The reprogramming of mitochondrial energy metabolism in Rice: The role of the TCA cycle and GABA shunt under Arsenic exposure
Abstract
Arsenic (As) pollution is hazardous for all living organisms. Plants growing in As-contaminated soil are less performant. Recently, seed priming has attracted significant attention for its beneficial effects on plants. Numerous studies have associated priming technique with the induction of stress memory against traumatic conditions, but its relation to crop's energy balance is under-researched. The present study highlights variations in energy metabolic pathways, particularly TCA (tricarboxylic acid) cycle and GABA (gamma-aminobutyric acid)-shunt in multi-nutrient [silicon (Si), iron (Fe), zinc (Zn), and ascorbic acid (AA)] primed rice seedlings under As stress. Arsenic exposure decreased the TCA cycle enzymes' activity while intensifying GABA accumulation and metabolism. This enhancement in GABA shunt provides an alternative energy source for As-stressed seedlings. However, priming and further Fe-supplementation reduced As-toxicity by accelerating the activity of both energy pathways, namely, TCA and GABA cycle. Improved energy regulation by priming and Fe-augmentation also enhanced the functionality of anabolic respiratory-interactive-pathway, i.e., carbon-nitrogen metabolism. The As-tolerance response of priming and Fe-augmentation was further analyzed from the expression profiling of nitrogen and phosphorous utilization genes. Out of 10 genes, particular enhancement was observed in OsNiR, OsGS, and OsGOGAT expression with improved macro-micronutrient content of the seedlings. Thus, irrespective of As infestations, multi-nutrient (Si, Fe, Zn, and AA) seed priming approach along with Fe-supplementation may emerge as a beneficial strategy to improve plant metabolic capacity in terms of nitrogen, phosphorous, and carbon assimilation to support normal vigor because they balance cellular energy levels.
1 INTRODUCTION
Arsenic (As) is classified as a group I human carcinogen (Khan & Gupta, 2018). Prolonged ingestion of As-contaminated drinking water and food is associated with an enhanced risk of bladder and skin cancer (Wei et al., 2019). It enters the environment through anthropogenic activities, weathering of mineral rocks, and microbial activities. Several studies on As contamination in the soil–plant interface have suggested that As toxicity in plants varies with its speciation (Chen et al., 2017; Mirza et al., 2022). The inorganic As species [i.e., arsenate (AsV) and arsenite (AsIII)] are considered the most abundant, toxic, and mobile forms (Feng et al., 2021). They enter the plant through high-affinity phosphate transporters (OsPht), aquaporins, and silicon transporters (OsLsi). Arsenate and arsenite both disrupt plant metabolism, but As(III) is considered more lethal than As(V) (Praveen et al., 2019). Arsenate is a structural analog of phosphate and interferes with several phosphate-dependent metabolic processes in plants. These effects include phosphate transporter protein disruption, phosphate supply imbalance, inhibition of phosphorylation events, and decreased ATP (adenosine triphosphate) synthesis. On the other hand, As(III) has a strong affinity for sulfhydryl groups and may bind to cysteine residues and dithiol co-factors in proteins, which can change their recruitment patterns and even the way those proteins interact with other proteins (Wei et al., 2019).
Plants usually deploy a wide range of physiological strategies to cope with metalloid toxicity, such as cellular resistance, ion exclusion, osmotic, and oxidative tolerance (Tyerman et al., 2019). All these strategies are energy-requiring active mechanisms, thus, controlled by diverse respiratory pathways, particularly mitochondrial respiration for ATP (Huang et al., 2016). Mitochondrial respiration is often considered at the center of several plant metabolic networks as it plays a pivotal role in underpinning growth, oxidative phosphorylation, and cell death. Tyerman et al. (2019) demonstrated the influential role played by mitochondria in plant resistance, by acting as ‘sensors’ against stress. Modulation in the activity of TCA (tricarboxylic acid) cycle's enzymes has further been observed in plants exposed to metalloid stress (Zhong et al., 2016; Samanta et al., 2020). A decrease in the activity of TCA cycle enzymes also cuts the outer flow of vital molecules and intermediates, leading to disruption of the total carbon pool of the cell, including photosynthesis at the level of both photochemical and biochemical steps (Huang et al., 2016). Therefore, any kind of dilapidation in the photosynthetic rate would be beneficial in manifesting the magnitude of devastations provoked by As exposure. Photosynthesis relies on two organelles: mitochondria and chloroplast. Both organelles contain membrane-bound enzymatic ATPase synthase, which utilizes the proton gradient to produce ATP, the energy currency of the cell. Exposure to As collapses the membrane architecture of both organelles, resulting in inhibited ATP production but also altered chlorophyll biosynthesis and fluorescence, as well as decreased photosynthetic O₂ evolution and phosphorylation. The interaction between the photosynthetic by-products (i.e., sugar-starch content) and the performance of plants has also been investigated in previous studies (Dai et al., 2016; Gupta et al., 2021). Higher sugar content has been shown to give plants more resistance to metalloid exposure (Wang et al., 2018; Gupta et al., 2021). A common characteristic that happens both at the organ and whole plant level when several PTEs (potent toxic elements, including As) impact plant functioning is the modification in carbon partitioning (Wang et al., 2018). Soluble sugars not only function as cell structural constituents and metabolic resources but also act as signals under toxic conditions. To modify plant metabolic responses, the sugar signalling system interacts with the stress tolerance pathways, namely the SNF-1 kinase and CDK (calcium-dependent protein kinase) pathways (Kaur et al., 2021). The fluctuating content of soluble sugar, disturbed sugar-starch interconversion, and its ratio sensing mechanism negatively affect plant productivity in terms of yield, grain weight, and even nutritional quality (Luo et al., 2021).
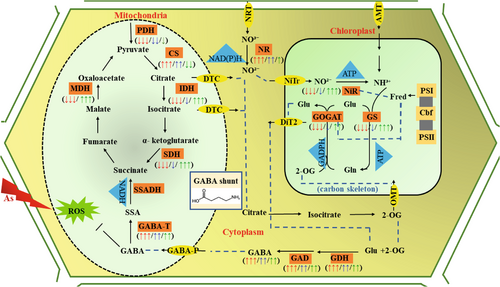
In soil, iron (Fe), zinc (Zn), and silicon (Si) have the greatest affinity for As interaction (Saini et al., 2023). Iron, an element of biological significance, appears to immobilize and restrict As uptake in plants via the formation of an inner sphere complex with As(V) and an outer and multiple inner sphere complex with As(III) species (Panthri & Gupta, 2022). In addition to the As-Fe interaction in soil, iron oxides were also found to exert domineering control over As transport via the Nramp metal transporter, ABCC, and ATP-binding cassette (Li et al., 2016). Additionally, Si inhibited As toxicity by competing with Lsi1 and Lsi2 transporters for their uptake (Das et al., 2022). Similarly, Zn manifests contrasting behavior against As toxicity by forming insoluble precipitates in soil and reducing its availability for uptake in plants, especially by ZIP transporter family members (Sun et al., 2021). Expression pattern analysis illustrated that ZIP was mainly expressed under Zn-deficiency conditions and has been found to be involved in the transportation of cadmium (CdII), manganese (MnII), and As(III) (Huang et al., 2020; Loyola et al., 2020). Interestingly, all these elements are also essential co-factors of several metabolic reactions and prerequisites for the normal functioning of Kreb cycle respiration. Differential respiration responses, namely, extensive metabolite content changes, are among the common responses of plants to As toxicity (Saini et al., 2023). 2-oxoglutarate dehydrogenase complex (2-OGDC) is a key metabolic enzyme necessary for the cyclic operation of the TCA cycle (Alamri et al., 2021). This enzyme is found to be one of the prime targets of metalloids and is physiochemically inhibited by them (Wei et al., 2022). GABA (gamma-aminobutyric acid) shunt activity, however, overcomes this suppression, as Che-Othman et al. (2020) showed in wheat (Triticum aestivum) seedlings under salt stress. Similar findings were also observed by Hijaz and Klinny (2019), where exogenous GABA application to citrus (Citrus aurantifolia) seedlings activated both TCA and GABA pathways to generate more energy. Several studies have further proposed that GABA shunt activity is also involved in regulating cytosolic pH, limiting ROS (reactive oxygen species) production, stress adaptation, and N-metabolism (Hijaz & Klinny, 2019; Das et al., 2022).
The relationship between the primary nitrogen (N) metabolism, mitochondrial respiration, and photosynthetic pathways has been thoroughly understood for decades. The reliance of N-metabolism for the requirements of cellular energy needs on mitochondrial respiration is another paradigm of how the underlying mechanisms coordinately function in synergism. Also, mitochondrial TCA cycle reaction molecules are required to support vascular nitrate assimilation (Erdal, 2019). The final step in the ammonium assimilating route is catalysed via glutamine-oxoglutarate-aminotransferase (GOGAT) by utilizing the carbon skeleton, i.e., 2-OG (2-oxoglutarate), and this 2-OG is provided through partial respiration of sugars in the TCA cycle. So, any manipulation in the metabolic route of the TCA cycle and electron transport directly or indirectly impacts N-metabolism and related processes (Diab & Limami, 2016). Thus, the TCA cycle has been found to be strongly associated with GABA shunt activity and N-assimilation.
The regulation of key plant metabolic processes, including N-assimilation, photosynthesis, and sugar-starch partitioning, particularly under stressful conditions, depends on the mitochondrial TCA cycle. However, the majority of the publications available have concentrated on the separate modulations in these biosynthetic processes brought on by As exposure (Shahid et al., 2019; Samanta et al., 2020) but only a few of them have taken into account the simultaneous variations in energy homeostasis in the plant cell. Similarly, literature is available regarding the useful effect of micronutrient seed priming (Bhatia & Gupta, 2022; Sherin et al., 2022). However, few studies have examined how multi-nutrient priming and further Fe-supplementation contribute to reconfiguring energy metabolism to facilitate the increased energy demand of the As-stressed seedlings. The goal of the current study is to fill this gap by investigating the effects of As and priming approach alone, as well as with Fe-supplementation, on the energy biosynthesis pathway. We hypothesized that priming application and additional Fe-augmentation might mitigate As toxicity by affecting respiratory enzymes and their relationship to GABA. This could be utilized as a potential tool for enhancing the function of several anabolic respiratory interacting pathways, i.e., C and N metabolism, for boosting crop growth and yield.
2 MATERIALS AND METHODS
2.1 Experimental layout
Rice seeds (Oryza sativa var. IR-64) procured from the Indian Agricultural Research Institute, New Delhi (IARI), were surface-sterilized using 30% ethanol for 3 min and subsequently washed thoroughly with distilled water. The seeds were primed with the aqueous solution containing an optimized concentration of nutrients: 2 mM zinc sulphate (ZnSO4), 2 mM iron sulphate (FeSO4), 3 mM silicic acid (H4SiO4) and 40 ppm ascorbic acid (AA), for 24 h (Table S1). The set of seeds soaked in distilled water served as control. After priming treatment, seeds were washed with distilled water to remove the extra trace of nutrients adhered to the seed coat and subsequently dried at room temperature according to the method of Sheteiwy et al. (2016). The seeds (20 seeds per pot) were spread equally in plastic pots (width x height;15 cm x 13 cm) and allowed to germinate in the dark at 25°C for 2 days. After germination, pots were transferred to a controlled growth chamber with 16 h photoperiod, 70% relative humidity, and 25 ± 2°Cday/night temperature. The treatment conditions were as follows:
- Control(C)- received 5% Hoagland solution (HM),
- As- 5% HM with 150μMAs (NaAsO2)
- Primed (P′)- 5% HM,
- P′ + As- 5% HM with 150 μM As, and
- P′ + As+Fe- 5% HM with 150 μM As and additionally supplemented with 1.5 mM FeSO4 (Table S2) in order to further explore the additive effect of Fe in As-stressed primed seedlings.
After 15 days, the plantlets were harvested, washed, and stored at −80°C or dried as required for experiments. All the treatments were performed in three biological replicates. We selected 150 μM As concentration as it significantly hampers plant growth based on our previous results (Khan & Gupta, 2018).
2.2 Estimation of phenotypic growth and photosynthetic pigment content
Growth parameters, namely, root length (RL), shoot length (SL), fresh weight (FW), and dry weight (DW) were analyzed for each treatment. Chlorophyll content was monitored following Arnon (1949). Leaves (100 mg) were homogenized in 80% chilled acetone, followed by centrifugation at 8,000 g. The absorbance of the supernatant was taken at 645 and 663 nm. Carotenoid content was calculated according to Kirk and Allen (1965). Anthocyanin content was measured using the method of Fuleki and Frankis (1968). Briefly, plant tissues (0.2 g) were homogenized in 2 mL acidified ethanol, and centrifuged at 8,000 g for 10 min. The absorbance of the supernatant was taken at 535 nm. The computed content of anthocyanin was estimated in mg gˉ1 FW.
2.3 Analysis of toxicity modulators and indicators
The method of Gaitonde (1967) was followed for cysteine estimation. Briefly, plant tissues (0.5 g) were crushed in perchloric acid, followed by centrifugation for 20 min at 10,000 g. Ninhydrin was added to the supernatant and incubated. Cysteine content was estimated from the standard curve using L-cysteine. Proline content was measured according to Bates et al. (1973). Roots and shoots (0.5 g) were ground in sulfosalicylic acid (3%), followed by centrifugation for 10 min at 6,000 g. To the supernatant, ninhydrin and glacial acetic acid were added. The amount of proline content was estimated by recording the absorbance at 560 nm. Malondialdehyde (MDA) content was estimated following Heath and Packer (1968). Roots and shoots tissue (0.2 g) were homogenized in 1% tri-chloroacetic acid and centrifuged for 10 min at 12,000 g. The supernatant was incubated along with thiobarbituric acid (TBA), and absorbance was measured at 532 and 600 nm. The method of Alexieva et al. (2001) was used to determine the hydrogen peroxide (H₂O₂) content by homogenizing the plant samples in 0.5% tri-chloroacetic acid, centrifuged at 5,000 g for 25 min at 4°C. To the supernatant, 0.1 M phosphate buffer and 1 M potassium iodide was added. The absorbance was taken at 390 nm.
2.4 Determination of energy metabolism via targeting the TCA cycle and GABA shunt activity
2.4.1 TCA cycle enzyme assay
Plant tissues (0.2 g) were homogenized in 0.2 M phosphate buffer containing MgCl2, cysteine, and β-mercaptoethanol, followed by centrifugation at 12,000 g. The supernatant was used to analyze enzyme activities. Pyruvate dehydrogenase (PDH, EC 1.4.2.1) activity was assayed following Williams and Randall (1979). An increase in absorbance was recorded at 340 nm, which corresponds to the oxidation of NADH. The citrate synthase (CS, EC 2.3.3.1) activity was monitored according to Srere (1969). Oxaloacetic acid (10 mM) was added to the reaction mixture to initiate the reaction. An increase in absorbance at 412 nm was monitored for 2 min. The isocitrate dehydrogenase (IDH, EC 1.1.1.42) activity was determined following Zhou et al. (2012) protocol. The change in absorbance was analyzed for 2 min at 340 nm using sodium citrate as substrate. The succinate dehydrogenase (SDH, EC 1.3.5.1) activity was monitored according to Green and Narahara (1980). Enzyme activity was monitored at 458 nm after adding 3 mM sodium succinate and 3-indophenyl-2-nitro-tetrazolium chloride to initiate the reaction. Malate dehydrogenase (MDH, EC 1.1.1.37) activity was determined using the Kumar et al. (2000) protocol. The change in absorbance was reported at 340 nm by monitoring the oxidation of NADH.
2.4.2 GABA content and GABA shunt pathway enzymes assay
The GABA content was determined according to the protocol of Kitaoka and Nikano (1969). Plant samples (0.1 g) homogenized in 80% ethanol were centrifuged at 10,000 g for 10 min. To the supernatant, 10% sodium hypochlorite was added and absorbance was read at 630 nm.
For estimation of enzymes assay, plant tissues (0.2 g) were homogenized in 0.1 mM Tris–HCl buffer containing ethylenediamine tetra-acetic acid (EDTA), dithiothreitol and pyridoxal-5-phosphate. After centrifugation at 10,000 g, the supernatant was used for further analysis. The glutamate dehydrogenase (GDH, EC 1.4.1.2) activity was estimated following Akihiro et al. (2005). The absorbance was recorded at 430 nm after adding 50 mM ammonium sulphate. According to Bartyzel et al. (2003), glutamate decarboxylase (GAD, EC 4.1.1.15) activity was analyzed. L-glutamate and 35% (w/v) ninhydrin solution were utilized to derivatize the supernatant. Absorbance was taken at 340 nm to analyze enzyme activity. The activity of GABA transaminase (GABA-T, EC 2.6.1.19) was analyzed using the Yazdanparast and Qujeq (1994) protocol. NADH formed during the reaction was quantified by recording the absorbance at 340 nm.
2.5 Estimation of chlorophyll a fluorescence and gas exchange attribute
Chlorophyll fluorometer (PAR-Fluor Pen FP 110 S/N: 001285) was used to measure the fluorescence metrics, namely, actual PSII efficiency (Fm′-Fs/Fm′), maximum PSII efficiency (Fm-Fo/Fm), non-photochemical quenching (Fm-Fm′/Fm′), and electron transport chain (ETC). Variable Fm is maximal fluorescence in dark-adapted conditions, while Fm´ is maximum fluorescence in light-adapted conditions, Fo is minimal fluorescence in dark-adapted conditions, Fs is steady-state fluorescence.
Gas exchange parameters in terms of internal CO2 concentration (Ci), transpiration rate (Tr), stomatal conductance (Gs), net photosynthetic rate (Pn), and water use efficiency (WUE) were analyzed from each treatment. Water use efficiency was determined as a ratio of Pn to Tr. Measurements were done using an InfraRed Gas Analyzer (IRGA) portable photosynthetic instrument. (LI-COR 6400, LI-COR).
2.6 Assessment of sugar and starch metabolism
2.6.1 Sugar estimation
Total soluble sugar (TSS) and reducing sugar (RS) contents were quantified using phenol-sulphuric acid assay as explained by Dubois et al. (1956). Reducing sugar content was estimated using the Miller (1959) protocol, which is based on the oxidation of the aldehyde group to the ketone group using 3,5-dinitrosalicylic acid. The concentration of RS was estimated by recording the absorbance at 570 nm. Measurement of non-reducing sugar (NRS) was done by subtracting the values of RS from TSS.
2.6.2 Sugar metabolizing enzyme assays
Plant tissues (0.5 g) homogenized in the extraction buffer (containing HEPES–NaOH buffer EDTA, dithiothreitol, and Triton X) were centrifuged at 17,000 g for 10 min. Sucrose phosphate synthase (SPS, EC 2.4.1.14) activity was estimated using the Miron and Schaffer (1991) protocol. Enzyme activity was monitored at 490 nm after the addition of 25 mM fructose-6-phosphate, glucose-6-phosphate, and UDP-glucose as substrate. For sucrose synthase (SS, EC 2.4.1.13) activity, the enzyme extract was incubated with 25 mM fructose and UDP-glucose at 37°C for 30 min to initiate the reaction. The amount of sucrose hydrolyzed was quantified at 470 nm. Acid invertase (AI, EC 3.2.1.26) activity was analyzed following Bozena and Szczerba (1991) protocol. The absorbance was recorded at 470 nm using 0.4 M sucrose as substrate.
2.6.3 Starch estimation
Starch concentration was determined following McCready et al. (1950). Plant samples (0.2 g) were crushed in 80% ethanol and centrifuged at 5,000 g. Starch quantity was determined in terms of glucose as described by Sil et al. (2019), and the factor 0.9 was used to get the value of starch from glucose.
2.6.4 Starch-metabolizing enzymes assay
Plant tissues (0.2 g) grounded in 0.1 M sodium acetate buffer (pH 4.8), containing cysteine and CaCl2, were centrifuged at 10,000 g. α-amylase (EC 3.2.1.1) activity was analyzed using the Bush et al. (1989) protocol. The activity of the enzyme was estimated at 440 nm after adding starch. For β-amylase (EC 3.2.1.2) activity, the amount of sugar hydrolyzed from starch was estimated using 5-dinitrosalicylic acid reagent, and absorbance was recorded at 570 nm. Starch phosphorylase (SP, EC 2.4.1.1) activity was assayed following Dubey and Singh (1999). Enzyme activity was recorded at 530 nm using glucose-1-phosphate as substrate.
2.7 Estimation of nitrogen and phosphorous metabolism
2.7.1 Nitrogen assimilation enzyme assays
Plant tissues (0.2 g) were homogenized in phosphate buffer (pH = 7.4) containing casein, cysteine, EDTA, Triton X-100, and mercaptoethanol. After centrifugation at 16,000 g, the supernatant was used to estimate the enzyme activities. Nitrite reductase (NiR, EC 1.7.2.1) activity was examined according to Robin (1979) by recording the absorbance at 540 nm after the addition of sulphanilamide and N-naphthyl ethylene diamine. For nitrate reductase (NR, EC 1.6.6.1) activity, after the addition of Griess reagent, absorbance was observed at 540 nm. The activity was measured from the standard curve of sodium nitrite (Robin, 1979). For glutamine synthase (GS, EC 1.4.1.2) activity, Singh and Srivastava (1986) protocol was followed. The reaction was initiated after the addition of hydroxyl amine hydrochloride and ATP to the extraction mixture, followed by incubation at 30°C. The absorbance was recorded at 540 nm, which corresponds to the formation of γ-glutamyl-hydroxamate (γ-GMH), and results were expressed as nmol γ-GMH formed g−1 FW h−1. The activity of GOGAT (EC 1.4.1.14) was determined according to Singh and Srivastava (1986). L-glutamine was added to initiate the reaction and the decrease in absorbance was recorded at 340 nm for 3 min. The activity of GOGAT was estimated by preparing a calibration curve using NADH.
2.7.2 Inorganic nitrogen content estimation
For the estimation of nitrate (NO₃ˉ) and ammonia (NH₄+) content, Cataldo et al. (1975) method was adopted. Fresh tissues (0.2 g) macerated in distilled water were heated for 15 min at 80°C. Nitrate content was determined by incubating the reaction mixture with 0.5% salicylic acid and absorbance was recorded at 340 nm. For NH₄+ content, the whole mixture was incubated for 5 min with Nessler's reagent, and the absorbance was observed at 425 nm.
2.7.3 Phosphorous assimilation enzymes assay
Phosphatase and phytase activities were assayed according to the Hayes et al. (1999). Sample materials (0.2 g) were macerated in an extraction buffer containing KCl, sucrose, and polyvinylpyrrolidone. The homogenate was centrifuged at 15,000 g. To assay alkaline phosphatase (EC 3.1.3.1) activity, the supernatant was incubated with Tris–HCl (pH 7.8) and p-nitrophenyl phosphate. Liberated p-nitrophenol was observed at 410 nm. For acid phosphatase (EC 3.1.3.2), a similar protocol was followed, except that Tris buffer is replaced by acidic 0.1 M acetate buffer (pH 4.8). For phytase (EC 3.1.3.8) activity, the protocol of Ascensio (1996) was followed. Enzyme activity was measured at 450 nm using potassium myoInsP6 as substrate.
2.8 Gene expression analysis
Total RNA was extracted from shoots and roots of 15-day-old rice seedlings using Trizol reagent. The RNA was treated with DNaseI followed by quantification using a Nanodrop-spectrophotometer (Thermo Scientific). The RNA quality was checked using 1.2% agarose gel. Approximately 2 μg RNA was utilized for first-strand cDNA synthesis using Revert Aid H Minus First Strand cDNA synthesis kit (Fermentas), as per kit's protocol. The qRT-PCR was performed in triplicate with the ABI Prism 7000 sequence detection system (Applied Biosystems) as described previously by Khan and Gupta (2018). For internal control, the endogenous ACTIN gene of rice was utilized. The primers used in the qRT-PCR study are listed in Table S6.
2.9 Analysis of As and mineral nutrients
Inductively Coupled Plasma Mass Spectrometer (ICP-MS, Agilent 7900 cx) was performed following the standard protocol of Praveen et al. (2019) to estimate nutrient content. Silicon content was monitored following the colorimetric molybdenum blue method as described by Khan and Gupta (2018). The concentration of AA was measured according to Roshanak et al. (2016). Plant samples (0.2 g) were homogenized in orthophosphoric-acetic acid solution, bromine water, and 2,4-DNAPH solution, which was added to assist the formation of osazone. To observe the oxidation of ascorbic acid to dehydroascorbic acid, absorbance was taken at 521 nm.
2.10 Statistical analysis
Data were subjected to one-way ANOVA using SPSS 20.0 to analyze the significant differences in the means of three replicates at p ≤ 0.05. The data illustrated are the value of the mean of three replicates (n = 3). The replication was analyzed by a random selection of samples with similar treatment conditions from three biological replicates. The three technical replicates were considered for each experiment and their average was counted as one. Statistical differences among the mean values were appraised using Duncan's multiple range test (DMRT).
3 RESULTS
3.1 Multi-nutrient priming influences the growth of rice seedlings and photosynthetic pigments under As stress
Arsenic exposure reduced RL and SL by 56% and 23%, respectively, compared to control (Table S3). Similarly, a reduction of 69% in FW and 74% in DW in roots and 90% and 64% in shoots, respectively, was observed when compared to the control. However, the priming treatment (P′ + As) was found to recover seedlings more effectively than As alone. Fe-supplemented seedlings had the highest SL, RL, FW, and DW values.
Exposure of seedlings to As had a significant influence on photosynthetic pigment contents (Table S4). When compared to control, As-stressed plants showed a decline of 45% in chlorophyll, 70% in carotenoid and 23%, in anthocyanin contents. In contrast, the seedlings raised from multi-nutrient primed seeds, under As stress, showed recovery in pigment content. Fe-augmented seedlings were found to have the highest concentration of photosynthetic pigments compared to control.
3.2 Modulation in toxicity modulators and markers under priming and As exposure
Arsenic exposure caused higher accumulation of H2O2 and cysteine in shoots by 379% and 605%, and in roots by 344% and 283%, respectively, compared to control (Figure S1). However, in P′ + As treatment, a substantial reduction of H2O2 and cysteine concentration was observed over As alone. Conversely, in P′ + As+Fe seedlings, Fe supplementation caused an increase in H2O2 and cysteine content, as compared to control. Overall, the trend of their accumulation under different treatments was as follows: As> P′ + As+Fe > P′ + As> P′ > C.
Moreover, As-supplemented seedlings also exhibited the highest levels of MDA and proline, with upregulation by 94% and 294% in shoots and 96% and 210% in roots, respectively, compared to the control (Figure S1). Over As, priming treatment notably decreased MDA and proline content in (P′ + As) seedlings. The lowest level of MDA was observed in Fe-supplemented seedlings. However, in contrast with the pattern of MDA content observed in P′ + As+Fe seedlings, proline content increased in shoots and roots, over control.
3.3 Regulatory effect of priming treatment and Fe-supplementation on energy metabolism via targeting TCA cycle and GABA metabolism
Following As stress, the lowering in the enzyme activity of the TCA cycle becomes more pronounced. In roots, PDH, IDH, SDH, and MDH activity decreased by 64%, 63%, 55%, and 33%, respectively, under As stress compared to control. Similarly, in shoots, reductions of 61%, 53%, 54%, and 37% were observed in the above-mentioned enzyme order, respectively, under As stress (Figure 1). In contrast to other enzymes, CS activity increased under As exposure by 33% in roots and 35% in shoots compared to control. However, priming treatment and Fe-supplementation lowered As-induced inhibition of enzymatic activity. Iron augmentation lowered PDH and CS activity by 23% and 28% in roots and 20% and 28% in shoots, compared to control.
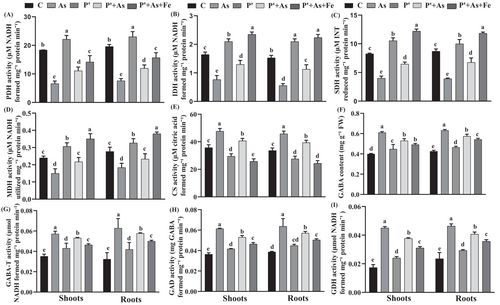
Upon As exposure, rice seedlings exhibited enhancement in GABA content, where the roots and shoots displayed about 49% and 52% elevation, respectively, over control (Figure 1). GABA-T, GAD, and GDH activity also increased by 94%, 65% and 97% in roots, and 62%, 68%, and 160% in shoots, respectively, under As stress, over control. However, in P′ + As treatment, a substantial reduction in GABA content by 13% in roots and 10% in shoots was observed, over As alone. Overall, the level of GABA accumulation as well as the activity of the related enzymes were observed in the following order; C < P′ < P′ + As+Fe < P′ + As< As.
3.4 Priming mediated changes in gas exchange, chlorophyll a fluorescence, sugar, and starch metabolism during As treatment
Considering the gas exchange parameters, Pn, Gs, Ci, and Tr decreased by 31%, 6.3%, 5.7%, and 11%, respectively, in As-stressed seedlings over control (Table 1). Treating rice seeds with multi-nutrients mitigated As toxicity, as revealed by the enhancement in parameters in P′ + As seedlings compared to their As counterparts. Fe-supplementation to As stressed-primed seedlings further enhanced the gas exchange parameters by 70%, 13%, 12.8%, and 24%, respectively, over As seedlings alone.
| Parameters | Treatment | P′ + As | P′ + As+Fe | ||
|---|---|---|---|---|---|
| C | As | P′ | |||
| Pn (μmol CO₂ mˉ2 sˉ1) | 15.60 ± 0.26ab | 10.70 ± 0.30c | 16.17 ± 0.25ab | 13.87 ± 0.57b | 18.20 ± 0.10a |
| Gs (mmol CO₂ /m2s) | 345 ± 0.70c | 326.67 ± 0.58e | 353.33 ± 0.29b | 334.67 ± 0.42d | 368.49 ± 0.29a |
| Ci (ppm) | 265.33 ± 0.49c | 250 ± 0.10e | 269.67 ± 0.31b | 259.67 ± 0.91d | 282.21 ± 0.70a |
| Tr (mmol mˉ2 sˉ1) | 2.86 ± 0.04c | 2.54 ± 0.01e | 3.00 ± 0.02b | 2.73 ± 0.01d | 3.15 ± 0.02a |
| WUE | 5.41 ± 0.27b | 4.48 ± 0.16d | 5.39 ± 0.04b | 4.82 ± 0.21c | 5.46 ± 0.01a |
| Fv/Fo | 0.63 ± 0.02b | 0.43 ± 0.01d | 0.64 ± 0.01b | 0.56 ± 0.01c | 0.68 ± 0.02a |
| Fv/Fm | 0.81 ± 0.02c | 0.65 ± 0.02e | 0.84 ± 0.01b | 0.74 ± 0.01d | 0.91 ± 0.01a |
| nqP | 0.64 ± 0.01c | 0.83 ± 0.02a | 0.62 ± 0.02cd | 0.75 ± 0.01b | 0.58 ± 0.02d |
| ETR | 121.63 ± 1.18b | 104.33 ± 2.08d | 124.67 ± 1.65ab | 113.33 ± 1.31c | 129.67 ± 1.68a |
- Pn, net photosynthetic rate; Gs, stomatal conductance; Ci, intercellular CO2; Tr, transpiration rate; WUE, water use efficiency; Fv/Fo, actual efficiency of PSII, Fv/Fm, maximum efficiency of PSII; nqP, non-photochemical quenching, ETR, electron transport rate.
Exposure of As led to the reduction of chlorophyll fluorescence attributes, viz., Fv/Fo, Fv/Fm, and ETR by 32%, 20%, and 14%, respectively, compared to control (Table 1). Treating rice seeds with multi-nutrients mitigated As toxicity and led to an increase in Fv/Fo, Fv/Fm, and ETR by 30%, 14%, and 8.5%, respectively, as observed in P′ + As seedlings, over As alone. Fe-supplemented seedlings were found to exhibit the highest chlorophyll fluorescence attributes.
Exposure of seedlings to As stress increased the concentration of TSS by 229% in shoots and 264% in roots compared to control (Figure 2). However, in P′, P′ + As, and P′ + As+Fe treatments, TSS was observed to increase by 27%, 61% and 114% in shoots, while by 33%, 83% and 149% in roots, respectively, over control. In comparison with As counterparts, reduction in TSS in P′, P′ + As, and P′ + As+Fe seedlings was also evident. A similar trend was followed in the case of RS and NRS. Additionally, in As-stressed seedlings, RS was found to contribute more to the increasing TSS content. The concentration of RS was found to be enhanced by 50% in shoots and 26% in roots, over NRS under As exposure. In contrast, in P′, P′ + As, and P′ + As+Fe, the content of NRS was higher than the RS content.
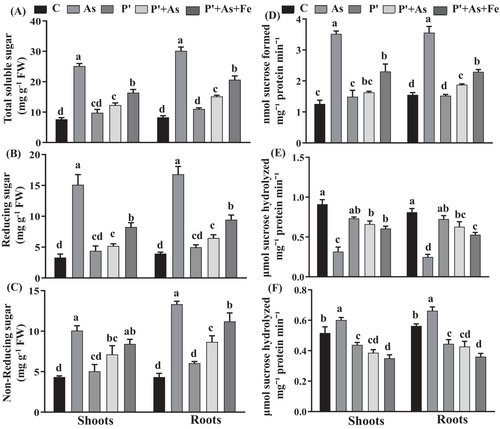
Seedlings grown in As-spiked soil was found to have the lowest SS activity (Figure 2). A reduction of 65% in shoots and 69% in roots in SS activity was observed in As-exposed seedlings, which was found to be significant over control. Under priming treatment and co-supplementation of As and Fe, the trend of reduction of SS activity was as follows: P′ > P′ + As> P′ + As+Fe.
In the case of SPS activity, it enhanced significantly by 179% in shoots and 129% in roots under As treatment compared to control. However, the SPS activity of P′ seedlings was not significant over control in shoots. On the contrary, in Fe-supplemented seedlings, augmentation in SPS activity was observed by 83% in shoots and 47% in roots, over control.
The activity of AI increased by 17% in both shoots and roots in As-treated seedlings when compared to control. Under priming and Fe-supplementation, the reduction in AI activity was in the following order: As>C > P′ > P′ + As>P′ + As+Fe. Reduction by 15%, 25%, and 32% in shoots and 20%, 24%, and 35% in roots was observed in P′, P′ + As, and P′ + As+Fe seedlings, respectively, compared to control.
The lowest concentration of starch was observed in As-exposed seedlings, wherein a decrease of 51% and 42% was noticed in roots and shoots, respectively, compared to control seedlings (Figure 3). However, the trend of increment in starch concentration observed in seedlings was as follows: As<C < P′ + As<P′ < P′ + As+Fe. Further, P′ + As+Fe seedlings revealed the highest starch content with significant increments of 81% and 54% in shoots and roots, respectively, over control.
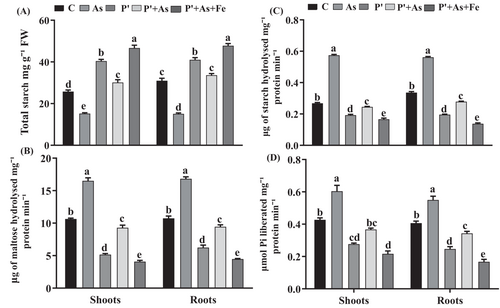
Statistically significant increments in α and β amylase and SP activity were observed under As exposure in comparison to control (Figure 3). Activity of α- and β-amylase, and SP increased by 114%, 55%, and 41.5% in shoots and 67%, 56% and 35% in roots under As stress, over control plants. Decreased activity of aforementioned enzymes in P′ + As seedlings by 57%, 43%, and 39% in shoots and 50%, 44%, and 37% in roots, respectively, were observed in comparison with their As counterparts. Fe-supplementation to As stressed-primed seedlings reported to have the lowest enzyme activity.
3.5 Influence of priming and As on nitrogen and phosphorus metabolism
Exposure of As to seedlings caused loss of function of all the studied N-assimilating enzymes, i.e., NiR, GS, GOGAT, except NR (Figure 4). Compared to control, As stress enhanced NR activity in both roots and shoots by 169% and 194%, respectively, over control. Priming and Fe treatment were found to successfully mitigate As toxicity and recover enzymatic activity in comparison with As alone. In P′ seedlings, the activity of NR, NiR, GS, and GOGAT in shoots was enhanced by 35%, 22%, 25%, and 14% over control seedlings, while, they increased by 37%, 45%, and 28% in shoots and 35%, 46%, and 25% in roots in P′ + As+Fe seedlings compared to control (Figure 4).
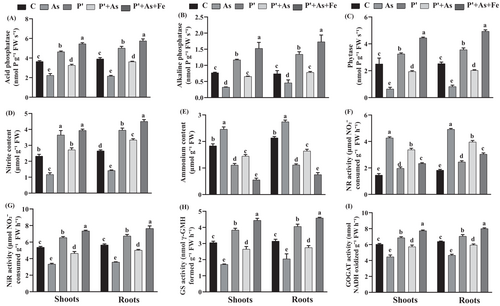
The NH₄+ content increased by of 34% in shoots and 28% in roots in As seedlings compared to the control value (Figure 4). On the contrary, in comparison with control, P′ and P′ + As+Fe reduced NH₄+ concentration effectively by 39.5% and 70% in shoots and 47% and 64% in roots, respectively. Opposed to NH₄+concentration, NO₃ˉconcentration exhibited an opposite trend with a lower value under As-stressed seedlings. A reduction of 46.5% and 50.1% in NO₃ˉ content was observed in the roots and shoots of As-treated seedlings, overcontrol. The pattern of accumulation of NO₃ˉcontent was as follows: As< C < P′ + As< P′ < P′ + As+Fe.
The activity of both phosphatase and phytase enzymes was reported to be lowest under As-stressed seedlings (Figure 4). A reduction of 39%, 58%, and 74% in shoots and 45%, 38%, and 68% in roots was observed in the activity of acid phosphatase, alkaline phosphatase, and phytase enzyme, respectively, in As-exposed seedlings, over control. However, priming treatment improved the enzymatic activities in roots and shoots, as compared to both control and As seedlings. However, the highest enzymatic activity was observed in Fe-supplemented seedlings. The enzyme activity of acid phosphatase, alkaline phosphatase, and phytase enzyme in P′ + As+Fe seedlings increased by 47%, 133%, and 95% in roots and 50%, 98%, and 77% in shoots compared to control.
3.6 Gene expression analysis
The relative gene expression of the N and P uptake and assimilation genes were assessed using qRT-PCR (Figure 5). Under As stress, nearly all the genes, including OsNRT2, OsAMT1, OsNR, OsPHT1, OsPHT2, OsPt, and OsAPase, had their expression levels upregulated in comparison to those of control seedlings. Among the N-assimilation genes, three of the genes (OsNiR, OsGS, and OsGOGAT) were downregulated under As stress, over control, with a gradual increase in P′ + As, P′ and P′ + As+Fe treated seedlings. All the P uptake-related genes showed a similar trend in expression pattern, with the highest expression in As treatment and the lowest in control.

3.7 Level of As and other mineral nutrients
Multi-nutrient seed priming treatment and Fe-supplementation significantly enhanced nutrient accumulation in rice seedlings (Table S5). K, Ca, Mg, and P contents in P′ + As+Fe seedlings were increased by 96%, 136%, 17.2%, and 20% in shoots and 105.7%, 211%, 29%, and 19% in roots, respectively, when compared against control. Similarly, in P′ seedlings, nutrient accumulation in the aforementioned order is enhanced by 62%, 100%, 7%, and 11% in shoots and 74%, 133%, 18%, and 15% in roots, respectively, over control. Contrasted with the nutrient uptake pattern of all other nutrients, Na showed a reverse order. Its accumulation was highest in As seedlings by 52% in shoots and 42% in roots in comparison to control. However, the reduced content of Na in P′ + As and P′ + As+Fe seedlings by 16% and 25% in shoots and 13% and 25% in roots, respectively, indicated the reduced As-induced toxicity over As alone. Primed seedlings were found to have the lowest Na content when compared with control.
Seed treatment had a positive effect on the uptake and absorption of nutrients used in the priming experiment (Table 2). Out of four, the content of three nutrients (Fe, Zn, and AA) were found to be higher in P′ + As+Fe and P′ seedlings by 47%, 61%, and 25% and 15%, 30% and 8% in shoots, respectively, compared to control. A similar pattern of accumulation of nutrients was observed in roots. In comparison to control, Fe, Zn, and AA decreased by 27%, 19%, and 19% in shoots and 20%, 32%, and 20% in roots, respectively, in As-exposed seedlings. In contrast, Si increased under As stress.
| Nutrient content (mg g−1 DW) | ||||||
|---|---|---|---|---|---|---|
| Fe | Si | AA | Zn | As | ||
| C | Shoot | 0.279 ± 0.38c | 1.52 ± 0.03e | 0.68 ± 0.42c | 0.068 ± 0.28bc | - |
| Root | 0.243 ± 0.03c | 1.63 ± 0.36e | 0.59 ± 0.29c | 0.063 ± 0.35c | - | |
| As | Shoot | 0.203 ± 0.83d | 2.34 ± 0.42a | 0.55 ± 0.08e | 0.0511 ± 0.18c | 0.06 ± 0.0.3a |
| Root | 0.194 ± 0.11d | 2.68 ± 0.15a | 0.47 ± 0.18e | 0.043 ± 0.31d | 0.076 ± 0.08a | |
| P′ | Shoot | 0.321 ± 0.28b | 1.71 ± 0.57d | 0.74 ± 0.52b | 0.082 ± 0.04ab | - |
| Root | 0.316 ± 0.72b | 1.85 ± 0.63d | 0.67 ± 0.09b | 0.078 ± 0.11b | - | |
| P′ + As | Shoot | 0.272 ± 0.74c | 2.07 ± 0.84b | 0.62 ± 0.14d | 0.063 ± 0.40bc | 0.038 ± .017b |
| Root | 0.238 ± 0.33c | 2.24 ± 0.72b | 0.53 ± 0.31d | 0.058 ± 0.23c | 0.042 ± 0.24b | |
| P′ + As + Fe | Shoot | 0.413 ± 0.88a | 1.83 ± 0.41c | 0.85 ± 0.25a | 0.102 ± 0.07a | 0.016 ± 0.19c |
| Root | 0.427 ± 0.75a | 2.04 ± 0.12c | 0.81 ± 0.63a | 0.110 ± 0.13a | 0.027 ± 0.10c | |
Among different treatments, As concentration was found to be maximum in non-primed As exposed seedlings (Table 2). Priming treatment was found to be effective in significantly reducing As concentration, as depicted by its decreased concentration in P′ + As and P′ + As+Fe treated seedlings. P′ + As and P′ + As+Fe exposed seedlings showed 36% and 73% decrease in shoots, and 45% and 64% reduction in roots As accumulation in comparison to As.
4 DISCUSSION
Considering the toxic nature of As, it has attained the utmost position on the Substance Priority List issued by the Bureau for Toxic Substance and Disease Registry (ATSDR) (Richardson, 2017). Approximately 140 million people in a minimum of 70 countries have been consuming food and water containing arsenic at a level greater than the WHO provisional guideline range of 10-12 μg/L (Praveen et al., 2020). Rice is a major constituent of the basic diet for half of the world's population and has high efficiency for acquiring As from contaminated soil. A loss of 60% in grain yield was reported in rice due to As-pollution in the soil (Muehe et al., 2019). Its exposure prompts a bottleneck effect on plant growth and development by hindering cellular function and metabolism (Praveen et al., 2020). Our study also revealed a significant reduction in plant growth parameters (RL, SL, FW, and DW) in As-stressed seedlings (Table S3). However, priming treatment resulted in a dearth of significant differences between P′ + As and C seedlings. These non-significant differences might be due to the accumulation of multi-nutrients in seeds, which are applied via priming approach, enabling seedlings to grow irrespective of As stress (Sherin et al., 2022). The modulations in physiological parameters i.e., chlorophyll, carotenoids, and anthocyanin contents, were observed under As exposure (Table S4). Reduced photosynthetic pigment under As stress might be due to augmented ROS production under As, which generated arbitrations in pigment metabolism (Sil et al., 2019). However, inhibition in chlorophyll synthesis was found to be recovered in primed and Fe-supplemented seedlings. These findings correlated with the reduced accumulation of As, decreased ROS production, protected chloroplast pigment molecules, and increased Fe and Mg content in P′, P′ + As, and P′ + As+Fe seedlings (Panthri & Gupta, 2019; Sherin et al., 2022).
A redox system imbalance causes oxidative stress, which deteriorates cellular membrane lipids and eventually causes MDA aggregation, the primary indicator of oxidative damage (Ren et al., 2018). Our results revealed the lower content of MDA in primed and Fe-supplemented seedlings (Figure S1). This finding is consistent with Chen et al. (2019) results, which demonstrated that Fe and Zn inhibit membrane peroxidation and stabilize cell membranes by interacting with both phospholipids and membrane proteins sulfhydryl groups. Another common consequence of As toxicity includes excessive production of ROS such as superoxide radicals (O2ˉ), H2O2, and hydroxyl radicals (OH), which is a prime outcome of several metabolite reactions occurring at multiple sites in plant cells (Praveen et al., 2020). In this study, the highest H2O2 content was observed in the As-stressed seedlings, which might be attributed to the As-mediated elicitation of free radicals. Besides acting as an indicator of stressful conditions, H2O2 also acts as a central participant in the stress signal transduction pathway. If H2O2 content in the cell is maintained at a non-toxic level via a delicate balancing act between H2O2 generation and scavenging, then it can be utilized as a signal molecule against stress by activating multiple acclamatory responses (Hossain et al., 2015). In this study, enhanced levels of H2O2 in P′, P′ + As, and P′ + As+Fe treated seedlings over control owe to the protective role of H2O2.
Cysteine, a thiol-containing amino acid, serves as an antioxidant and scavenges ROS. Enhanced cysteine under As exposure depicted tolerance mechanism in rice. Apart from imparting tolerance against ROS, it takes on different functions such as regulation, catalysis, electron transport, and structural roles (Sheteiwy et al., 2016). Our observation of augmented cysteine content in P′ + As, and P′ + As+Fe treated seedlings, over control, owes to the above-mentioned facts. Proline acts as an osmoprotectant. Its higher accumulation under As exposure lowers the water potential and regulates osmotic equilibrium, thus facilitating self-protection under traumatic conditions (Ren et al., 2018). However, its high concentration in P′, P′ + As, and P′ + As+Fe treated seedlings over control might be due to the proline's role in plant growth and development.
The TCA cycle comprises an important protection system under stress conditions. Its constituents help to restore the amino acid content, which in turn regulates osmotic homeostasis. PDH is regarded as the first enzymatic component of the TCA cycle, which maintains carbon entry into the cycle (Hijaz & Killiny, 2019). Its activity was significantly decreased in both roots and shoots of As-stressed seedlings, as As binds directly to the dihydrolipoyl group of transacetylase moiety in PDH enzyme (Das et al., 2022). Decreased PDH activity also ultimately narrowed down the carbon flow in the cycle and reduced energy production. After PDH, CS and IDH are the next enzymes in the TCA cycle, which catalyzed the conversion of acetyl-CoA to citric acid and citric acid to isocitrate, respectively. The formation of succinate from α-ketoglutarate is mediated by SDH (Zhong et al., 2016). In the current study, under As exposure, the CS activity was found to be raised over control. This increment might be owed to the enhanced demand for citric acid, which would provide energy to the cell under stress conditions (Hu et al., 2016). However, in contrast to CS, a significant inhibition in enzymatic activity of IDH, MDH, and SDH was observed in roots and shoots of As-stressed seedlings (Figure 1). Inhibition in SDH activity was attributed to the As-induced oxidization of the thiol group (Samanta et al., 2020). Higher As concentration decreased the IDH and MDH activity by altering their gene expression (Singh et al., 2020). However, P′ + As seedlings showed efficient mitigation of As toxicity by minimizing its destructive effects on enzymatic activity and restoring their activity. For supporting escalated respiratory chain metabolism, sufficient substrate availability for oxidation was maintained in the cell, which is also depicted by increased sugar accumulation in P′, P′ + As, and P′ + As+Fe seedlings over control. Iron-supplemented seedlings were found to have the highest enzymatic activity except for PDH and CS. The decrease in PDH activity in roots and shoots of P′ + As+Fe seedlings, over control, corresponded to the Fe-induced Fenton's reaction, which prompts more H2O2 and ROS production, thus disrupting PDH protein structure (Das et al., 2022).
A mitochondrial GABA permease links the TCA cycle to the GABA shunt and is crucial for normal carbon metabolism. In the cytosol, GABA is generated through the activities of two enzymes (GAD and GDH) by using glutamate and 2-OG as substrate (Hijaz &Killiny, 2019). In this study, GABA content significantly increased in both roots and shoots of As-exposed seedlings (Figure 1). Raised GABA content corresponded well with the increased activity of GAD and GDH. Several studies have demonstrated a negative correlation between GABA accumulation and the growth of seedlings (Che-Othman et al., 2020; Das et al., 2022). This is in line with the findings of the current study too. Increased GABA content and enhanced GAD, GDH activity indicated that extensive efforts had been made by As-stressed seedlings to limit ROS production, oxidative damage, and maintain cytosolic pH (Das et al., 2022). Upon GABA formation, it is catalyzed to succinate, which principally donates electrons to mitochondrial ETC (electron transport chain) with the help of GABA-T (Hijaz & Killiny, 2019). In the current study, GABA-T activity was increased during As stress in order to support continuous ATP synthesis and maintain succinate supply to mitochondrial ETC. Upon priming treatment and Fe-supplementation, we observed less GABA accumulation and low GAD as well as GDH activity over As, which might be attributed to the reduced intensity of As-induced stress. However, compared to controls, greater GAD, GDH, and GABA-T activity in P′ and P′ + As+Fe treated seedlings may suggest that both GABA shunt route and TCA cycle were functional to support increased respiration and energy demand.
Mitochondrial respiration is intricately coupled to chloroplast function and photosynthesis. Reduced chlorophyll fluorescence attributes upon As exposure indicated damage to photosynthetic apparatus, compromised functionality of PSII, and photoinhibition. This reduction was attributed to the excess accumulation of reducing equivalents in the cell due to impeded redox shuttling machinery of mitochondrial oxidation (De Andrade et al., 2015). Depreciation in Pn and Gs under As stress might be ascribed to the inactivation of RuBisCo (Ribulose bis-phosphate-carboxylase oxygenase) and As-induced phosphorous deficiency (Yang et al., 2015). Repression in SDH and MDH activity would also be a reason for decreased photosynthesis and slower stomatal function (Foyer et al., 2011). Nevertheless, priming treatment and Fe-supplementation protected PSII center machinery from As-induced damage by promoting Rubisco carboxylation, RuBP regeneration, and enhanced phosphorous uptake (Chen et al., 2017).
Sugar, the ultimate product of photosynthesis, besides only acting as a substrate in cellular respiration, also plays a central role in osmotic homeostasis, growth regulation, and membrane stabilization (Luo et al., 2021). Further, a large proportion of sucrose is transported to sink tissues, where it is converted to starch granules and stored for the fulfilment of future needs. Upon exposure to stressful conditions, the breakdown of starch is carried out for the accumulation of sugar to sustain basal metabolism (Dai et al., 2016). The enhancement in the concentration of TSS and reduction in starch content under As stress in the present study were in good line with the above statements (Figure 2). This fact is further supported by increased activity of starch-degrading enzymes (i.e., α and β amylase and SP) under As stress. Also, elevated concentrations of TSS provoke a feedback mechanism of Pn inhibition (Gupta et al., 2021), as also noticed in our study in As-exposed seedlings. Contrary to this, seedlings grown from multi-nutrient pre-treated and Fe-supplementation have shown a mitigation effect of As toxicity, as evidenced by increased starch content. This observation might be a consequence of the reduced activity of starch-degrading enzymes in P′, P′ + As, and P′ + As+Fe seedlings. However, over control, increased levels of sugar in P′ and P′ + As+Fe might be owed to the fact that seedlings escape the hydrolysis of sucrose so that it could be made irresistibly more available for respiration activities required for plant development (Luo et., 2021). Better regulation of sucrose-starch in P′ and P′ + As+Fe seedlings might also be because of induced kinase activity via upregulation of Ca2+-dependent kinase that creates increased sucrose flux in the cells (Sherin et al., 2022). Another reason for the reversal of As toxicity might be the efficient establishment of both TCA and GABA activity, which would restore the full rate of cytoplasmic starch synthesis and sugar consumption. Thus, the findings of the present study establish the link between the interdependency of sugar-starch metabolism with photosynthesis and mitochondrial respiration.
The enhancement in NRS and RS together contributed to the augmented concentration of TSS. The increase in concentration of TSS and RS in response to As is in good correlation with enhanced activities of sucrose synthesizing enzymes (SPS) and starch breakdown, as indicated by the increased activity of starch-degrading enzymes in this study (Figure 3). By regulating the carbon partitioning between sugar accumulation and starch degradation, SPS plays an important role in stressful conditions, especially under metalloid stress. Plant exposure to As, Cd, and chromium (Cr) leads to enhancement in the SPS activity (Feng et al., 2019; Li et al., 2020; Samanta et al., 2020). The change in SPS activity is often correlated with the altered sugar requirement of seedlings (Sil et al., 2019). Its reduced activity indicated a low requirement of sugar and, thus, lower stress intensity. The non-significant increase in SPS activity in primed seedlings over control is due to the above-mentioned fact. Further, increased AI activity under metalloid stress facilitates the generation of reducing hexose, which aids in regulating ROS metabolism and quenching free radicals (Dai et al., 2016). Interestingly, the ratio of NRS/RS was higher in primed and Fe-supplemented plants, while the ratio of RS/NRS was higher in non-primed As-stressed plants. The modulation in the relative concentration of sugar can be explained by the fact that non-primed plants require RS to quench As-induced ROS. However, P′ and P′ + As+Fe plants are already endowed with enhanced enzymatic and non-enzymatic antioxidants to constrain As toxicity (Gupta et al., 2021).
The chloroplast and mitochondrial capacity to deliver reducing equivalents in cytosol influenced the N-assimilation rate of the plant cell (Erdal, 2019). In the current study, the activity of N-assimilating enzymes was downregulated under As exposure, except NR activity (Figure 4). The highest NR activity under As stress is probably due to the low availability of its substrate (NO3−) in the cell. However, the lower NiR activity after As exposure might be attributed to contracted NADH and NADPH supply due to hampered TCA cycle function (Erdal, 2019). Decreased GS and GOGAT activity in As-spiked seedlings might correspond to the decreased IDH activity, which limited the production of 2-OG, used as fuel in the GS/GOGAT cycle (Che-Othman et al., 2020). Decreased GS/GOGAT activity also leads to increased accumulation of NH₄+, which was in line with the results of the present study. However, priming treatment and Fe-supplementation reverted the deadly effects of As toxicity and finely tuned the N-metabolism. This function might be attributed to the healthy functioning of TCA cycle enzymes.
Relevant studies have revealed that along with N, P is also closely related to the performance of plants (Adnan et al., 2020; Bechtaoui et al., 2021). Numerous carbohydrate conversions within plants are catalyzed in phosphorylated form. This role of phosphorous seems vital in regulating several metabolic pathways via phosphorylation and dephosphorylation reactions. The dephosphorylation reaction is catalyzed via phytase and phosphatase enzymes, which hydrolyze the phospho-ester bond from organic-phosphorous-containing substrates (Kong et al., 2018). In the present study, the activity of phytase enzyme, alkaline and acid phosphatase enzymes decreased under As stress, which in turn contracted the activity of several TCA cycle and N-metabolic enzymes. This reduction in enzyme activity might be attributed to As-stress-induced phosphorous deficiency. Mndzebele et al. (2020) also demonstrated that phosphatase and phytase activity is one of the fundamental traits revealing phosphorous deficiency. However, the release of competitive reactions between As and phosphorous in primed and Fe-supplemented seedlings makes sufficient availability of P to the seedlings, which might increase the enzyme activities both over control and As.
Heavy metal exposure and distressed mitochondrial activity are both reported to influence N uptake and assimilation (Diab & Limami, 2016). However, there is very little research available on the transcriptional regulation of N-metabolism genes in As-stressed rice genotypes under multi-nutrient priming and Fe-supplementation. Plants uptake nitrate via high-affinity nitrate transporter OsNRT2 and ammonium via OsAMT1 transporter (Praveen et al., 2019). Here, we found the upregulation of OsNRT2 and OsAMT1 transporters in the roots and shoots of As-stressed seedlings over control (Figure 5). The upregulation of OsNRT2 expression was not positively correlated with NO₃ˉ content. This observation might be due to the fact that NRT2 is associated with partner protein NAR2 to assist NO₃ˉ transport, and alteration in the expression of NAR2 probably be the reason for contracted NO₃ˉ content in the cell (Chen et al., 2020). Enhanced expression of AMT1 under As correlated well with increased NH₄+ content in seedlings. Once NO₃ˉ entered the cell, NR converted it to NO₂ˉ. Our results showed upregulation of the expression of OsNR in both roots and shoots under As stress but low OsNiR expression, which might be due to improper regulation at the translational level (Praveen et al., 2019). Furthermore, downregulation of GS and GOGAT was observed in both roots and shoots under As treatment. Similar results were also reported by Khan and Gupta (2018). On the contrary, GS and GOGAT expression were upregulated in primed and Fe-supplemented seedlings over control, which might be due to the reduction of As uptake and its toxicity. Enhanced GS and GOGAT activity might accelerate the incorporation of NH₄+ ions into amino acids, thus reducing the NH₄+ ion accumulation-induced toxicity in the cell under P′, P′ + As, and P′ + As+Fe treatments.
We also analyzed the regulation of P transporter genes. Phosphorous uptake is usually facilitated by PHT family proteins. Current data revealed the upregulation of PHT1, PHT2, Pt, and APase in As-stressed seedlings (Figure 5). This upregulation might be attributed to low P content, which triggers the expression of P-accumulating genes (Khan & Gupta, 2018). However, priming treatment and Fe-supplementation also upregulated the expression of PHT genes over control, which probably indicated accelerated P-metabolism in the plant cell.
Sustaining ion homeostasis is fundamental for almost all cellular and metabolic functions. In this study, As stress subsequently leads to nutrient deprivation (Table S5). In contrast to Na content, the K status of the As-stressed seedlings was significantly decreased over control. Reduced K content activates NAD(P)H oxidase, which causes membrane damage, hampered stomatal conductance, and net photosynthesis, which is also parallel to our findings (Panthri & Gupta, 2019). Priming treatment and Fe-supplementation were found to improve Na/K homeostasis, which eventually improved photosynthesis. Calcium plays an important role in the acclimatization of plants to stress by maintaining their cellular structure and activating plasma membrane ATPase (Sil et al., 2019). Its content declined significantly in As-exposed seedlings, and this is in accordance with the results of Panthri and Gupta (2019). Magnesium (Mg) and P uptake were lowest in As-stressed seedlings. This decrease in Mg level is probably associated with As-prompted decouplation of oxidative phosphorylation. However, the reduction in P content was attributed to the similarity in their chemical analogy, which leads to competitive inhibition for their partner transporter (Feng et al., 2021). The reduction in Fe and Zn content is noteworthy in As-treated seedlings (Table 2), which revealed As-induced hindrance in Fe as well as Zn uptake and translocation. However, their improved content in P′, P′ + As, and P′ + As+Fe might be due to the enhanced production of transporter proteins and better nutrient interplay (Khan & Gupta, 2018). On the contrary, Si content was higher under As exposure. This observation might be attributed to the role of Si in increasing the activity of SOD, APX, and CAT (Khan et al., 2023). Ascorbic acid is known to control cell division, differentiation, and elongation in plants (Alamri et al., 2018). Low AA concentration in As seedlings was stipulated from hampered seedling growth. On the contrary, the healthy growth of P′, P′ + As, and P′ + As+Fe seedlings indicated its enhanced concentration in respective seedlings.
5 CONCLUSION AND FUTURE PROSPECTS
We demonstrated that rice mitochondrial respiration modulated its substrate source under As stress. Arsenic toxicity induced reduction in the cyclic events of the TCA cycle. However, this inhibitory effect on the TCA cycle was compensated by enhanced GABA shunt activity to supply respiratory substrate and facilitate the energy demand. This indicates that GABA shunt, under As stress, makes a great contribution to respiratory metabolism. However, priming rice seeds with a consortium of nutrients before sowing and later supplementing with iron aided seedlings to withstand As toxicity by channeling energy sources. An increase in GAD, GABA-T, GDH enzyme activities and improved activity of PDH, CS, IDH, and SDH along with MDH, indicated the contribution of both energy regulatory pathways. Improved TCA cycle regulation by priming and Fe-augmentation generated indirect signals for the enhancement of several anabolic respiratory interactive pathways, i.e., photosynthesis, sugar-starch metabolism, and N-metabolism (Figure 6). The exploitation of flexibility in respiratory pathways can be used as a potential tool for enhancing N and P uptake and assimilation, as depicted from the expression analysis of OsNRT2, OsAMT1, OsNR, OsNiR, OsGS, OsGOGAT, OsPHT1, OsPHT2, OsPt and OsAPase. Synthesizing our findings, the current study has not only provided insightful information about the critical function of energy balance in controlling cellular coordination, but it has also suggested that components of respiration are an exciting potential target to improve carbon-nitrogen-phosphorous metabolism for boosting future crop growth and yield.
AUTHOR CONTRIBUTIONS
PB: Conceptualization, Investigation, Writing- original draft and executed the manuscript. HS: Data curation; ZM: Methodology; MG; Visualization, Resources, Supervision, Revision and Editing.
ACKNOWLEDGMENTS
PB thanks Jamia Millia Islamia, New Delhi, India for providing the UGC Non-NET fellowship.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as all newly created data is already contained within this article and in a supporting file.




