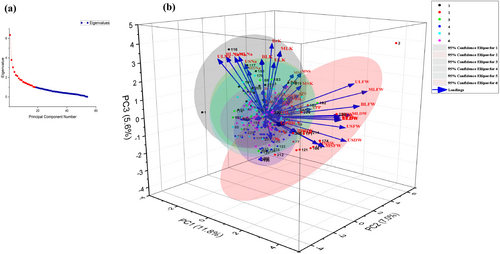
The genotypes CS 56, R224, R172, R168, R190, R164, R212, R222, R203, and R169 were supported by PC1; and CS 56, R53, R55, R31, R87, R202, R223, R250, R201, and R228 in favor of high PC2. PC3 favored CS 56, R114, R115, R151, R181, R176, R238, R186, R179, and R217 when sorting done for top ten percent [Table S10 and Figure 6 (b)].
RILs viz., R114, and R210 positioned themselves on the extreme right side of the X and Y axis but high along the Z axis i.e. away from the cluster and in favour of PC1, PC2 and PC3 [Table S10 and Figure 6 (b)]. PC1 and PC2 combinedly favor R80, R82, R150, and R228; PC2 and PC3 sort out R133, R134, R41, R75, R94, R181, R201, R202, R204, and R206; and PC1 and PC3 selected R16, R19, R111, R119, R139, R164, R170, R173, R210, and R238.
3.7 Screening of RILs based on new index Composite Selection Index
To characterize tolerant and sensitive RILs over the environment, the composite selection Index (CSI) was calculated. The new index CSI was computed for 250 RILs, and both parents based on MP, GMP, HM, and STI from pooled YPP. The top-ranked RILs with high CSI value (tolerant RILs) were RIL 217, RIL 236, RIL 221, RIL 96, RIL 168, RIL 87, RIL 93, RIL 34, RIL 53, and RIL 104 (Table S5). Lowest ranked RILs with low CSI values (sensitive RILs) scored were RIL 155, RIL 179, RIL 157, RIL 199, RIL 154, RIL 134, RIL 40, RIL 44, RIL 42, and CS 614.
4 DISCUSSION
4.1 Analysis of variance
The pooled ANOVA of the 250 RILs with both parents revealed that environment, genotypes × environment interaction (G × E), and genotypes showed significant mean sum of square (p < 0.05) for all the studied traits indicating significant variability presented among genotypes and differential environments have significant impact for its expression and categorizing the genotypes for salt tolerance capability through variable response of genotypes toward environments (Singh & Sharma 2016; Singh et al., 2019). The mean of all the studied traits of both parents (Table S1) showed a statistical difference between both parents (p ≤ 0.05), indicating the contrasting nature to each other.
4.2 Effect of salinity on morphological traits
There was a noticeable reduction in almost all morphological traits under the saline condition as compared to a control condition in both parents and RILs except, DF (Table S2; Figures 2, 3). Seed germination is the most important trait of plant growth and development. In this study, the germination percentage significantly reduced and delayed the germination rate. Plant loss causes a drop in plant density, which lowers production. Water consumption triggers the germination of seeds. Salinity hinders water absorption, which stops seed germination from starting. Previous research demonstrated that higher salt delays the start of germination, resulting in lower germination rates (Jia et al., 2020).
NaCl stress affects DF in mustard. Salt stress creates an osmotic effect and accumulation of toxic ions. It has a negative impact on the physiological and metabolic systems. This results in longer seed germination times, seed initiation delays, shorter plumule, and radical lengths, and ultimately days to flowering delayed (Dolatabadi and Toorchi 2017; Mohamed et al., 2020).
The productivity traits, i.e., DM, PH, PBr, SBr, NSPP, MSL, MSS, SL, SPS, TW, and YPP, all were reduced in almost all RILs and both parents with variable extend under saline conditions due to salt stress. Reduced plant height is the result of excessive salt buildup in the cell wall, which limits cell wall flexibility and causes cellular stiffness. Insufficient turgor pressure also prevents cell expansion in a salty environment (Kumari et al., 2019).
Salinity greatly reduced the formation of dry matter. Although the PBr and SBr were dramatically reduced under saline conditions, the effect of salinity on test weight was allegedly more pronounced in Brassica juncea (Table S2) (Tripathi et al., 2019). The current study showed that YPP in RILs was reduced by salt treatment up to 38.9% (Table S2). The CS 56 and tolerant RILs based on reduction percentage of YPP (RIL 144, RIL 186, RIL 127, RIL 80, and RIL 137), who have a better physiological and molecular salt tolerance mechanism than CS 614–1–1-100-13 and sensitive RILs (RIL 250, RIL 247, RIL 158, RIL 225, and RIL 154), exhibited the least decrease (Table S4). Salt-induced chloroplast shrinkage and even destruction, a drop in photosynthates in the phloem, and a lack of water in the growth area were all blamed for the low seed yield under a saline environment in Brassica spp. (Jan et al., 2017; Singh et al., 2019).
4.3 Effect of salinity on photosynthetic traits
Photosynthesis is the primary determinant of plant growth and productivity in all crops, including Indian mustard. Reduced photosynthetic capacity under salt stress is frequently linked to a decline in productivity in many plant species (Singh & Sharma 2016). Almost all the 250 RILs and both parents evaluated showed a significant reduction in Pn, E, and gSW under saline condition ECiw 12 dS m−1, as compared to the control condition (Table S2 and Figure 2). Under salt stress, the net photosynthetic rate was reduced more drastically in the salt-sensitive RILs (RIL 226, RIL 215, RIL 235, RIL 202, and RIL 247) than in the salt-tolerant RILs (RIL 118, RIL 157, RIL 88, RIL 117, and RIL 70) (Table S4). This decrease is due to salt-induced injury to photosynthetic tissue and inhibition of mesophyll conductance, which results in a limitation of CO2 availability for carboxylation and accelerates senescence under salinity stress (Singh et al., 2022). Furthermore, decreased transpiration in salt-sensitive under increasing salinity compared to salt-tolerant genotypes may be caused by the toxicity of too much salt in the transpirational path, which damages cells in the transpiring leaves and inhibits growth (Table S4) (Singh & Sharma 2016). Salt-sensitive genotypes with increased ionic buildup have decreased leaf turgor, altered leaf shape, and reduced stomatal aperture, which in turn causes partial stomatal closure to limit water loss by transpiration and, as a result, lower stomatal conductance (Table S4) (Singh et al., 2019).
Increasing salinity significantly reduced iWUE and inWUE in sensitive RILs and enhanced in tolerant RILs. This decrease in iWUE and inWUE may be caused by a larger drop-in carboxylation rate brought on by non-stomatal variables, such as dwindling Rubisco and chlorophyll levels at high salinity (Singh et al., 2019). As a result, with rising salt stress compared to the control, all RILs showed a significantly lower CO2 assimilation rate (P < 0.05). Harmful effects of salinity on the photosynthetic system directly resulted in non-stomatal suppression of photosynthesis, which is independent of stomatal closure, decreased stomatal conductance, and restricted availability of CO2 for carboxylation process in the salt-sensitive mustard RILs and sensitive parent (Singh et al., 2021). Other potential causes for the salinity-induced decline in photosynthetic features include changes to cytoplasmic architectures, negative feedback of lower sink activity associated with sluggish transport of photosynthates, and changes in the activities of enzymes (Singh et al., 2021).
4.4 Effect of salinity on growth attributing traits and ionic concentration in different plant parts
A reduction in growth-attributing traits was observed in almost all RILs and both parents (Table S2, S4, and Figure 2) under a saline environment as compared to control. At the flowering stage, the highest reduction was observed in the fresh weight of the root and among dry weights, in the middle stem dry weight under saline conditions. These reductions brought on by the adverse effects of salinity on plants, triggered by osmotic potential, preventing the root cells from obtaining the necessary water from the soil (Singh et al., 2019). As a result, some mineral nutrients that are dissolved in soil water cannot be taken up by plants. Thus, the resulting metabolic deficiency impeded the growth and development of plants. Some studies hypothesize that decreased growth is caused by ion buildup as a result of altered membrane permeability (Sehrish et al., 2021). Most of the crops exposed to the salinity experienced decreased growth. Hence, the detrimental effects of salinity might be due to ion toxicities, ion imbalance, or a combination of all these mechanisms (Naheed et al., 2021).
The concentration of K+ in the different plant parts of CS 614–1–1-100-13 and sensitive RILs is dramatically lower than that of CS 56 and tolerant RILs under a saline environment when compared to the control. Salinity imposed elevated values of Na+ concentration, and penalty in K+ concentration in all RILs with parents (Figures 2 and 3). At the flowering stage, the highest increment percentage of Na+ and reduction percentage of K+ concentrations were recorded in middle leaves (Table S2, S4 and Figures 4 and 5) similar results reported by (Priya et al., 2021). Since Na+ and K+ have comparable hydrated ionic radii, the transporter finds it challenging to distinguish between the two ions, thus larger Na+ accumulation in the roots of salt-sensitive parent and RILs can be related to the differential cellular entrance of ions under high salinity (Priya et al., 2021). The salt tolerance at the cellular level may have been influenced by the increased internal K+ concentration of the salt-tolerant CS 56 and RILs (Volkov & Beilby 2017). To maintain intracellular ion homeostasis, which is essential for cytoplasmic metabolic activity and to raise cellular osmolality to combat osmotic stress, it may be assumed that the majority of detrimental ions are compartmentalized into the vacuoles (Singh et al., 2019). Instead of only sustaining low Na+ concentrations, salinity tolerance is connected to the plant's capacity to maintain a reduced Na+ concentration in plant parts (Keisham et al., 2018).
4.5 Effect of irrigation water salinity on seed quality traits
The oil, protein, and crude fiber content of mustard seeds decreased in a saline climate while erucic acid content climbed. (Table S2, S4 and Figure 2). According to (Singh & Sharma 2016) the decrease in seed oil content may be caused by imbalances in nutrients and important components, an increase in the osmotic pressure of the soil solution, or the early maturity of plants and delayed seed development in high salinity treatments (Singh et al., 2014). The decrease in the protein and crude fiber content could be due to the plant's inability to utilize nitrogen molecules to their maximum potential or a reduction in nitrogen supply for the synthesis of amino acids and proteins (Singh et al., 2019).
A sharp rise in erucic acid content with rising salinity may be caused by changing fatty acid composition and the ratio of saturated to unsaturated fatty acids in Brassica. Additionally, fatty acid concentrations were higher under salt stress than under normal circumstances, perhaps because of the involvement of certain fatty acids in cell wall integrity. Some of the enzymes involved in the resistance to salt stress are given more energy by these fatty acids. Different fatty acid compositions are crucial for the transportation of protective substances like glycine-betaine. In the Indian mustard breeding program for seed quality criteria, the genotypes that demonstrated the high amount of oil, protein, crude fiber content, and least amount of increase in erucic acid content may be employed as possible donors (Singh et al., 2013; Singh et al., 2014; Singh et al., 2021).
4.6 Selection of best stress index
Seed production is significantly impacted negatively by salt stress, which has resulted in a substantial decrease in seed yield due to climate change; therefore, a key breeding goal is to increase seed yield during saline stress (Korshid 2016; Kumawat et al., 2023). In general, enhanced yield potential must be paired with better salt tolerance in mustard breeding (Thiry et al., 2016). A desired selection indices should make it possible to differentiate between genotypes that produce well under stress and those that do not. To achieve better seed production under both non-saline and saline conditions, a high value of indices viz., STI, YSI, YI, GMP, MP, and HM is required in Indian mustard (Table S5, S6). Meanwhile, among all stress indices, STI showed a highly positively significant correlation with Yp and Ys, So, selection based on STI will improve seed yield.
According to (Singh et al., 2018), under waterlogging conditions, several indices, including GMP, MP, HM, and STI, showed positive and very significant correlations with wheat grain production. To find the potential lines, they used the indices GMP, STI, MP, and HM. STI, MP, GMP, and HM were introduced by (Mariey & Khedr 2017) as important and useful indices for choosing barley cultivars with high yields in both conditions. In addition, (Singh et al., 2017) used GMP, HP, MP, and STI as the best indices for choosing the best tolerant bread wheat genotypes. The selection indices STI were successful in finding mustard genotypes for salt stress. The findings presented here further supported the finding that throughout time, STI showed the largest common connection with Yp and Ys.
4.7 Correlation among different STI
There was a significant negative association of seed yield STI with RNa indicating that RILs with low Na+ concentration have high seed yield (Table S7 and Figure 5). These results are in favour of (Priya et al., 2021; Wang et al., 2022) which stated that seed yield negatively associated with the Na+ concentration of different plant parts. STI of YPP showed a positively significant association with yield attributing traits STI viz., MSL, NSPP, TW, PH, SPS, SL, PBr, and SBr and growth attributing traits STI viz., USFW, BrFW, BLFW, and MLFW and photosynthetic traits STI viz., Pn, inWUE indicating that by improving these traits seed yield will be increased. These results are in agreement with different previous research: for yield attributing traits by (Naheed et al., 2021); growth attributing traits by (Jia et al., 2020), and photosynthetic traits by (Mohamed et al., 2020) conducted in different Brassica spp.
4.8 Selection of tolerant RILs based on PCA and CSI
The genotypes located on the extremely upbeat X and Y axis were thought to be superior genotypes against the characteristics that made up PCs 1 and 2, respectively (Tejaswini et al., 2018). The genotypes pulled higher along the Z-axis will also be deemed to perform better against the contributing trait in the 3D (three-dimensional) scattered diagram, where it was considered an extra vertical Z-axis. The genotypes located on the right side or loaded with higher coefficient value of the PC1 axis (X-axis) of the 3D scattered diagram [Table S10 and Figure 6 (b)] may be regarded as promising RILs for salt tolerance in terms of growth attributing traits because PC1 was loaded with high coefficient values (regardless of direction) of the majority of the FW and DW of different plant parts (Verma et al., 2019). Similarly, because PC2 was enriched with the high coefficient values of seed yield and its related traits, RILs located on the relatively right side of the Y-axis may also be chosen. The results are consistent with (Gour et al., 2017). The RILs that plotted higher loaded coefficient values along the Z-axis on PC3 were also to be taken into consideration with the appropriate gravity because PC3 was laden with high factors of Na+ and K+ ion concentration traits in different plant parts. The 3D PCA plot for STI depicts RILs viz., R114 and R210, which were positioned on the extreme right side of the X and Y axis but high along the Z axis, away from the cluster. The RIL's positions on the Y and Z axes demonstrated that they were steady yielders with little expression of salt-escaping features while under pressure (Acharjee et al., 2021).
The STI method, developed by (Fernandez, 1992) is more effective than other stress indices in identifying genotypes that perform well under stress and stress-free conditions and those that do not alone STI unable to characterize tolerant and high-yield genotypes under both conditions, despite some of the indices being more effective in discriminating desirable genotypes under both environments. However, a combination of indices might offer a more suitable selection criterion for breeding programs. The suggested index, called CSI, offers selection based on a combination of the top indices. Significant agreement between the CSI findings and the results of the PCA visual display biplot confirmed the CSI may be deemed useful for more quickly and easily selecting the best genotypes under stressful situations like salt stress (Sabouri et al., 2022). Based on the results of PCA and CSI index RIL viz., R114, R150, R164, R170, and R206 with showed stable performance under control and saline condition.
Plant breeders have made it a top priority to create mustard varieties that can withstand salinity stress to address the issue of salt-affected soil. Additionally, using better RILs as donor parents in hybridization might be quite effective in creating tolerant cultivars of mustard.
5 CONCLUSION
According to our knowledge, this was the first experiment report describing the accurate phenotyping of large-scale quantitatively controlled traits in an experimental setup that can provide on the dynamic response of plants to salt stress in Indian mustard. Integrating the mega data phenomics and high-throughput data might provide a more comprehensive understanding of the plant response and developmental changes under salt stress. In the combination of forward genetics with the identified functional traits, it will be useful to researchers to identify the genomic region/genes/QTLs underlying early response to salt stress with the aim of providing new target genes for salt tolerant cultivar development programs. Further, the enhanced salt tolerant lines will perform a dual function, allowing for both vertical expansion by enhancing additional seed production and lateral expansion by turning salt-affected areas into cultivable land.
AUTHOR CONTRIBUTIONS
Gayatri Kumawat: Conceptualization, Methodology, Data acquisition, Validation, Analysis of data, Investigation, Data curation, Drafting of manuscript, Writing – review and editing. Mohan Lal Jakhar and Vijayata Singh: Conceptualization, Methodology, Drafting of manuscript. Jogendra Singh: Conceptualization, Methodology, Data acquisition, Validation, Analysis of data, Investigation, Resources, Data curation, Drafting of manuscript, Writing – review and editing. All authors have read and agreed to the published version of the manuscript. Dinesh Kumar Gothwal and Devendra Kumar Yadava: Drafting of manuscript, Writing – review and editing.
ACKNOWLEDGMENTS
The authors are thankful to the Indian Council of Agricultural Research (ICAR), India for the support to carry out the work through ‘Incentivizing Research in Agriculture-Component 4: Mustard’, Ministry of Agriculture and Farmers Welfare, Govt. of India.
CONFLICT OF INTEREST STATEMENT
The authors confirm that they have no known financial or interpersonal conflicts that might have looked to have an impact on the research presented in this article.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




