Inorganic carbon sensing and signalling in cyanobacteria
Abstract
Cyanobacteria utilize CO2 and HCO3− as inorganic carbon (Ci) sources. In low Ci, like in ambient air, cyanobacteria efficiently collect Ci using a carbon concentrating mechanism (CCM). The CCM includes bicarbonate transporters SbtA, BicA and BCT1; the specialized NDH complexes NDH-13 and NDH-14, which convert CO2 to HCO3− in the cytoplasm; and carboxysomes that are protein shell encapsulated ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCo) and carbonic anhydrase containing bodies in which the first reaction of carbon fixation occurs. Ci-dependent regulation of bicarbonate transporters and specialized NDH complexes, especially the regulation of the SbtA transporter, are well understood. CcmR (also called NdhR), CyAbrB2, CmpR and RbcR act as transcription factors regulating CCM genes. Ci signalling molecules detecting the metabolic status of the cells include 2-oxoglutarate, which accumulates when the Ci/nitrogen ratio of the cell is high, and 2-phosphoglycolate, the first intermediate of the photorespiration pathway, whose accumulation indicates low Ci. These signalling molecules act as corepressors and coactivators of the CcmR repressor protein, whereas 2-phosphoglycolate and ribulose-1,5-bisphosphate activate transcription activator CmpR. In addition, bicarbonate or CO2 activates the adenylyl cyclase that produces cAMP, and ATP/ADP/AMP provide information about the energy status of the cell. Less is known about the molecular mechanisms regulating carboxysome dynamics or how production, activity and degradation of photosynthetic complexes are regulated by prevailing Ci conditions or which mechanisms adjust cell division according to Ci. This minireview summarizes the present knowledge about molecular mechanisms regulating cyanobacterial acclimation to prevailing Ci.
1 INTRODUCTION
Life on Earth depends on photosynthetic reactions fixing CO2 to organic carbon. When cyanobacteria invented oxygenic photosynthesis, the CO2 concentration of the atmosphere was 20–100 times higher than nowadays, and oxygen concentration was negligible. In the subsequent aeons, CO2 concentration decreased and O2 concentration increased (Berner 2003). As the first enzyme of the Calvin-Benson-Bassham cycle, Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCo) functions both as a carboxylase and an oxygenase (for a recent review see Prywes et al. 2023), a carbon concentrating mechanism (CCM) evolved in cyanobacteria to actively concentrate CO2 near the active centre of RuBisCo to avoid the futile oxygenase reaction. Figure 1 summarizes the overall carbon flow in cyanobacteria.
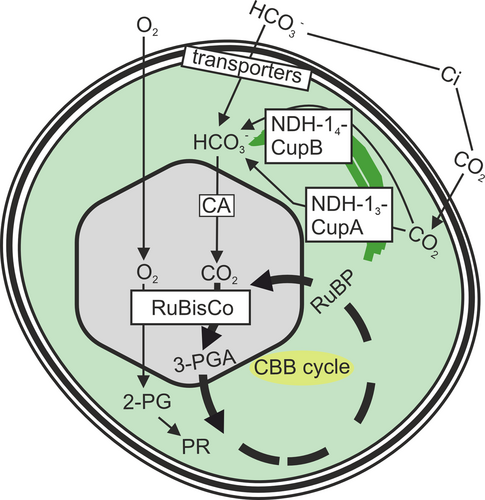
Cyanobacteria can utilize both CO2 and HCO3− as inorganic carbon (Ci) sources, the available form of Ci being highly dependent on pH. Cyanobacteria can efficiently collect Ci from aquatic environments and concentrate CO2 close to the active centre of RuBisCo using the CCM. The CCM includes three bicarbonate transporters in the cytoplasmic membrane and two specialized NDH-1 complexes facilitating CO2 uptake and conversion to HCO3− in the cytoplasm (Figure 1), although all cyanobacterial species do not contain all these complexes (Okawa and Kaplan 2003). Furthermore, protein-shielded carboxysomes, fully packed with RuBisCo and carbonic anhydrase, increase CO2 concentration and reduce O2 levels close to RuBisCo (Figure 1). If RuBisCo uses O2 as a substrate, the product, 2-phosphoglycolate (2-PG), needs to be metabolized via photorespiratory routes (Eisenhut et al. 2008). In addition, numerous auxiliary factors and regulators are required to regulate carbon metabolism according to available Ci and to balance carbon fixation with photosynthetic light reactions and nitrogen assimilation. Here, we aim to summarize what is currently known about the molecular mechanism(s) by which cyanobacteria measure the amount of available Ci and how this information is transmitted to adjust cellular functions accordingly. Understanding cyanobacterial carbon regulation will help predict the effects of climate change on primary production and offer practical solutions for construction of better cyanobacteria-based systems for carbon-neutral production and wastewater treatment in the circular economy.
2 CARBON CONCENTRATING MECHANISMS
The CCM functioning in cyanobacteria is summarized in Figure 2. The inactivation of two specialized NDH complexes and three bicarbonate transporter systems results in mutant cells that cannot grow in ambient air but grow in high CO2 conditions (Xu et al. 2008a). In acidic pH, CO2 is the prevailing form of inorganic carbon. After diffusing into the cell, CO2 is converted to HCO3− in the cytoplasm. No carbonic anhydrase has been detected in the cytoplasm of cyanobacteria, and contrary to the original expectations, expressing carbonic anhydrase in the cytoplasm leads to a high CO2 requiring phenotype, further indicating that in the cytoplasm, bicarbonate concentration is high (Price and Badger 1989). Instead of carbonic anhydrase, two specialized NDH complexes, an inducible NDH-13 (NDH-1MS) and a constitutively expressed NDH-14 (NDH-1MS’) complex have been suggested to convert CO2 to HCO3− in the cytoplasm, thus increasing the net influx of CO2 and minimizing the efflux of CO2 (Price et al. 2002; Han et al. 2017; Schuller et al. 2020; Artier et al. 2022). Both specialized NDH complexes contain a carbonic anhydrase-like protein (Shibata et al. 2001): CupA is associated with the NDH-13 complex and CupB with the NDH-14 complex (Zhang et al. 2004; Xu et al. 2008b; Han et al. 2017). Recently, the NDH-13 complex was shown to utilize a redox-driven proton-pumping system and a CO2 channel to convert CO2 to HCO3− (Schuller et al. 2020). A carbonic anhydrase-like protein, EcaB, has been shown to interact with CupA and CupB subunits and was suggested to regulate the conversion of CO2 to HCO3− by the NDH-13 and NDH-14 complexes (Sun et al. 2019a).
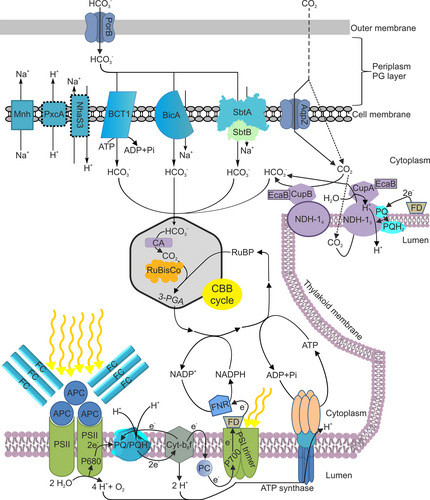
Bicarbonate dominates in alkaline conditions. HCO3− enters the periplasmic space via PorB channels, and then three bicarbonate transporters transfer it across the cell membrane (Omata et al. 1999; Shibata et al. 2002; Price et al. 2004; Woodger et al. 2007). BicA and SbtA transporters function as Na+/HCO3− symporters, requiring at least 1 mM Na+ concentration to function (Shibata et al. 2002; Price et al. 2004). SbtA is a medium affinity, low flux transporter, whereas BicA is a low affinity, high flux transporter (Shibata et al. 2002; Price et al. 2004). Knockout mutants of either Na+/HCO3− symporter alone do not show any clear growth phenotype, but simultaneous inactivation of sbtA and bicA genes decreases growth in alkaline pH (Price et al. 2004). Over-expression of the bicA gene in cyanobacteria increases photosynthetic activity, glycogen production and biomass accumulation (Kamennaya et al. 2015; Gupta et al. 2020), indicating that the availability of Ci in ambient air might be a growth limiting factor. In addition to Na+/HCO3− symporters, bicarbonate is transported via the BCT1 transporter, an ABC-type high-affinity HCO3− pump directly fuelled by ATP (Omata et al. 1999). The BCT1 subunits are encoded by the cmp operon (Omata et al. 1999). In addition, a few auxiliary complexes, including the specialized NDH-1 complex Mnh and the Na+-H+ antiporter NhaS3, whose function is required for Na+ gradient formation, and the PcxA proton pump, which maintains the cellular pH, are supporting bicarbonate influx (Kupriyanova et al. 2023).
From cytoplasm, HCO3− diffuses into carboxysomes where it is converted to CO2, and then Rubisco catalyses the fixation of CO2 to ribulose-1,5-bisphosphate (RuBP) by forming two molecules of 3-phosphoglycerate (3PGA). Cells lacking carboxysome shell proteins die in ambient air but survive in high CO2 (Cameron et al. 2013), which indicates the essential role of carboxysomes for the CCM, and on the other hand, shows that when CO2 is abundant, RuBisCo can function without being sequestered inside the carboxysome. Cyanobacterial carboxysomes have been classified into two categories: β carboxysomes and α carboxysomes (Badger et al. 2002). Both types of carboxysomes are particles where a proteinaceous shell encloses RuBisCo, carbonic anhydrase and a few auxiliary proteins, and despite their convergent evolution, α carboxysomes might have originated from gammaproteobacteria via horizontal gene transfer (Rae et al. 2013), their function and responses to environmental cues are similar (Whitehead et al. 2014). α-carboxysomes contain the 1A form of RuBisCo and the β-carbonic anhydrase CsoSCA, while β-carboxysomes contain the 1B form of RuBisCo and the carbonic anhydrase is either the γ-carbonic anhydrase domain of the scaffolding protein CcmM or a β-carbonic anhydrase CcaA (Turmo et al. 2017). The shell proteins are also distinct to α and β carboxysomes. Ci enters from the cytoplasm to carboxysomes as HCO3−, the pH inside the carboxysome is lower than in the cytoplasm, and carbonic anhydrase converts HCO3− to CO2, thus increasing CO2 concentration in the vicinity of RuBisCo active site, especially because carboxysome shell also decreases diffusion of CO2 out of the carboxysome (Turmo et al. 2017). In silico modeling of carboxysome pores (Mahinthichaichan et al. 2018; Faulkner et al. 2020) has suggested that the carboxysome shell provides a barrier against free mobility of O2, thereby reducing O2 concentration inside the carboxysome and lowering the oxygenase activity of the RuBisCo enzyme. The substrate and product of RuBisCO, RuBP and 3PGA, respectively, are assumed to move in and out of carboxysomes via pores (Turmo et al. 2017).
3 REGULATION OF BICARBONATE TRANSPORTERS
3.1 SbtA bicarbonate transporter
Regulation of bicarbonate transporters is summarized in Figure 3. SbtA is a medium affinity, low flux Na+/HCO3− symporter (Shibata et al. 2002; Price et al. 2004). The sbtA gene is non-essential, and deletion of the sbtA gene alone does not disturb the growth of cells in ambient air or in high CO2 (Shibata et al. 2002). The sbtA gene (slr1512) forms an operon with the sbtB gene (slr1513) that encodes a PII-type signalling protein (Selim et al. 2018). The expression of the sbtA operon is rapidly upregulated when cells are transferred from high CO2 to ambient air (Wang et al. 2004) and rapidly and permanently downregulated when cells are transferred from ambient air to high CO2 (Kurkela 2020). A carbon responsive transcription factor, CcmR (also called NdhR), has been shown to act as a repressor of the sbtA operon in high CO2 conditions (Wang et al. 2004), and cells without CcmR repressor protein have shown to be preadapted to low Ci even in high CO2 conditions (Daley et al. 2012; Klähn et al. 2015). CcmR is activated by 2-oxoglutarate (2-OG) that accumulates in cells when the Ci/nitrogen balance is high (Jiang et al. 2018) and by the oxidized form of nicotinamide adenine dinucleotide phosphate (NADP+) (Daley et al. 2012). On the other hand, 2-phosphoglycolate (2-PG), the first intermediate of the photorespiration pathway, inhibits CcmR when cells suffer from low Ci (Jiang et al. 2018). Thus, CcmR senses the metabolic balance of the cell.
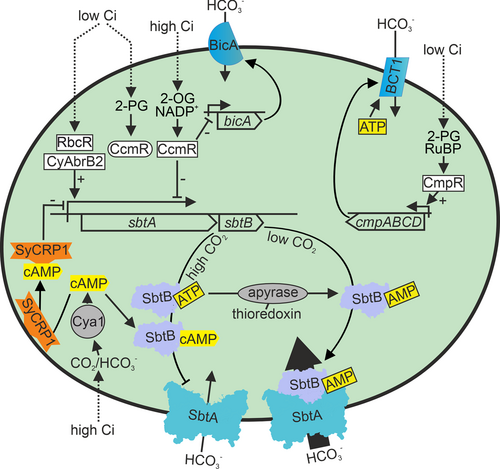
In addition to CcmR, two other transcription factors have been shown to regulate the sbtA operon, although the actual carbon signal they detect is not known. Upon high CO2 to ambient air transition, the sbtA operon is not normally up-regulated if the CyAbrB2 transcription factor is missing (Orf et al. 2016). In low carbon conditions, the RbcR transcription factor upregulates the expression of the sbtA operon (Bolay et al. 2022).
In addition to the co-repressor 2-OG that detects the carbon/nitrogen balance and the co-activator 2-PG that is related to overall Ci availability, also cyclic adenosine monophosphate (cAMP) acts as a Ci sensor molecule for the sbtA operon (Bantu et al. 2022). Adenylyl cyclases are known as evolutionary conserved bicarbonate sensors (Chen et al. 2000). Adenylyl cyclases of Spirulina platensis (Chen et al. 2000) and Anabaena sp. PCC 7120 (Cann et al. 2003) are activated by bicarbonate, although repression by bicarbonate was reported in Synechocystis (Masuda and Ono 2005). Activation by CO2 has also been suggested (Hammer et al. 2006). The cAMP receptor SyCRP1 is a membrane-bound protein in low carbon conditions but is released to the cytoplasm upon high CO2 treatment or by the addition of cAMP (Bantu et al. 2022). SyCRP1 has a binding site on the promoter region of the sbtA gene, and the accumulation of cAMP in high CO2 might thus downregulate the sbtA operon via binding of the SyCRP1 protein in the promoter region (Bantu et al. 2022).
In addition to the transcriptional response of the sbtA operon to Ci, the activity of the SbtA bicarbonate transporter is regulated. The key regulator is the PII-like protein SbtB. In high CO2 conditions, when SbtA activity is low, SbtB is mainly localized in the cytoplasm, whereas in low Ci conditions, a high portion of SbtB is found from the membrane fraction (Fang et al. 2021). In the membrane fraction, SbtB interacts with the cell membrane-localized SbtA, facilitating bicarbonate transportation (Fang et al. 2021). Structural studies show symmetric binding of the trimeric SbtB protein to a trimeric SbtA (Fang et al. 2021; Liu et al. 2021). The interaction of the SbtA transporter with the SbtB protein is regulated by numerous different adenylyl nucleotides, including AMP, ADP, ATP, cAMP, and c-di-AMP, all of them interacting with the same cleft of the SbtB protein, and competing for the binding with each other (Mantovani et al. 2022; Selim et al. 2018, 2021, 2023; Liu et al. 2021). However, only SbtB: AMP forms a complex with the membrane-bound SbtA and activates bicarbonate transporter activity (Selim et al. 2018; Fang et al. 2021). Upon high CO2 treatment, the cAMP/AMP ratio increases, less SbtB: AMP complex is formed, and the SbtA transporter activity is reduced (Selim et al. 2018). Furthermore, adenyl nucleotide control is related to the ATP/ADP diphosphohydrolase activity of the SbtB; this reaction allows the transition from an inactive SbtB: ATP complex to the active SbtB: AMP form that can interact with the SbtA complex (Selim et al. 2023). The diphosphohydrolase activity of SbtB is controlled by the redox state of the cell via thioredoxin, and is suggested to be important for diurnal regulation of bicarbonate uptake (Selim et al. 2023). In the dark, both reduction of the ATP level of the cell and activated diphosphohydrolase activity of SbtB favours the formation of SbtB-AMP, whose interaction with SbtA closes the bicarbonate channel when demand for bicarbonate is low (Selim et al. 2023). In addition to the SbtA transporter, SbtB interacts and activates the glycogen branching enzyme GlgB (Selim et al. 2021). Interaction of SbtB with GlgB requires the binding of c-di-AMP to the cytosolic SbtB protein (Selim et al. 2021). Both SbtB and c-di-AMP cyclase knockout mutants show defects in glycogen synthesis and have reduced survival rates when grown in a diurnal light rhythm (Selim et al. 2021).
3.2 BicA bicarbonate transporter
BicA is a high-flux, low-affinity sodium-bicarbonate symporter (Price et al. 2004). The BicA transporter alone allows autotrophic growth of Synechocystis cells in ambient air, albeit with reduced growth rate, if SbtA and BCT1 bicarbonate transporters and both CO2 uptake facilitating specialized NDH complexes are knocked out (Xu et al. 2008a). The BicA transporter has been suggested to function constantly, as a similar moderate bicarbonate transport rate is detected in ambient air and in 3% CO2 if BicA is the only functional bicarbonate transporter (Xu et al. 2008a). Transcripts of bicA respond differently to Ci levels in different cyanobacterial species; in Synechocystis, the amount of the bicA transcripts does not highly vary between ambient air and high CO2, whereas bicA transcripts are highly upregulated when Synechococcus sp. PCC 7002 cells are transferred from high CO2 to ambient air (Price et al. 2004; Woodger et al. 2007). The bicA gene belongs to the CcmR repressor protein regulon, but unlike the sbtA operon, the bicA gene is not regulated by the CyAbrB2 or RbcR transcription factor (Woodger et al. 2007; Klähn et al. 2015; Jiang et al. 2018).
3.3 BCT1 bicarbonate transporter
The BCT1 transporter is encoded by the cmpABCD operon (Omata et al. 1999). The cmp operon is activated under low CO2 conditions by the CmpR transcription factor (Omata et al. 2001). Two metabolite intermediates, 2-PG and RuBP, function as co-activators of CmpR (Nishimura et al. 2008; Daley et al. 2012), suggesting that low Ci, sensed by activation of the photorespiratory pathway or by accumulation of the RuBisCo substrate RuBP, activates the ATP-fuelled BCT1 bicarbonate pump (Figure 3).
4 REGULATION OF SPECIALIZED NDH-1 COMPLEXES
The regulation of NDH-1 complexes functioning in the CCM is summarized in Figure 4. The high-affinity NDH-13 and the low-affinity NDH-14 complexes are located in the thylakoid membrane (Xu et al. 2008b). Most NDH complex proteins are shared with NDH-13 and NDH-14 complexes and with the NDH complexes functioning in cyclic electron flow. The special subunits for NDH-14 are NdhD4, NdhF4 and CupB, which do not form a single operon in the genome of Synechocystis sp. PCC 6803, unlike the NDH-13 specific operon comprising ndhF3, ndhD3 and cupA genes. Overall, the shared ndh genes are moderately down-regulated when cells are transferred from ambient air to high CO2 (Kurkela 2020) and up-regulated when cells are transferred from high CO2 to ambient air (Wang et al. 2004). The NDH-14 complex specific ndhD4 and ndhF4 genes do not show a clear response to CO2 levels, whereas the cupB gene is upregulated upon high CO2 treatment and down-regulated when cells are transferred from high CO2 to ambient air. Recently, the RbcR transcription factor was shown to up-regulate many ndh genes in low carbon conditions (Bolay et al. 2022). The ndhF3-ndhD3-cupA operon belongs to CcmR and CyAbrB2 regulons and is strongly down-regulated in high CO2 and up-regulated upon transfer of cells from high CO2 to ambient air. Belonging to the CcmR operon points to metabolic control of the NDH13 complex. Possible post-transcriptional or complex level regulation of NDH-14 and NDH-13 complexes remains to be solved; biochemical regulation by proton motive force and redox state of the plastoquinone pool (Schuller et al. 2020) and by pH and light via the EcaB protein (Sun et al. 2019) have been suggested.
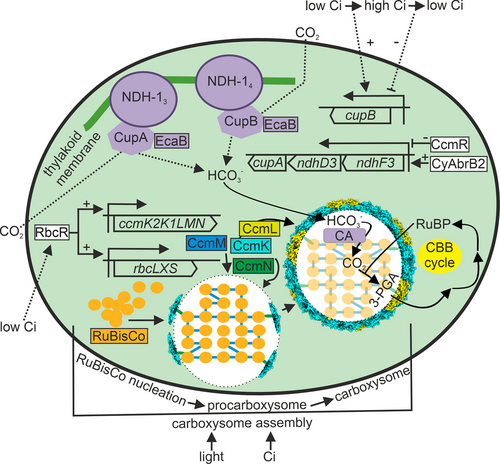
5 REGULATION OF CARBOXYSOMES
Carboxysome assembly and regulation is summarized in Figure 4. Carboxysome assembly starts with Rubisco nucleation, in which free RuBisCo enzymes form aggregates with the help of CcmM proteins to form procarboxysomes (Cameron et al. 2013). The CcmN protein joins the procarboxysome via interactions with CcmM, and then CcmN recruits shell proteins, leading to the encapsulation of part of the procarbosysome by the CcmK2 and CcmO proteins. Full encapsulation of the carboxysome further requires the CcmL protein, and finally, the fully encapsulated carboxysome is separated from the procarboxysome. The package of Rubisco inside a carboxysome is highly ordered both in α and β carboxysomes, although differently organized (Wang et al. 2019; Ni et al. 2022). The process of the formation of new carboxysomes is repeated until the procarboxysome has been depleted. Regulation of this process is not well understood.
Regulation of carboxysome degradation and dynamics are not well understood, although it is known that carboxysome formation and degradation are highly dynamic processes. During cell division, the carboxysomes of the mother cell are distributed to the daughter cells, and the carboxysome number per cell is transiently reduced (Hill et al. 2020). Furthermore, the size and RuBisCo content of a single carboxysome vary according to environmental conditions. In ambient air, the size and RuBisCo content of carboxysomes increase with light intensity, whereas high CO2 treatment leads to reduced carboxysome diameter and RuBisCo content (Sun et al. 2019b). In addition, the number of carboxysomes increases with light intensity, and inhibition of photosynthetic electron flow blocks the synthesis of new carboxysomes (Sun et al. 2016). The number of carboxysomes per cell is higher in ambient air than in high CO2 (McKay et al. 1993; Whitehead et al. 2014). The addition of a sugar efflux transporter to the cyanobacterium Synechococcus elongates PCC 7942 increased the number of carboxysomes (Singh et al. 2022). All these findings suggest a metabolic control of the production of new carboxysomes and inactivation and degradation of unnecessary ones. Breakage of the carboxysome shell might be the main mechanism of the inactivation of the carboxysome (Hill et al. 2020). Release of free RuBisCo occurs during carboxysome breakage, and released RuBisCo enzymes have been suggested to be recirculated (Hill et al. 2020).
The RuBisCo operon rbcLXS and the main carboxysome shell protein operon ccmK2K1LMN show only moderate downregulation upon transfer from ambient air to high CO2 (Kurkela 2020) and moderate upregulation upon transfer of cells from high CO2 to ambient air (Wang et al. 2004). For known carbon-responding transcriptional regulators, the RbcR transcription factor is the main regulator of the carboxysome genes, as RbcR activates expression of the rbcLXS and ccmK2K1LMN operons in low Ci, whereas the rbcLXS and ccmK2K1LMN operons do not belong to CcmR, CyAbrB2 or CmpR regulons (Bolay et al. 2022).
6 REGULATION OF PHOTOSYNTHESIS AND GROWTH
As described above, molecular mechanisms regulating the CCM according to available Ci are already partially resolved. However, molecular mechanisms regulating growth and photosynthesis according to available Ci are less well understood. A direct link between CO2 and photosynthetic light harvesting was recently discovered as CO2 was shown to be bound to the α subunit of allophycocyanin, causing a structural change that enhances the energy transfer efficiency within the phycobilisome (Guillén-García et al. 2022). Furthermore, Photosystem II activity is influenced by two bicarbonate molecules that bind to the acceptor and donor sides of Photosystem II (Shevala et al. 2020) and might provide a direct link to how scarcity of inorganic carbon could regulate light reactions. The actual signals controlling cell division in cyanobacteria are not known, and how cell division is linked to photosynthetic activity remains to be solved as well. Obviously, the amount of Ci limits growth in ambient air despite the function of the CCM, as growth is highly upregulated when cyanobacteria are transferred from ambient air to high CO2 (Kurkela et al. 2017).
Only two mutants have been reported to have a high-CO2 lethal phenotype (Figure 5). The first mutant is a merodiploid Synechococcus sp. PCC 7942 strain, containing the topA topoisomerase gene inactivated by an antibiotic resistance cassette (Gabay et al. 1998). The reasons for the CO2 lethality of the merodiploid topA inactivation strain remain to be solved, but fast-growing cells may need a more efficiently functioning topoisomerase than slowly growing cells. Topoisomerases change DNA topology and play central roles during DNA replication, transcription, recombination and chromosome segregation. The other CO2 lethal mutant is the ΔrpoZ strain of Synechocystis sp. PCC 6803, in which the small ω subunit of the RNA polymerase has been knocked out (Gunnelius et al. 2014; Kurkela et al. 2017). In ambient air, ΔrpoZ cells grow like control strain cells, although most genes encoding CCM proteins are down-regulated (Gunnelius et al. 2014). In many cases, cells with malfunction of the CCM show impaired growth in ambient air but grow well in high CO2 (Shibata et al. 2001; Xu et al. 2008a; Cameron et al. 2013). To our surprise, ΔrpoZ cells did not benefit from high CO2, but instead, the ΔrpoZ culture survived only a few days in high CO2 (Gunnelius et al. 2014; Kurkela et al. 2017). Further analysis of ΔrpoZ cells in high CO2 showed that typical acclimation responses of photosynthesis to high CO2 are largely missing in the ΔrpoZ strain (Kurkela et al. 2017). Furthermore, carbon skeletons that are produced in high CO2 are used to synthesize the storage compound glycogen, which accumulates in high quantities (Kurkela et al. 2017), indicating that low photosynthetic activity is not the only reason for slow growth, but in addition, something else prevents growth of ΔrpoZ cells in high CO2.
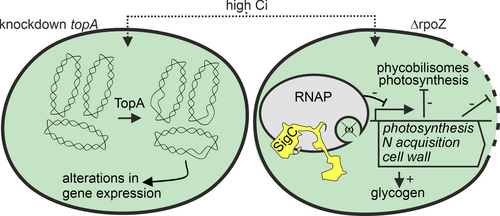
Deletion of the ω subunit from the RNA polymerase core seems to affect the recruitment of different sigma subunits (σ) by the RNA polymerase core, not only in cyanobacteria but also in other bacteria (for a review see (Kurkela et al. 2020). The RNA polymerase core recruits one of the σ subunits to form the transcription initiation competent RNA polymerase holoenzyme, and depending on which of the numerous σ subunits the RNA polymerase holoenzyme is bearing, the holoenzyme will recognize a certain set of promoters. Thus, replacement of one σ subunit with another one in the RNAP holoenzyme will change the transcriptome of the cell. Indeed, the σ subunits are considered as main regulators of gene expression in cyanobacteria. The SigC factor has been connected to low carbon acclimation (Gunnelius et al. 2010) and to growth restriction during nitrogen deficiency (Antal et al. 2016; Heilmann et al. 2017) or the stationary growth phase (Asayama et al. 2004). In the ΔrpoZ strain, SigC containing RNA polymerase holoenzyme is more abundant in high CO2 than in the control strain, and expression of many phycobilisome, photosynthetic light reactions, cell division and nitrogen metabolism genes are down-regulated at the transcriptional level (Figure 5; Kurkela 2020).
Carbon and nitrogen metabolism are known to be tightly balanced under different environmental conditions, although molecular mechanisms are not yet fully understood; for an excellent review see Forchhammer and Selim (2020). The metabolite 2-OG plays a central role in signalling the C: N status of the cells, as it regulates both CO2 metabolism by controlling the transcription factor CcmR and nitrogen metabolism via the PII protein and the NtcA transcription factor (Forchhammer and Selim 2020). In addition, adenosine nucleotides directly bind to the PII protein, regulating nitrogen metabolism according to the energy status of the cells (Forchhammer and Selim 2020), and also control the expression and activity of the bicarbonate transporter SbtA (Selim et al. 2018, 2023; Mantovani et al. 2022). However, more research is required to find out which signals and signalling routes regulate light harvesting processes and photosynthetic light reactions, balance light reactions and carbon fixating reactions, and adjust cell divisions according to prevailing Ci concentration.
7 CONCLUSIONS
CCM efficiently collect CO2 and HCO3− in low Ci conditions. The functioning of the CCM is highly regulated and depends not only on the amount of available Ci but also on the energy and nutrient status of the cells. The molecular mechanisms regulating the CCM, photosynthesis and growth according to Ci levels are only partially known. By now, regulation of the bicarbonate transporter SbtA is known best. The signalling molecules include 2-OG (measure of Ci/nitrogen balance), 2-PG (accumulates when RuBisCo functions as oxygenase due to low amount of CO2) and adenylyl nucleotides (energy status of the cell); the regulation functions at the transcriptional level by transcription factors CcmR, CyAbrB2 and RbcR, and in addition the regulatory protein SbtB directly modifies the activity of the SbtA transporter. These same transcription factors also regulate the bicA gene, whereas the metabolites 2-PG and RuBP function as co-activators of the transcription activator protein CmpR, which, in turn, activates the operon encoding the BCT1 transporter in low CO2. Transporter level regulation of BicA and BCT1 remains to be solved.
The operon encoding NDH-13 complex belongs to CcmR and CyAbrB2 regulons and is highly activated in low Ci, whereas the expression of NDH-14 encoding genes does not show as clear of a response to Ci level. Understanding the functional-level regulation of these specialized NDH-1 complexes is only in the beginning.
Regulation of RuBisCo and the main carboxysome shell operon in β cyanobacteria was recently shown to be activated by the RbcR transcription factor in low CO2, but the signal sensed by RbcR remains to be solved. The molecular mechanisms regulating carboxysome dynamics, photosynthetic complexes and growth according to prevailing Ci conditions are not yet understood. Similarly, further studies are required to understand how light and carbon fixing reactions are balanced at the molecular level, how carbon and nitrogen metabolisms are balanced and which signalling routes are involved.
AUTHOR CONTRIBUTIONS
Juha Kurkela designed and prepared the illustrations, Taina Tyystjärvi conceptualized the idea of the mini-review and wrote the manuscript together with Juha Kurkela.
ACKNOWLEDGEMENTS
We thank the Research Council of Finland (grant 347172) for financial support.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analysed in this study.




