The potential of Nordic microalgae in nutrient removal from anaerobic digestion effluents
Abstract
Anaerobic digestion is a promising method for organic waste treatment. While the obtained digestate can function as fertilizer, the liquid fraction produced is rather problematic to discharge due to its high nitrogen and chemical oxygen demand contents. Microalgae have great potential in sustainable nutrient removal from wastewater. This study aimed at evaluating native Swedish microalgae cultivation (batch operation mode, 25°C and continuous light of 80 μmol m−2 s−1) on anaerobic digestion effluent of pulp and paper sludge (PPS) or chicken manure (CKM) to remove ammonium and volatile fatty acids (VFAs). While algal strains, Chlorella vulgaris, Chlorococcum sp., Coelastrella sp., Scotiellopsis reticulata and Desmodesmus sp., could assimilate VFAs as carbon source, acetic acid was the most preferred. Higher algal biomass and cell densities were achieved using PPS compared to CKM. In PPS, Coelastrella sp. and Chlorella vulgaris reached the highest cell densities after 15 days, about 79 × 106 and 43 × 106 cells mL−1, respectively. Although in PPS, ammonium was completely assimilated (195 mg L−1), this was only 46% (172 mg L−1) in CKM. Coelastrella sp. produced the highest biomass concentration independently of the medium (1.84 g L−1 in PPS and 1.99 g L−1 in CKM). This strain is a promising candidate for nutrient removal and biomass production in the aforementioned media, followed by Chlorella vulgaris and Chlorococcum sp. They have great potential to reduce the environmental impact of industrial anaerobic digestion effluents in Nordic countries.
1 INTRODUCTION
The continued growth of the world's population results in the generation of immense amounts of different wastes (Parchami et al. 2020). One of the major waste streams is organic (Aini et al. 2002), including household food waste and agri-food industrial waste (Patel et al. 2021). Improper organic waste management can contribute to environmental issues such as air, water and soil contamination, in addition to setting the stage for adverse impact on public health (Singh et al. 2018). Therefore, such waste streams must be treated efficiently to reach stability in social, economic, and environmental sustainability (Parchami et al. 2020). Organic wastes can be treated in different ways, such as direct landfilling, incineration, composting, and anaerobic digestion (Schüch et al. 2016). Anaerobic digestion (AD) is a promising biological treatment process that converts residual organic matter into biogas and digestate. While the obtained digestate can be used as fertilizer (Cesaro et al. 2019), the produced liquid effluent has a high chemical oxygen demand (COD) and nitrogen content. Therefore, it has to be treated as wastewater, imposing great costs on wastewater treatment plants (WWTP) prior to reaching regulated discharge levels.
Building on the previous point, the pulp and paper business (Santos et al. 2020), alongside the animal breeding (Gu et al. 2019) sector, are two of the primary industries producing wastewater and organic waste to a remarkable extent, respectively. The pulp and paper sector is closely related to human life and plays a significant role in the global economy (Liang et al. 2023). This industry is an important player in Sweden's industrial sector (Plöhn et al. 2022). In 2021, Europe produced a quarter of the global pulp, and Sweden was the largest pulp producer within Europe (Statista December 16, 2022) with around 10.1 million metric tons. Together with Finland producing 9.3 million metric tons of pulp, these two countries accounted for approximately 60% of the pulp produced in European countries (Statista July 7, 2022). The pulp and paper industry generates huge quantities of wastewater. Only in Sweden, 870 million m3 of wastewater per year are generated during the production of pulp, paper, and paperboard leading to the generation of pulp and paper sludge (PPS) through the treatment process (Munthe et al. 2011). Animal breeding is another important sector, which generates organic waste (manure) at large extent. In 2020, 40% of global meat production was related to poultry meat (Food and Agriculture Organization of the United Nations (FAO). Gateway to Poultry Production and Products 2020), resulting in the production of around 267 million metric tons of chicken manure (CKM). Roughly 40 kg manure are produced per laying hen per year and 3 kg of manure per broiler (Yin et al. 2022a). A common approach to treat PPS (Lin et al. 2017) and/or CKM (Yin et al. 2022a, Yin et al. 2022b) is anaerobic digestion for biogas production.
Microalgae are highly productive; in the photosynthetic processes, they consume CO2 and nutrients (e.g., nitrogen and phosphorous) to produce biomass. They are also able to grow heterotrophic on different carbon sources or mixotrophic using both light energy and organic carbon as energy source (Anbuchezhian et al. 2015, Gojkovic et al. 2019, Plöhn et al. 2022). Liquid digestate arising from anaerobic digestion of different substrates such as dairy wastewater (Dębowski et al. 2020) or agro-industrial liquid digestate (Sánchez-Quintero et al. 2023) have been successfully used as the nutritional growth medium for microalgae. Moreover, liquid digestate may contain volatile fatty acids (VFAs). VFAs have shorter metabolic pathways compared to sugar-based carbon sources such as glucose, sucrose or glycerol, making them good candidates for microalgae growth (Su et al. 2021). The algal biomass obtained during the treatment process can be used as feedstock for various applications, such as biofuel (Sánchez-Quintero et al. 2023), bio-oil (Dębowski et al. 2020), biopolymer (Mehariya et al. 2023), biochemicals and fertilizer. It can also be added to the digester as a co-substrate to enhance biogas production, resulting in improved economic and environmental benefits of AD (Passos et al. 2014, Stiles et al. 2018).
In this study, five native Nordic microalgae strains (Ferro et al., 2018) were cultivated on anaerobic digestion effluents (AD-effluents) obtained from either PPS or CKM in an effort to reduce their high COD, nitrogen and phosphate contents. Among the strains studied, Coelastrella sp. proved to be capable of producing the highest biomass concentration in both medium (1.84 g L−1 in PPS and 1.99 g L−1 in CKM). This investigation not only highlights strategies for treating the organic load in industrial organic-rich effluents, but also presents opportunities to produce algal biomass for value-added applications such as bio-oil and biopolymer production.
2 MATERIALS AND METHODS
2.1 Waste streams
Anaerobic digestion effluent obtained from PPS (Abdla 2022) was collected from Östrand pulp mill (Östrand, Sweden), CKM (Yin et al. 2022a) was obtained from the egg-laying farm Sjömarkens Hönsgård AB (Borås, Sweden). VFAs were produced of both waste streams in an immersed membrane bioreactor; the separated liquid part (Abdla 2022, Yin et al. 2022a) was first filtered using filter paper (Ahlstrom-Munksjo Munktell, Thermofisher scientific) with the pore size of 10 μm, then autoclaved. The pH was adjusted to 7.1 using 1% H2SO4 and 2 M NaOH. BG11 medium (Thermofisher scientific) was used to prepare pre-inoculum.
2.2 Algal cultivation
Five native Nordic microalgae strains were used in this study; Desmodesmus sp. (2–6) and Chlorococcum sp. (MC-1) were collected from fresh water, Chlorella vulgaris (13–1), Coelastrella sp. (3–4) and Scotiellopsis reticulata (UFA-2) from municipal wastewater (Ferro et al. 2018). To prepare a pre-inoculum, the microalgae strains were grown in 250 mL Erlenmeyer flasks filled with 75 mL BG11 medium in an open-air shaker under sterile conditions at 120 rpm, room temperature (25°C) and continuous light (80 μmol m−2 s−1). After 5 days, PPS or CKM were inoculated with the starter culture at an optical density (OD680) of 0.1 in 125 mL Erlenmeyer flasks with the volume of 35 mL. Scotiellopsis reticulata was more diluted than the other cultures. All experiments were performed in biological triplicates and cultivations were conducted for 15 days. Every other day the optical densities of all cultures were measured (collecting 1 mL sample from each flask) spectrophotometrically at 530 nm, 680 nm and 750 nm (Thermo Scientific™ GENESYS 140 Visible Spectrophotometer), the number of cells mL−1 measured using CellDrop FL (Denovix) and the pH was also measured (pH-meter METTLER TOLEDO). Optical densities were measured directly if they were in the range of 0–1, and they were diluted if they were higher. To harvest at the end of the cultivation period, the microalgae were centrifuged (5 minutes at 3000 × g) and washed once with Milli-Q water. The obtained biomass was oven-dried at 105°C for 24 hours to obtain dry biomass, further kept in a freezer until analysis.
2.3 Analytical methods
Soluble COD (sCOD) and ammonium content were determined using the CSB 15000 test kit and Ammonium 100 test kit, respectively (Nanocolor, MACHEREY-NAGEL GmbH & Co. KG, Germany). The concentrations of sCOD and ammonium nitrogen were determined using a Nanocolor 500D Photometer (MACHEREY-NAGEL GmbH & Co. KG, Germany). VFA concentrations were measured using gas chromatography (GC) (Clarus 550; Perkin-Elmer, Norwalk), equipped with a capillary column (Elite-WAX ETR, 30 m × 0.32 mm × 1.00 μm, Perkin-Elmer) as well as a flame ionization detector (FID). The injection and detection temperatures were 250°C and 300°C, respectively. The carrier gas was nitrogen with a flow rate of 2 mL min−1 and a pressure of 20 psi. The GC oven temperature was set at 200°C. For analysis by GC, to each 1.0 mL sample was added 200 μL of acid mix followed by centrifugation (10000 × g, 5 min). The acid mix was 25% (v/v) formic acid and 25% (v/v) of ortho-phosphoric acid at a ratio of 1:3. Afterwards, the supernatant was passed through a syringe filter having 0.22 μm pore size (ABLUO syringe filter) to remove undissolved particles. Finally, 250 μL of filtered sample, 250 μL of butanol (1 g L−1) as the internal standard and 500 μL Milli-Q water was mixed and used for VFA measurement (Jomnonkhaow et al. 2021).
2.4 Statistical analysis
All experiments were conducted in biological triplicates. Regarding technical replicates, for GC analysis, cell counting, pH and OD measurement, the number of data replicates was three, while sCOD and ammonium measurement were carried out in duplicate. Average values are reported in the text, while the graphs depict the average values along with error bars illustrating two standard deviation (95% confidence interval). The data was analyzed using Minitab® 21 (Minitab Ltd.). The level of significance in the difference between two mean values was determined using 2 sample t-test with 95% probability.
3 RESULTS AND DISCUSSION
3.1 Algal cultivation and growth analysis
Five native Nordic microalgae strains (Ferro et al. 2018) were cultivated on anaerobic digestion effluents obtained from either PPS or CKM in an effort to reduce their high COD, nitrogen and phosphate contents. The initial VFAs concentrations and ammonium contents in the cultivation media are provided in Table 1, as are total COD, COD from VFAs and COD from ammonium. The COD conversion factors used to determine the COD equivalent of VFAs were 1.07 for acetic acid, 1.51 for propionic acid, 1.82 for butyric and iso-butyric acids, 2.04 for valeric and iso-valeric acids and 2.2 for caproic and iso-caproic acids (Khatami et al. 2021). The conversion factor used to calculate the COD equivalent of ammonium was 1.78.
| Medium: PPS | Medium: CCKM | |||
|---|---|---|---|---|
| Strains | C. vul, Chlo, Coel, Des | S. ret | C. vul, Chlo, Coel, Des | S. ret |
| Acetic acid (g L−1) | 0.66 ± 0.02 | 0.60 ± 0.02 | 0.22 ± 0.00 | 0.19 ± 0.00 |
| Propionic acid (g L−1) | 0.10 ± 0.00 | 0.09 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| Isobutyric acid (g L−1) | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 | 0.00 |
| Butiric acid (g L−1) | 0.00 | 0.00 | 0.00 | 0.00 |
| Iso Valeric acid (g L−1) | 0.07 ± 0.00 | 0.06 ± 0.00 | 0.03 ± 0.00 | 0.02 ± 0.00 |
| Valeric acid (g L−1) | 0.00 | 0.00 | 0.00 | 0.00 |
| Caproic acid (g L−1) | 0.00 | 0.00 | 0.00 | 0.00 |
| Total VFAs (g L−1) | 0.84 ± 0.02 | 0.76 ± 0.04 | 0.27 ± 0.00 | 0.23 ± 0.01 |
| Ammonium (mg L−1) | 195 ± 7.07 | 160 ± 14.14 | 375.00 ± 7.07 | 330.00 ± 14.14 |
| Total COD (g L−1 O2) | 3.2 ± 0.00 | 3.00 ± 0.07 | 3.1 ± 0.00 | 2.85 ± 0.14 |
| COD from VFAs (g COD L−1) | 1.02 ± 0.03 | 0.92 ± 0.04 | 0.32 ± 0.00 | 0.28 ± 0.01 |
| COD from ammonium (g COD L−1) | 0.35 ± 0.00 | 0.28 ± 0.02 | 0.67 ± 0.00 | 0.59 ± 0.02 |
While growth of Chlorococcum sp. and Scotiellopsis reticulata was slow in PPS (Figure 1A), Chlorella vulgaris, Coelastrella sp. and Desmodesmus sp. reached high densities quickly. Coelastrella sp. and Chlorella vulgaris reached the highest cell densities of 79.00 × 106 and 43.20 × 106 cells mL−1 after 15 days, respectively. Compared to the BG11 control, these cell densities are increased by factor 6.5 and factor 2, respectively (Figure S1). However, the cell density of Desmodesmus sp. only increased by factor 1.3. Chlorococcum sp. and Scotiellopsis reticulata yielded the lowest cell densities, with 16.00 × 106 and 6.94 × 106 cells mL−1 in PPS, which can be attributed to the larger cell size of these two strains. An inverse relationship between microalgal maximal population density and cell size has been reported previously (Agusti et al. 1987, Ferro et al. 2018). Nonetheless, Chlorococcum sp. and Scotiellopsis reticulata were able to maintain growth and remove 100% of the available ammonium (see chapter 3.2). Cultured in CKM, similar results were obtained (Figure 1B). While Scotiellopsis reticulata (1.51 × 106 cells mL−1) and Desmodesmus sp. (3.02 × 106 cells mL−1) had the lowest cell densities, the highest cell densities after 15 days were obtained for Chlorella vulgaris and Coelastrella sp. with 37.5 × 106 cells mL−1 and 34.5 × 106 cells mL−1, respectively. Compared to the BG11 control, this corresponds to an increase in cell density by factor 1.5 and 2.8, respectively (Figure S2). In addition, Chlorella vulgaris, Coelastrella sp. and Desmodesmus sp. had shorter lag phases compared to the two other strains. In general, the five Nordic microalgal strains reached higher cell densities in the PPS medium.
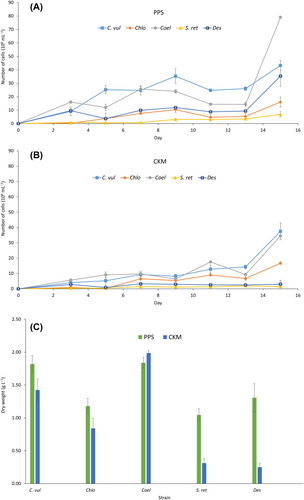
Coelastrella sp. produced the highest biomass dry weight in both PPS and CKM (Figure 1C), 1.84 ± 0.08 and 1.99 ± 0.04 (g L−1), respectively. This was also higher than the biomass dry weight produced in BG11 (Figure S3). The biomass concentration of Coelastrella sp. in CKM was significantly higher (p-value ≤0.05, assumption of sample size ≥3) than its biomass produced in municipal wastewater (1.46 ± 0.16 g L−1) after 13 days of cultivation (Ferro et al. 2018), showing the great potential of this strain in anaerobic digestion compared to other strains in this study. Coelastrella sp. was observed to have a very high lipid production capability (0.45 g L−1 or around 30% of dry biomass) in wastewater (Ferro et al. 2018). Although Desmodesmus sp., together with Scotiellopsis reticulata, produced the lowest amount of biomass in CKM, Desmodesmus sp. produced 1.31 ± 0.21 g L−1 of biomass in PPS, which is in line with the maximal cell density results. Moreover, this value of biomass production by Desmodesmus sp. is higher than in the above-mentioned study (Ferro et al. 2018). Providing optimal ammonium concentration (110 mg L−1) in manure-free piggery wastewater, Chlorella vulgaris from a Chinese culture collection produced 1.26 ± 0.09 g L−1 biomass within 7 days of cultivation (Zheng et al. 2019). This is comparable to the biomass produced in CKM by the Nordic Chlorella vulgaris strain in this study (1.42 ± 0.17 g L−1) and significantly (p-value ≤0.05) lower than its biomass produced in PPS (1.82 ± 0.12 g L−1). The amount of biomass produced in PPS by Chlorella vulgaris is also higher than in municipal wastewater after 13 days (1.15 ± 0.06 g L−1; Ferro et al. (2018) or 8 days (1.25 ± 0.9 g L−1; Nzayisenga and Sellstedt (2021) of cultivation. In a study on microalgal cultivation in liquid digestates from anaerobic digestion of pulp and paper sludge, it was shown that biomass concentrations of Chlorella vulgaris can be achieved between 2.02 and 2.91 g L−1 within 15 days, depending on the growth medium. In the same study, a Scenedesmus strain, which is a close relative to Desmodesmus sp., reached concentrations between 2.91 and 8.22 g L−1 (Tao et al. 2017). The higher biomass dry weight production in PPS medium compared to CKM may be explained by the higher initial acetic acid content in PPS medium; a direct relationship between biomass production and the amount of acetic acid in the medium has been observed earlier (Kim et al. 2019).
Table 2 summarizes pH values and optical densities measured during cultivation in PPS and CKM. In PPS, the Coelastrella sp. culture showed a slightly reduced pH (from 8.9 to 8.6) from day 7 to day 11, probably caused by the depletion of ammonium and VFAs. The pH values of the other cultures in PPS increased during the 15 days of cultivation. In contrast, in CKM, the pH values decreased during the course of time, even though CKM contains higher ammonium levels, which should enhance its buffering capacity at alkaline pH.
| Strain | CKM | PPS | |||
|---|---|---|---|---|---|
| Day | OD (680 nm) | pH | OD (680 nm) | pH | |
| C. vulgaris | 0 | 1.22 ± 0.04 | 7.23 ± 0.01 | 0.42 ± 0.01 | 7.23 ± 0.03 |
| 3 | 1.44 ± 0.07 | 8.52 ± 0.02 | 2.35 ± 0.12 | 8.98 ± 0.01 | |
| 7 | 1.62 ± 0.35 | 8.47 ± 0.05 | 3.14 ± 0.11 | 9.66 ± 0.07 | |
| 11 | 2.78 ± 0.33 | 8.00 ± 0.12 | 3.90 ± 0.17 | 9.95 ± 0.21 | |
| 15 | 4.40 ± 0.66 | 7.44 ± 0.06 | 3.76 ± 0.39 | 9.99 ± 0.24 | |
| Chlorococcum sp. | 0 | 1.22 ± 0.03 | 7.21 ± 0.03 | 0.42 ± 0.00 | 7.22 ± 0.03 |
| 3 | 1.22 ± 0.38 | 8.66 ± 0.22 | 1.75 ± 0.05 | 8.67 ± 0.04 | |
| 7 | 1.20 ± 011 | 8.40 ± 0.01 | 2.10 ± 0.2 | 8.91 ± 0.11 | |
| 11 | 1.75 ± 0.23 | 8.05 ± 0.07 | 3.08 ± 0.51 | 9.24 ± 0.21 | |
| 15 | 3.13 ± 0.42 | 7.52 ± 0.1 | 4.12 ± 0.45 | 9.55 ± 0.14 | |
| Coelastrella sp. | 0 | 1.22 ± 0.03 | 7.22 ± 0.04 | 0.42 ± 0.01 | 7.21 ± 0.00 |
| 3 | 1.80 ± 0.13 | 8.45 ± 0.01 | 2.00 ± 0.13 | 8.78 ± 0.02 | |
| 7 | 3.06 ± 0.12 | 8.26 ± 0.01 | 3.13 ± 0.26 | 8.97 ± 0.03 | |
| 11 | 4.25 ± 0.04 | 7.60 ± 0.04 | 4.70 ± 0.33 | 8.63 ± 0.22 | |
| 15 | 5.93 ± 0.08 | 7.00 ± 0.02 | 5.25 ± 0.33 | 8.95 ± 0.17 | |
| S. reticulata | 0 | 1.22 ± 0.00 | 7.32 ± 0.02 | 0.39 ± 0.05 | 7.32 ± 0.06 |
| 3 | 0.85 ± 0.03 | 8.41 ± 0.01 | 1.02 ± 0.08 | 8.66 ± 0.02 | |
| 7 | 0.76 ± 0.04 | 8.41 ± 0.01 | 1.04 ± 0.08 | 8.91 ± 0.04 | |
| 11 | 0.68 ± 0.4 | 8.20 ± 0.06 | 1.66 ± 0.08 | 9.24 ± 0.19 | |
| 15 | 0.75 ± 0.04 | 8.00 ± 0.01 | 1.96 ± 0.12 | 10.08 ± 0.14 | |
| Desmodesmus sp. | 0 | 1.22 ± 0.04 | 7.23 ± 0.02 | 0.42 ± 0.00 | 7.2 ± 0.05 |
| 3 | 0.92 ± 0.1 | 8.49 ± 0.02 | 1.39 ± 0.02 | 8.63 ± 0.26 | |
| 7 | 0.76 ± 0.07 | 8.43 ± 0.04 | 1.87 ± 0.11 | 8.75 ± 0.05 | |
| 11 | 0.72 ± 0.06 | 9.21 ± 0.03 | 2.54 ± 0.28 | 9.26 ± 0.17 | |
| 15 | 0.71 ± 0.07 | 8.12 ± 0.06 | 3.6 ± 0.76 | 10.49 ± 0.42 | |
3.2 Ammonium nitrogen removal
Nitrogen release into natural water bodies is the major cause of eutrophication (Chislock et al. 2013). Algae have the capability to consume nitrogen in a controlled environment and, therefore, can alleviate these consequences. Most microalgae prefer ammonium compared to other nitrogen sources (Zhao et al. 2019), which first must be converted to ammonium inside the algal cells, a process that requires energy. Therefore, integrating algal culture with anaerobic effluent treatment brings advantages (Zhao et al. 2019). The results of ammonium assimilation from PPS or CKM medium by Nordic microalgal strains during 15 days of cultivation are presented in Figure 2.
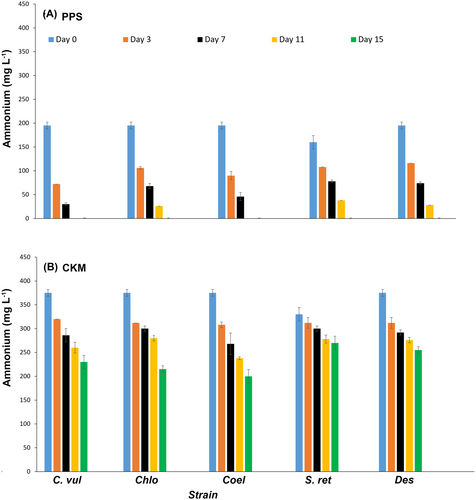
In PPS, all five strains were capable of removing 100% of the ammonium; Chlorella vulgaris and Coelastrella sp. consumed all ammonium already after 11 days. Nordic microalgae therefore outcompeted other microalgae. In the study by Zheng et al. (2019), ammonium assimilation was stopped after 6 days of Chlorella vulgaris cultivation, with an initial ammonium concentration of 220 mg L−1. The Nordic Chlorella vulgaris could consume 200 mg L−1 of ammonium within 11 days. As indicated in Table 1, the initial ammonium content of PPS was 160 mg L−1 or higher, whereas CKM contained almost 330 mg L−1 or higher of ammonium. None of the algal strains could assimilate the total ammonium in CKM within 15 days. While Coelastrella sp. showed the highest ammonium removal of 46% after 15 days of cultivation in CKM, Scotiellopsis reticulata removed only 18% (Table 3). The higher initial ammonium concentration in CKM might inhibit algal cell growth. One of the major problems in animal manure digestion, particularly poultry manure, is the high nitrogen content (Jasińska et al. 2023). The main nitrogen sources in chicken manure are uric acid (around 70%) and undigested proteins (around 30%), resulting from chickens' diet. Absorbed amino acids that cannot be utilized for protein deposition are broken down, resulting in nitrogen excretion in form of uric acid in chicken manure (Nahm 2003).
| Media | Strain | Biomass (g L−1) | Consumed NH4+- N (mg L−1) | Biomass/consumed NH4+-N | NH4+-N removal % |
|---|---|---|---|---|---|
| PPS | C. vulgaris | 1.82 ± 0.12 | 195 ± 7.07 | 9.33 | 100.00 |
| Chlorococcum sp. | 1.18 ± 0.12 | 195 ± 7.07 | 6.05 | 100.00 | |
| Coelastrella sp. | 1.84 ± 0.08 | 195 ± 7.07 | 9.44 | 100.00 | |
| S. reticulata | 1.05 ± 0.09 | 160 ± 14.14 | 6.56 | 100.00 | |
| Desmodesmus sp | 1.31 ± 0.21 | 195 ± 7.07 | 6.72 | 100.00 | |
| CKM | C. vulgaris | 1.42 ± 0.17 | 145.00 ± 5.00 | 9.79 | 38.69 ± 1.85 |
| Chlorococcum sp. | 0.84 ± 0.15 | 160.00 ± 0.00 | 5.25 | 42.67 ± 0.57 | |
| Coelastrella sp. | 1.99 ± 0.04 | 175.00 ± 15.00 | 11.37 | 46.73 ± 4.62 | |
| S. reticulata | 0.31 ± 0.07 | 160 .00 ± 0.00 | 1.94 | 18.20 ± 0.55 | |
| Desmodesmus sp | 0.25 ± 0.06 | 120.00 ± 0.00 | 2.08 | 32.01 ± 0.43 |
Jiang et al. (2021) cultivated Chlorella vulgaris on artificial digestate (modified BG-11 medium, in which nitrate was replaced by one of five different concentrations of total ammonium ranging from 50 to 500 mg L−1). At pH 7.5, which is rather comparable to the initial pH (7.1) of this study, microalgae in the cultures with 120 to 360 mg L−1 exhibited continuous rapid growth. However, noticeable inhibition occurred in the culture with an ammonium concentration of 500 mg L−1. The removal efficiencies of ammonium at concentrations of 120, 240, 360 and 500 mg L−1 were 100%, 100%, 72% and 42%, respectively. These results are comparable with the 100% ammonium removal of Chlorella vulgaris observed in PPS (initial ammonium content of 195 mg L−1), and 46% ammonium removal in CKM (initial ammonium concentration of 375 mg L−1). While the average inhibitory and toxic ammonium concentrations reported for Chlorophytes are 332 mg L−1 and 548 mg L−1, respectively (Collos and Harrison 2014), Zhao et al. (2019) reported that C. pyrenoidosa has the ability to tolerate 350 mg L−1 ammonium, when cultivated in artificial anaerobic effluent.
Comparing the ratio of produced biomass to consumed ammonium, Coelastrella sp. showed the highest yield in PPS and CKM, which were 9.44 and 11.37 (g biomass per g consumed ammonium), respectively. As expected, also Chlorella vulgaris was highly efficient in both media. The three other strains obtained similar results in the biomass per consumed ammonium ratio in PPS (Table 3). However, Scotiellopsis reticulata and Desmodesmus sp. showed the lowest value of biomass per consumed ammonium in CKM (Table 3).
3.3 VFAs consumption
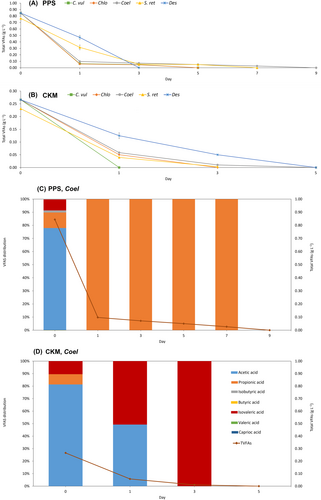
When VFAs act as carbon source for microalgae, the VFAs ratio can have a noticeable effect on growth and biomass production (Fei et al. 2011). All VFAs in PPS were consumed by the applied algal strain after 3 to 9 days of cultivation (Figure 3A). However, the VFAs in CKM effluent were consumed within just 1 to 5 days. The faster consumption in CKM medium can be attributed to the lower starting VFAs concentration, as shown in Table 1. Coelastrella sp. was the most promising algal strain in terms of biomass production and ammonium removal among all strains studied in this work. It consumed 100% of acetic acid on the first day of cultivation, while propionic acid needed 9 days to be completely assimilated (Figure 3C). This indicates that certain algal strains display a preference to assimilate specific types of organic acids, likely due to variations in their metabolic pathways for consuming VFAs (Perez-Garcia et al. 2011, Kim et al. 2019). Acetic acid can easily be transformed to acetyl-CoA as the precursor for fatty acid biosynthesis through tricarboxylic acid (TCA) cycle (Ramanan et al. 2013). Acetic acid requires less energy to be transported into cells compared to butyric acid, leading to a prioritization of acetic acid consumption over butyric acid. In addition, the energy required to transport butyric acid may be balanced by the energy produced by metabolizing butyric acid, resulting in no net energy benefit (Baroukh et al. 2017, Patel et al. 2022). Moreover, propionic and butyric acids have to pass through a more complex metabolic pathway compared to acetic acid. While 1 mol of butyric acid can be transformed to butyryl-CoA, further degraded to 2 mol of acetyl-CoA via β-oxidation, propionic acid can be a precursor of oxaloacetate (intermediate in the TCA cycle and glyoxylate pathway) (Venkata Mohan and Prathima Devi 2012, Kim et al. 2019). Propionic acid is slower assimilated by microalgae than butyric acid and this acid cannot be used as the sole carbon source (Venkata Mohan and Prathima Devi 2012, Patel et al. 2022). While, in CKM, acetic acid was completely consumed by Coelastrella sp. in the first 24 hours, propionic acid was assimilated faster than acetic acid (Figure 3D). The initial concentration of propionic acid in CKM was much lower (0.02 g L−1) than acetic acid (0.22 g L−1). In PPS and CKM, the dominant part of VFAs is acetic acid, which was consumed rapidly by all Nordic algal strains (data not shown). Acetic acid was completely assimilated within 1 day by all strains in both media, only Desmodesmus sp. grown in CKM needed 3 days for total consumption.
4 CONCLUSION
Five Nordic microalgae were studied to examine their ability to remove ammonium, VFAs and COD when cultivated in effluents from anaerobic digestion of pulp and paper sludge or chicken manure. Ammonium removal and biomass production were enhanced in PPS compared to CKM. All ammonium was assimilated in PPS (initially 195 mg L−1) within 11 or 15 days, whereas only 46% was consumed in CKM (initially 375 mg L−1) after 15 days. Coelastrella sp. cultivated in CKM generated the highest biomass concentration (1.99 g L−1), generating a biomass:consumed ammonium ratio of 11.37. While all of the strains could assimilate VFAs as carbon source, they demonstrated a preference for acetic acid in comparison to other VFAs. Coelastrella sp. (3–4), isolated recently from municipal wastewater in Northern Sweden was greatly effective in nutrient removal and has shown significant potential in sustainable biomass production. Following Coelastrella sp. also Chlorella vulgaris and Chlorococcum sp. were highly efficient; these strains are promising candidates for mitigating environmental impact of industrial anaerobic digestion effluents in Nordic countries by turning waste streams into resources.
AUTHORS CONTRIBUTION
Ghasem Mohammadkhani: Investigation, methodology, writing - original draft; Amir Mahboubi: supervision, conceptualization, writing – review & editing; Martin Plöhn: investigation, writing—review and editing, data analyzing; Christiane Funk: writing – review & editing and funding acquisition; Päivi Ylitervo: supervision, conceptualization, writing – review & editing and funding acquisition.
ACKNOWLEDGEMENTS
The authors would like to thank the University of Borås and Umeå University for their technical and financial supports.
FUNDING INFORMATION
The authors are thankful to ÅForsk (22–228) for the financial support of this project. The authors also are grateful to the Swedish Research Council FORMAS (2019–00492) to CF, Bio4Energy (www.bio4energy.se) to CF and Umeå University for their financial support.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the results reported in this study will be available from the corresponding author upon request.




