Effects of a dark septate endophyte and extracellular metabolites on alfalfa root exudates: a non-targeted metabolomics analysis
Abstract
Dark septate endophytes (DSE) are widespread colonizers of plant roots and have important ecological functions such as the regulation of plant growth and nutrient uptake. The mechanisms by which DSE mycelium and its extracellular metabolites promote plant growth have not yet been determined. Here, the growth-promoting effects of DSE mycelium (H) and extracellular metabolites (M) on alfalfa (Medicago sativa L.) were investigated. Treatments H, M and HM increased the total biomass of alfalfa by 23.9%, 47.2% and 47.1%, respectively. H and M modified root structure by increasing root volume and reducing root tissue density, and promoting nutrient uptake. Metabolomic analysis indicates that alfalfa root exudates contained 204 metabolites of different types, mainly lipids and lipid-like molecules, organic acids and derivatives, benzenoids. There were more organoheterocyclic compounds and fewer organic acids and derivatives in root exudates in treatment H than in treatment M. Pathway analysis shows that DSE and its extracellular metabolites had greater effects on glycerophospholipid metabolism and N-glycan biosynthesis pathway. The results provide information on the mechanisms involved in the metabolic regulation of plant growth promotion by DSE.
1 INTRODUCTION
Dark septate endophytes (DSE) are a class of nonclavicipitaceous endophytes (Rodriguez et al., 2009) that are closely associated with plant roots in different habitats and have important biological and ecological functions (Almario et al., 2017; Sietio et al., 2018). DSE have melanized hyphae and microsclerotia as characteristic structures and can colonize the root epidermis, cortex and vascular bundles in the intracellular or intercellular space (Jumpponen & Trappe, 1998). DSE colonization can enhance the growth and nutrition of host plants (Mayerhofer et al., 2013; Newsham, 2011). They can also transfer carbon from host plants to themselves (Usuki & Narisawa, 2007) and help the host in the acquisition of nitrogen and phosphorus (Nieva et al., 2021; Yakti et al., 2018). DSE have enzymes that catalyze the breakdown of complex organic substrates and aid host plants in taking up nutrients (Knapp et al., 2018; Ruotsalainen et al., 2022). However, the metabolic regulatory mechanisms by which DSE promote host plant growth and nutrient uptake remain unclear.
Bioactive metabolites produced by DSE may increase the availability of nutrients to host plants and this has led to studies of biostimulants and advances in metabolomics techniques (Godinez-Mendoza et al., 2023; Rinschen et al., 2019; Tienaho et al., 2019). Tellenbach et al. (2013) isolated the growth-promoting compounds sclerotinin A and sclerotinin B from DSE. When Birat et al. (2022) supplemented the callus culture medium of Catharanthus roseus with DSE culture extract, the vincristine content in C. roseus callus increased by 21.7%. Our previous studies also show promotion of maize seedling growth, nutrient uptake, and root development by DSE extracellular metabolites (Wang et al., 2023b). Therefore, utilizing DSE extracellular metabolites to encourage plant growth and development has great potential for development.
Root development is a trait that is important in plant growth and survival, and plays an important role in water and nutrient uptake (Freschet et al., 2021). Root structure is important in determining the composition of root exudates both quantitatively and qualitatively (Badri & Vivanco, 2009). Root exudates are composed mainly of protein, mucus and other high- and low- molecular weight compounds (Chai & Schachtman, 2021). The latter includes a range of primary metabolites (amino acids, sugars, carboxylic acids) and secondary metabolites (sorghum ketones, flavonoids, coumarins) (Bais et al., 2006). Wen et al. (2022) found that plant root exudates, root morphology and endophyte symbiosis together comprised underground resource acquisition strategies.
Alfalfa (Medicago sativa L.) is a widely cultivated, high-yielding and high-quality forage legume (Hawkins & Yu, 2018). It is also commonly used as a model plant in soil remediation (Tussipkan & Manabayeva, 2022), with characteristics such as drought resistance (Mouradi et al., 2016), high salinity resistance (Guiza et al., 2021), rapid growth, and a very effective root system (Clement et al., 2022). Numerous studies indicate that DSE can promote alfalfa growth (Gao et al., 2022), nutrient uptake (Hou et al., 2020) and root development (Deng et al., 2020; Newsham, 2011; Xie et al., 2021). During the growth of alfalfa in hydroponic conditions with the addition of DSE and extracellular metabolites, a constant regulation of the complex metabolic network is needed to obtain nutrient and water resources to promote growth. Comprehensive analysis of alfalfa root exudates can elucidate the underlying mechanisms by which plants respond to DSE mycelium and extracellular metabolites.
The aim here was to investigate the effects of DSE and extracellular metabolites on root exudates of alfalfa using non-targeted metabolomics methods and to analyze the similarities and differences between DSE mycelium and extracellular metabolites as biological and abiotic factors on the mechanism of plant growth promotion. Whether DSE extracellular metabolites can promote the colonization of DSE in host plants was also explored. This will further our understanding of how DSE and their extracellular metabolites promote plant growth and metabolism and provide a theoretical basis for DSE-mediated microbial remediation.
2 MATERIALS AND METHODS
2.1 Preparation of DSE mycelium and extracellular metabolites
The DSE strain was isolated from the roots of Stipa krylovii, a species growing in the grasslands around the Shengli opencast coal mine in Xilinhot, Inner Mongolia. The strain was isolated in pure culture at the microbiology laboratory of China University of Mining and Technology (Beijing). Based on morphological characteristics and ITS phylogenetic identification at the General Microbiology Center of China National Committee the DSE strain was identified as an Alternaria sp. (preservation number CGMCC 17463) (Xie et al., 2021). The DSE strain was grown on potato glucose agar (PDA) medium and stored in the dark at 27°C for later use. Two Petri dishes with a diameter of 7 mm were used to inoculate 150 mL Modified Melin-Norkra (MMN) medium under sterile conditions. The DSE fermentation broth was harvested after culturing with shaking (170 rpm) at 27°C in the dark for 8 days (Wang et al., 2022b). The DSE fermentation culture was divided into 10 mL centrifuge tubes and centrifuged at 5550 × g and 4°C for 15 min to obtain DSE mycelia and extracellular metabolites. The DSE mycelium was washed with sterile water and the extracellular metabolites were sterilized by passing through a 0.22-μm pore filter.
2.2 Plant materials and growth conditions
Alfalfa (Medicago sativa L.) seeds were surface-sterilized with 0.1% (w/v) sodium hypochlorite for 10 min, sterilized with 75% (v/v) ethanol for 30 min, washed with sterile water, and germinated in the dark on sterilized glass beads (250 g, diameter 1 mm, moisture content 16%) for 7 days. Seedlings with uniform growth were transplanted into hydroponic vessels containing nutrient solution. The nutrient concentrations of the 1/2 Hoagland's solution were (mg L−1): K2SO4 (303.5), NH4H2PO4 (57.5), MgSO4 (246.5), ferric sodium EDTA (10), FeSO4 (1.43), borax (2.25), MnSO4 (1.065), CuSO4 (0.025), ZnSO4 (0.11), (NH4)2SO4 (0.01), and calcium salt (472.5). The pH of the nutrient solution was adjusted to 7.0 with 0.1 M KOH. After 7 days of growth, the nutrient solution was changed and supplemented with DSE mycelium (H treatment), DSE extracellular metabolites (M treatment), both DSE mycelium and extracellular metabolites (HM treatment) or sterile water (CT control). There were six replicates of each treatment. Throughout the experiment, the plants were continuously aerated individually and connected to two 50-mm-diameter PTFE membrane filters with a pore size of 0.45 μm to prevent airborne microorganisms from entering the hydroponic vessel. Plants were cultivated at a temperature regime of 24°C/22°C, a relative humidity of 60%, a light intensity of ~8000 lux, and a day/night cycle of 16 h/8 h. The fluid level was maintained by supplying nutrient solution on a daily basis and the plants were grown for 21 days after treatment.
2.3 Determination of the DSE colonization rate
2.4 Measurement of growth index
The alfalfa was dried at 105°C in an oven for 15 min, and then at 60°C until constant weight was achieved. Underground and aboveground biomass of alfalfa was weighed and recorded, and the total biomass and root/shoot ratio were calculated. Root mean diameter, total length, root volume and root branch number were determined using a Modal GXY-A plant root phenotype analysis system and RhizoPheno software (both Zhejiang Top Cloud-Agri Technology Co., Ltd.). Root tissue density (RTD, ratio of dry weight to root volume), specific root length (SRL, ratio of root length to root dry weight) and the percentage of root length of different root diameters (< 0.5 mm, 0.5–1.0 mm, 1.1–2.0 mm, > 2.0 mm) were calculated.
2.5 Determination of nutrient indexes
Oven-dried plant samples were digested with H2SO4-H2O2 and the digests were analyzed by inductively coupled plasma emission spectroscopy (ICP-OES, Optima 5300DV, Perkin Elmer) to determine the concentrations of P and K. The N content was determined using the Kjeldahl method (Thomas et al., 1967).
2.6 Determination of chlorophyll and root activity
Chlorophyll content was determined by spectrophotometry according to Lichtenthaler and Wellburn (1983). The leaves were ground and 2 mL 80% acetone was added to extract chlorophyll. The extracts were transferred to new tubes and absorbance values were measured at 663 nm and 646 nm. The contents of chlorophyll a, chlorophyll b, total chlorophyll and carotenoid in the leaves were determined.
Root activity was determined by triphenyltetrazole chloride (TTC) reduction method (Clemensson-Lindell, 1994). A piece (0.25 g) of root was cut into small pieces (1-cm-long), placed in a 50-mL centrifuge tube, mixed with 0.4% TTC (5 mL) and 0.1 M Na2HPO4-KH2PO4 buffer (5 mL), incubated at 37°C for 1 h, and then 2 M H2SO4 (2 mL) was added to terminate the reaction. Rhizomes were placed in a mortar containing 5 mL ethyl acetate and ground thoroughly. The extract was transferred to a tube and the absorbance at 485 nm was determined.
2.7 Collection and extraction of root exudates
The plant roots in each hydroponic vessel underwent a series of sterile water washings. Each plant was then transferred to a centrifuge tube containing 50 mL sterile water. The roots were shielded from light with aluminum foil. After 6 h, root exudates were collected and filtered through a sterile 0.22-μm polyethersulfone syringe filter immediately to remove cell debris and contaminating microbes. The plants remained in the greenhouse during the collection of exudates to ensure that conditions remained consistent. The secretion-root biomass ratio was then calculated when the roots were collected and weighed. All samples were stored at −80°C until analysis.
2.8 Non-targeted metabolomics analysis
UPLC-QTOF-MS (Xevo G2-XS, Waters) was used to analyze the alfalfa root exudates extracted. An Acquity UPLC HSS T3 (2.1 × 100 mm, 1.8 μm, Waters) chromatography column was used. There were two mobile phases: mobile phase A (water and 0.1% (v/v) formic acid) and mobile phase B (acetonitrile and 0.1% (v/v) formic acid). The flow rate was maintained at 0.4 mL min−1 and the gradient was as follows: 0–1.5 min, 0% B; 1.5–2 min, 0–10% B; 2–3 min, 10–35% B; 3–5 min, 35–95% B; 5–14 min, 95% B; 14–17 min, 95–0% B. The column temperature was 40°C, and the sample size was 1 μL. The mass spectrometer range was set at 50–1200 Da and the detection parameters were: sprayer temperature 400°C; ion source temperature 120°C; atomizer gas flow rate 800 L h−1; and tapered voltage 40 V.
Each sample was scanned in positive and negative ion mode (ESI+ and ESI-) to obtain two sets of data. During operation, quality control sample (QC) was injected once every 10 samples to monitor the stability of the instrument. The device was operated by MassLynx 4.1(Waters) software. The original data collected were imported into Progenesis QI (V2.1, Nonlinear Dynamics) software for baseline correction, denoising, smoothing, time window segmentation, deconvolution, peak alignment and series processing. Principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were then used to statistically analyze the data, and VIP >2, P ≤ 0.05 differential metabolites were screened. The differential compounds and HMDB (http://www.hmdb.ca) NPATLAS (https://www.npatlas.org) database were identified, and the mass-to-charge ratio (m/z), retention time and peak area data tables were obtained.
2.9 Statistical analysis
Single factor analysis of variance (ANOVA) was performed on the growth data set using SPSS 22.0, followed by Tukey's post-hoc test (P < 0.05). Shapiro–Wilk's and Bartlett's tests were used to determine whether the data were normal and whether the variance was homogeneous. Square root or logarithmic transformations were conducted on the data when needed to satisfy parametric assumptions. Origin 2021 was used to obtain bar charts, principal component analysis (PCA) chart, heat map and correlation plot. E Venn software (http://www.ehbio.com/test/venn/#/) was used to obtain the Venn diagram (Chen et al., 2021). In order to compare the differences and changes of metabolites between different treatments and translate the metabolites identified in the experiment into differential metabolism-related pathways, MetaboAnalyst 5.0 was used to conduct partial least squares discriminant analysis (PLS-DA) analysis, and volcano plots and pathway analysis. The ‘global test’ and ‘relative intermediate centrality’ methods were based on the Arabidopsis thaliana metabolic pathway database (KEGG) (Pang et al., 2022). The Kyoto Encyclopedia of Genes and Genomes (KEGG) metabolic database was used to build metabolic pathways.
3 RESULTS
3.1 DSE colonization rate
DSE mycelium and microsclerotia colonized alfalfa root systems after DSE mycelium was added to the hydroponic solution. There was no significant difference in the colonization rate of DSE mycelium and microsclerotia in roots in treatments H and HM. The DSE mycelium infection rates of alfalfa treated with H and HM were 55.55% and 58.89%, respectively (Table 1). Compared with the mycelial infection rates, the microsclerotia infection rates in alfalfa roots treated with H and HM were low (8.89% and 5.55%, respectively) (Table 1).
| Treatment | Hyphal infection rate (%) | Microsclerotia infection rate (%) | Total infection rate (%) |
|---|---|---|---|
| H | 55.55±18.99 | 8.89±2.22 | 55.55±18.99 |
| HM | 58.89±4.84 | 5.55±2.22 | 58.89±18.99 |
- H, DSE mycelium treatment; HM, both DSE mycelium and extracellular metabolites treatment. No treatments are significantly different by Student's t-test (P < 0.05). Values are mean ± SE (n = 3).
3.2 Effects of DSE on plant growth and nutrient content
The addition of both DSE mycelium and extracellular metabolites in the hydroponic solution promoted plant growth. Total biomass of alfalfa in treatments H, M and HM increased by 23.88%, 47.20% and 47.14%, respectively, over the control (Figure 1C). Treatments M and HM significantly increased shoot and total oven-dried biomass (P < 0.05, Figure 1A, C). Treatment M significantly increased the root dry biomass (P < 0.05, Figure 1B) over the control. The root:shoot ratio in treatments H, M and HM decreased by 3.46%, 15.29% and 17.36%, respectively, compared to the control.
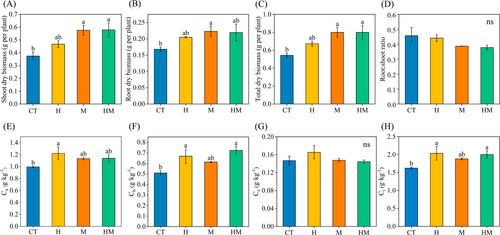
Total chlorophyll content in treatments H, M and HM increased by 25.45%, 15.90% and 23.27%, respectively, over the control (Figure 1H). Treatment H significantly increased the chlorophyll a content (P < 0.05, Figure 1E), with an increase of 22.81%. Treatments H and HM significantly increased chlorophyll b (P < 0.05, Figure 1F), with increases of 31.26% and 41.94%, respectively. There were no significant effects of DSE on carotenoid contents in the different treatments (Figure 1G).
Plant N content in treatments H, M and HM increased by 33.89%, 17.26% and 24.90%, respectively, over the control. Treatments H and HM significantly increased plant P content (P < 0.05), with an increase of 179.49% and 109.89%, respectively. Treatment H significantly increased plant K content over the control (P < 0.05, Table 2).
| Treatment | N (mg g−1) | P (mg g−1) | K (mg g−1) |
|---|---|---|---|
| CT | 38.33±3.21b | 1.24±0.18c | 19.42±0.30b |
| H | 51.33±3.32a | 3.46±0.35a | 31.33±2.30a |
| M | 44.95±1.68ab | 2.17±0.22bc | 23.26±0.54b |
| HM | 47.88±0.82ab | 2.60±0.25ab | 22.48±1.19b |
| F | 4.89 | 12.69 | 14.56 |
| P | 0.03 | 0.002 | 0.001 |
- N, nitrogen content; P, phosphorus content; K, potassium content; CT, sterile water control; H, DSE mycelium treatment; M, DSE extracellular metabolites treatment; HM, both DSE mycelium and extracellular metabolites treatment; F, test statistic; P, significance. For each set of treatments, different letters indicate significant differences at P < 0.05 levels, as estimated by Tukey's HSD test. Values are mean ± SE (n = 3).
3.3 Effects of DSE on root structure and root activity
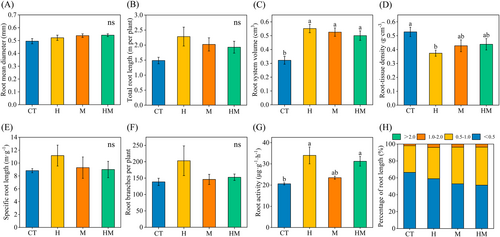
Treatment H significantly increased root volume and significantly decreased RTD (P < 0.05, Figure 2B-D). Treatments M and HM significantly increased root system volume (P < 0.05, Figure 2C). The percentages of root diameter >0.5 mm in treatments H, M and HM increased by 7.49%, 13.51% and 15.09%, respectively (Figure 2H). Treatments H, M and HM increased root activity by 65.00%, 14.01% and 51.53%, respectively (Figure 2G).
3.4 Metabolite profiles in root exudates
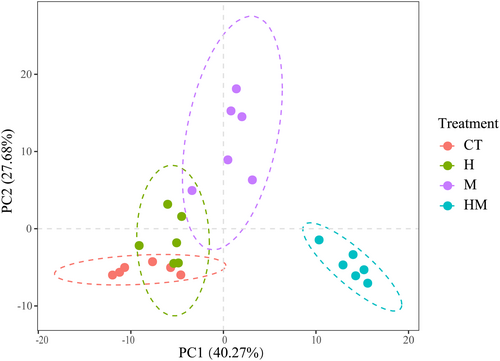
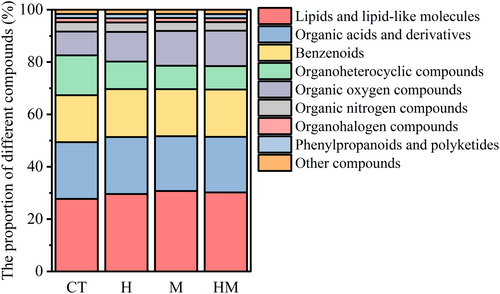
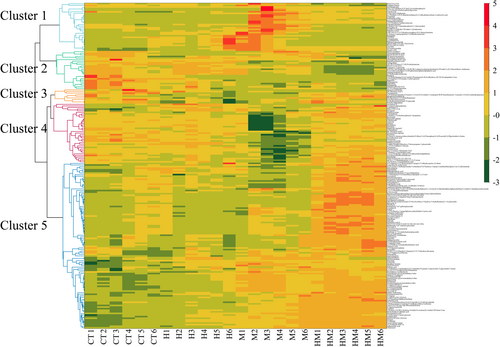
PCA shows differences in root exudates between different treatments. Treatment HM differed the most from the other treatments (Figure 3). A total of 204 metabolites were identified from the root exudates across treatments (Table S1). The compounds identified included lipids and lipid-like molecules (29.54%), organic acids and derivatives (21.40%), benzenoids (18.09%), and organic oxygen compounds (11.82%) and organoheterocyclic compounds (10.91%). The relative abundances of benzenoids, lipids and lipid-like molecules, organic oxygen compounds, organohalogen compounds, organosulfur compounds, phenylpropanoids and polyketides in the root exudates in treatments H, M and HM were higher than in the control (Figure 4). In contrast, the relative abundances of organophosphorus compounds in H, M and HM were lower than in the controls (Figure 4). Heat map analysis of differential metabolites reveals the presence of five clusters (Figure 5). Cluster 1 consists of exudates with the highest M abundance, cluster 2: CT > H > M > HM, cluster 3 and 4 had the lowest M, and cluster 5: M, HM > CT.
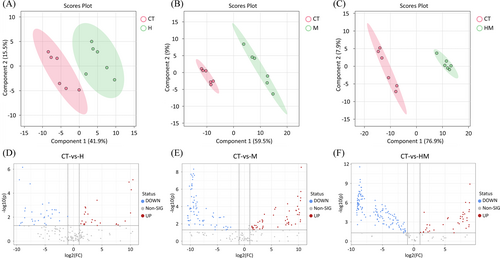
PLS-DA analysis (Figure 6, 7) shows differences in compounds between treatments and controls, including compounds that were up- and down-regulated (Figure 6D-F). The Venn diagram (Figure S1) shows that there were 34 substances in common in the different treatments, and only 3 specific different substances were found in treatment H. There were 47 different substances in treatment HM (|log2(FC)| > 1, P < 0.05). Overall, 97.55% of the compounds were different in at least one pairwise comparison. In order to compare the differences between DSE mycelium and extracellular metabolites in root exudates, volcanic plot analysis was conducted on root exudates treated with H and M (Figure S2A), and the results show that 48 compounds were up-regulated in treatment H and 58 compounds were down-regulated in treatment M. There were more organoheterocyclic compounds and fewer organic acids and derivatives in treatment H than in treatment M. (Figure S2B). Treatment H up-regulated and down-regulated organoheterocyclic compounds by 18.75% and 5.17%, respectively, and up-regulated and down-regulated organic acids and derivatives by 6.25% and 17.24%, respectively.
3.5 Analysis of plant metabolic pathways
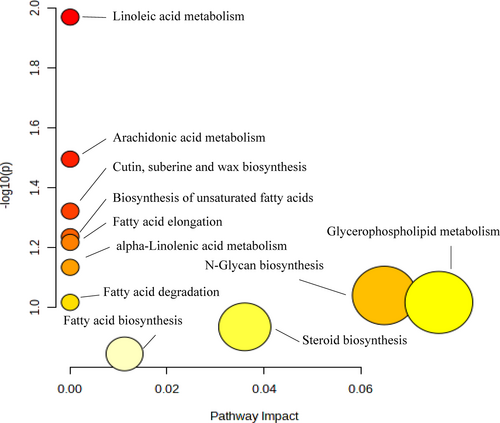
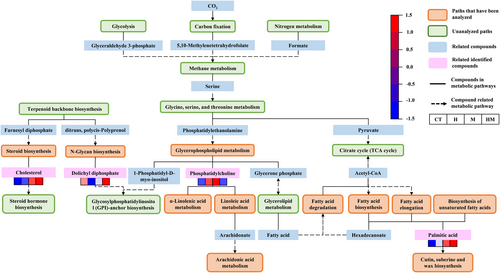
The KEGG pathway was used to analyze the differences in metabolic pathways between treatment H, M and HM and the controls (Figure 7). Treatments H, M and HM affected glycerophospholipid metabolism, N-glycan biosynthesis, steroid biosynthesis, fatty acid biosynthesis and arachidonic acid metabolism and other metabolic pathways (Figure 8). The main differential metabolic pathway was fatty acid metabolism. The largest difference among different treatments was in linoleic acid metabolism (P = 0.01).
4 DISCUSSION
4.1 Promoting effect of DSE mycelium and extracellular metabolites of plant growth
In this study, we showed that, after hydroponic inoculation, DSE could colonize the root system of alfalfa. This is consistent with previous studies. For example, Marian et al. (2022) conducted a hydroponics test by inoculating lisianthus with DSE, and found that the DNA content of DSE detected in roots under hydroponics was higher than that in pot experiment. Studies show that DSE may colonize aquatic plants (Kai & Zhao, 2006; Vohnik et al., 2015), but there are few laboratory hydroponic experiments on DSE inoculation. It is unclear how DSE conduct signal recognition of root colonization. The current results show that the colonization rate of alfalfa treated with HM was only 3.34% higher than that treated with H, indicating that DSE extracellular metabolites do not significantly promote root colonization. Nutrients are abundant in hydroponic environments but DSE are more capable of forming potentially symbiotic associations with host plants under harsh conditions (Malicka et al., 2022). In addition, since DSE form mutualistic relationships between mycorrhiza and saprophytic habits, there is little need for derived C (Ruotsalainen et al., 2022).
Here, treatments H and M both promoted plant growth. This is consistent with previous studies showing that DSE and DSE extracellular metabolites promote the growth of maize (Xie et al., 2021; Wang et al., 2023b). The nonsignificant effect of treatment HM on alfalfa biomass compared with the single treatment was unexpected. DSE extracellular metabolites contributed more to the biomass of alfalfa, possibly because the hormone-derived compounds in DSE extracellular metabolites had a more pronounced plant growth-promoting effect over a short period of time than mycelium (Wang et al., 2022b). Treatment H had more pronounced effects than treatment M on chlorophyll content. Previous studies also show that DSE treatment increased the chlorophyll content of licorice, wheat and barley (He et al., 2019; Qiang et al., 2019; Shadmani et al., 2021).
The root system determines the water and nutrients needed for photosynthesis and growth (Tracy et al., 2020), and the plant microbiome largely controls the characteristics of root development (Gonin et al., 2023). Numerous studies show that DSE can significantly promote root development (He et al., 2020; Li et al., 2022). Here, DSE mycelium and extracellular metabolites alone or in combination affected the root development of alfalfa. For example, DSE treatment increased the root oven-dried biomass, total root length, root system volume, coarse root percentage and root activity, and decreased RTD. The beneficial effect of DSE on roots may be due to the promotion of plant hormone production (Priyadharsini & Muthukumar, 2017). Our previous studies indicate that the presence of indole substances in DSE extracellular metabolites can also promote plant root development (Wang et al., 2022b).
This study agrees with a study by Kong et al. (2019) on relationships between root traits. Root diameter is negatively correlated with RTD and an increase in root diameter can increase root for nutrients. Correlation analysis shows (Figure S3) that DSE colonization rate was positively correlated with root diameter and negatively correlated with RTD under treatment H. Moreover, DSE colonization rate was positively correlated with root activity and nutrient content. Root activity can reflect the quality, metabolism and nutritional status of root development to a certain extent (Liu et al., 2014; Wang et al., 2006). Treatment H increased the root diameter, increased root vitality and promoted root nutrient absorption. Under the conditions of treatment M, the root oven-dried biomass of alfalfa increased significantly and the oven-dried root biomass was positively correlated with the root diameter and root volume, indicating that alfalfa increased its nutrient content by increasing the root diameter and root volume to expand the nutrient absorption area (Figure 9).
4.2 Variations in root exudates
DSE colonization significantly affects plant physiology and promotes plant growth by regulating plant hormones and metabolites (Liu & Wei, 2019; Zhang et al., 2017). Plants may also promote microbial growth by regulating the composition of root exudates (Zhalnina et al., 2018), and the presence of specific compounds in exudates or tissues may control interactions between microorganisms (McLaughlin et al., 2023). At present, how DSE further affect alfalfa root exudates remains unknown. On one hand, the effects of DSE mycelium and metabolites on root exudation by alfalfa are similar. Compared to the controls, the metabolites of alfalfa root exudates that were significantly altered under DSE treatment were lipids and lipid-like molecules, organic acids and derivatives and benzenoids. DSE can accumulate and disperse large amounts of lipids through the roots (Barrow, 2003). Lipid and lipid-like molecules are abundant in root exudates of all field-grown and hydroponically-grown plants (Heuermann et al., 2023). Lipids are major and essential components of all plant cells; they provide structural integrity and energy for various metabolic processes and also serve as signal transduction mediators (Lim et al., 2017). Their structural similarities among plant, fungal, and bacterial taxa suggest the potential for transboundary communication (Beccaccioli et al., 2022; Pohl & Kock, 2014). Lipids are important energy storage substances in both DSE and arbuscular mycorrhizal fungi (AMF) (Barrow, 2003; Keymer et al., 2017). Studies indicate that lipids are the main nutrients that plants synthesize and transfer to AMF (Jiang et al., 2017). However, there is no direct evidence for the exchange of lipids between host plants and DSE.
The current results show differences in alfalfa root exudates with the addition of DSE mycelium and metabolites alone or in combination. In order to clarify the specific mechanisms of different treatments on alfalfa metabolism, a comparative analysis was conducted between each treatment and the control (Figure 6). There were three different phenylpropanoid and polyketide metabolites in root exudates treated with H, and all three were up-regulated. Phenylpropanoids and polyketides typically regulate specific interactions between beans and their symbionts (Wang et al., 2023a). Lahlali et al. (2014) demonstrated that DSE can increase the activity of phenylalanine ammonia lyases (PAL) in Brassica napus L. The up-regulated compound, delphinidin 3-(6″-malonyl-Glucoside), belongs to the flavonoid glycosides. This result supports Zhang et al. (2017), who report that DSE inoculation in sorghum can promote the synthesis of flavonoids. Flavonoids play an important role in biological communication (Sugiyama & Yazaki, 2014). Flavonoids in tomato root exudates increased root AMF colonization (Scervino et al., 2007). Brassinolide and 28-norbrassinolide, whose direct precursor is brassinolide and its derivatives, are up-regulated. Brassinosteroid (BR) steroidal plant hormones are natural products that promote growth (Nemhauser et al., 2004). Compared with treatment M, treatment H also adds organoheterocyclic compounds (Figure S2). These include indoles and derivatives, which regulate plant growth and stimulate root formation (Sun et al., 2023).
Compared with the control, 75 compounds were up-regulated and 53 compounds were down-regulated in alfalfa root exudates of plants treated with M. A total of 18 compounds in root exudates of plants treated with M were found to be significantly different from those treated with H and HM. These include 4-O-deacetylvinflunine, a changchun alkaloid. This result supports previous studies showing that Alternaria sesami extract in rose wood callus increased the vinblastine content of rose callus (Birat et al. 2022).
HM-treated alfalfa root exudates had the most differential metabolites. Compared with treatments H and M, the percentages of amino acids, peptides, and analogues increased in HM treatment. The percentages of amino acids, peptides and analogues in treatment M also increased compared with treatment H. Studies demonstrate that microorganism-produced secondary chemicals can increase the release of amino acids by roots (Fernandez et al., 2012; Phillips et al., 2004). For example, AMF increased the amino acid content of Anchusa officinalis L. under semi-hydroponic conditions (Cartabia et al., 2021).
4.3 Regulation mechanism of DSE mycelium and extracellular metabolites on plant metabolism
Compounds detected in exudates are usually synthesized in roots (McLaughlin et al., 2023). Tricarboxylic acid (TCA) cycle is the basis of biological metabolism and the hub of metabolic pathways of sugars, fatty acids and amino acids. As shown in Figure 8, acetyl-CoA connects fatty acid degradation, fatty acid biosynthesis, and fatty acid elongation. After addition of DSE mycelium, the concentration of palmitic acid in single and combined treatment increased, promoting cutin, suberine and wax biosynthesis. Cutin, suberine and wax biosynthesis is associated with plant growth promotion (Grunhofer & Schreiber, 2023). In addition to its primary function as a transpiration barrier, the physiological functions of the stratum corneum also include important roles in processes such as development and interaction with microorganisms (Yeats & Rose, 2013). Wu et al. (2018) inoculated Streptomyces lydicus A01 to promote the growth of tomato seedlings, and found that all genes in the cutin, cork and wax biosynthesis pathways were significantly up-regulated.
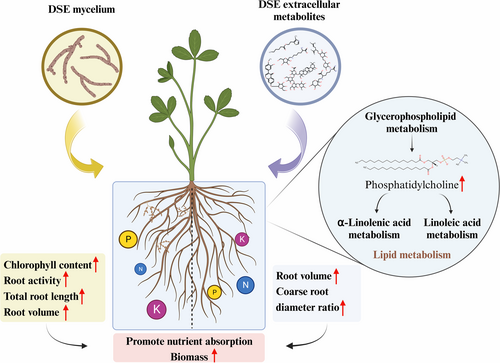
The phosphatidylcholine content of glycerophospholipid metabolism in root exudates of alfalfa treated with H and M increased, which promoted α-linolenic acid metabolism and linoleic acid metabolism. Goddard et al. (2021) also found that AMF colonization led to increased levels of linoleic acid and linolenic acid in plants. α-linolenic acid is an important polyunsaturated fatty acid precursor of jasmonic acid (JA) (Wasternack & Strnad, 2018). JA plays an important role in root growth, seed germination, leaf senescence and stomatal opening (Bari & Jones, 2009). Linoleic acid is the precursor of arachidonic acid, which can make plant tissues more metabolically active and increase regeneration ability (He et al., 2023).
Interestingly, the main metabolic cycles of alfalfa are up-regulated, including glycolysis and carbon fixation. In particular, we predicted that DSE mycelium and metabolites would increase the nitrogen content in alfalfa by promoting plant nitrogen cycling. Wu et al. (2023) found that DSE S16 could phosphorylate the high-affinity nitrate transporter PaNRT2.1 by activating the receptor-like kinase PaCRK10, thereby stabilizing the transport function of PaNRT2.1 and promoting the uptake of exogenous nitrate in the sweet cherry rootstock Gisela 5 seedlings. This conclusion can be further demonstrated by whole plant tissue core metabolomics.
Terpenoid backbone biosynthesis can increase plant nitrogen use efficiency (Wang et al., 2022a). The terpenoid skeleton biosynthesis of farnesyl diphosphate was used as a synthetic substrate for steroid biosynthesis. Cholesterol in treatment H, M and HM increased compared with the controls. As a substrate, cholesterol promotes steroid hormone biosynthesis. BR stands for a class of steroid hormones that control plant development, growth, and environmental adaption and are crucial for plant development (Wang et al., 2023c). HM increases dolichyl diphosphate and promotes glycosylphosphatidylinositol (GPI) -anchor biosynthesis, and this plays an important role in the deposition of cellulose in cell walls (Qiu et al., 2023).
5 CONCLUSION
The current study investigated the effects of DSE mycelium and extracellular metabolites on the growth of alfalfa and the underlying metabolic mechanism. The main conclusions are as follows. First, inoculation with DSE mycelium or addition of extracellular metabolites can promote the growth, root development and nutrient content of alfalfa, but the combined addition of DSE mycelium and extracellular metabolites had no discernible dual effects on alfalfa. DSE mainly promoted the nutrient uptake by roots by increasing root activity, while DSE extracellular metabolite treatment mainly increased root volume, expanded the nutrient contact area, and increased nutrient absorption. Secondly, lipids and lipid-like molecules were the main differential metabolites in treatments H and M. This may be closely related to the signal exchange between the DSE and host plants. Third, glycerophospholipid metabolism was the most significant metabolic pathway that affected DSE mycelium and extracellular metabolites of alfalfa, and the phosphatidylcholine content was significantly higher than in the controls. Finally, the root exudates of alfalfa treated with H and M were compared. The root exudates treated with H increased in organoheterocyclic compounds, while the organic acids and derivatives decreased. This study reveals mechanisms by which DSE and its extracellular metabolites promoted plant growth.
AUTHOR CONTRIBUTIONS
Yinli Bi: Conceptualization, writing - review & editing, supervision, validation, funding acquisition. Shuhui Wang: Writing - original draft, conceptualization, methodology, visualization, formal analysis and investigation. Yaning Song: Investigation, data curation. Peter Christie: Writing - correction and revision. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
We thank the National Key Research and Development Program of China (2022YFF1303300), the National Natural Science Foundation of China (51974326), the Joint Research program for ecological conservation and high-quality development of the Yellow River Basin (2022-YRUC-01-0304) and the Basic Research Fund of China University of Mining and Technology (Beijing) - Top-notch Innovative Talents Cultivation Fund for doctoral students (BBJ2023022). We also thank the editor and reviewers for their valuable suggestions on an earlier version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study, including metabolomics data, are available in the supplementary material of this article.




